- 1Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 2Nephrology Department, Kidney Research Institute, Chang Gung Memorial Hospital in Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 3Department of Medical Education, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 4Department of Dermatology, National Taiwan University Hospital, Taipei, Taiwan
- 5Division of Allergy, Asthma, and Rheumatology, Department of Pediatrics, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 6Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou, Taiwan
- 7Cardiovascular Department, Chang Gung Memorial Hospital at Linkou, Chang Gung University School of Medicine, Taoyuan, Taiwan
- 8Graduate Institute of Nursing, Chang Gung University of Science and Technology, Taoyuan, Taiwan
- 9Division of Nephrology, Department of Internal Medicine, Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan, Taiwan
- 10Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan
- 11Division of Critical Care Surgery, Department of Critical Care Medicine, Veterans General Hospital, Kaohsiung, Taiwan
Background: Advanced chronic kidney disease (CKD) patients are at higher risk of sepsis-related mortality following infection and bacteremia. Interestingly, the urate-lowering febuxostat and allopurinol, both xanthine oxidase inhibitors (XOis), have been suggested to influence the sepsis course in animal studies. In this study, we aim to investigate the relationship between XOis and infection/sepsis risk in pre-dialysis population.
Methods: Pre-dialysis stage 5 CKD patients with gout were identified through the National Health Insurance Research Database (NHIRD) in Taiwan from 2012 to 2016. Outcomes were also compared with national data.
Results: In our nationwide, population-based cohort study, 12,786 eligible pre-dialysis stage 5 CKD patients were enrolled. Compared to non-users, febuxostat users and allopurinol users were associated with reduced sepsis/infection risk [hazard ratio (HR), 0.93; 95% confidence interval (CI), 0.87–0.99; P = 0.0324 vs. HR, 0.92; 95% CI, 0.86–0.99; P = 0.0163]. Significant sepsis/infection-related mortality risk reduction was associated with febuxostat use (HR, 0.68; 95% CI, 0.52–0.87). Subgroup analysis demonstrated preference of febuxostat over allopurinol in sepsis/infection-related mortality among patients younger than 65 years of age, stain users, non-steroidal anti-inflammatory drug non-users, and non-diabetics. There was no significant difference in major adverse cardiac and cerebrovascular event (MACCE) risk between users and non-users while reduced risk of all-cause mortality was observed for XOi users.
Conclusions: Use of XOi in pre-dialysis stage 5 CKD patients may be associated with reduced risk of sepsis/infection and their related mortality without increased MACCE and overall mortality.
Introduction
Preventive measures against infection in chronic kidney disease (CKD) patients are vital, since infectious complications are significant sources of both morbidity and mortality among these patients (1). One such complication is sepsis, which is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (2). Interestingly, uric acid (UA), the end product of purine metabolism that is often elevated in CKD patients, has also been suggested as a biomarker for sepsis severity (3), while serum UA level at the initiation of dialysis has been associated with infection-related mortality (4). Furthermore, gout has been associated with increased risk of cancer while hyperuricemia predicts increased cancer mortality risk (5). Taken together the observed connection of gout with both infection and cancer, it is reasonable to hypothesize that gout patients may suffer an impairment or dysfunction of the immune system.
Not surprisingly, marked increase in the activity of xanthine oxidase, an enzyme necessary in the generation of uric acid, is also observed in non-surviving septic patients (6). Therefore, targeting xanthine oxidase and thus uric acid production may potentially influence infection and sepsis risk. Allopurinol and febuxostat are the two commonly prescribed xanthine oxidase inhibitors (XOis) used for the treatment of gout in CKD. In studies of septic mice and rats, allopurinol treatment has demonstrated both protection against sepsis and aggravation of sepsis (7). Mice treated with febuxostat has exhibited reduced sepsis-induced liver and kidney injuries and mortality (8, 9). In humans, one clinical trial has actually reported upper respiratory tract infection as a frequently occurring adverse effect for both allopurinol users and febuxostat users (10). Since studies on the role of XOis in infection/sepsis risk have been largely animal-based and scarce, no conclusion can be drawn at present.
In our nationwide population-based cohort study, we aimed to investigate the relationship between xanthine oxidase inhibitor use (either allopurinol or febuxostat) and the risk of infection/sepsis and their related mortality in CKD patients. Since lower estimated glomerular filtration rate (eGFR) in CKD has been correlated with higher risk of infection-related death (11), we exclusively selected stage 5 CKD patients, those predicted as most vulnerable to infectious complications, in this study. The commonly debated concerns of XOi, i.e., major adverse cardiac and cerebrovascular event (MACCEs) and all-cause mortality, were also evaluated.
Methods
Data Source
The present study used data from the NHIRD, which contained complete healthcare utilization data of ~24 million persons enrolled under the universal National Health Insurance (NHI) program in Taiwan and has been demonstrated to be a reliable source for population studies (12). This study complied with the Declaration of Helsinki and Declaration of Taipei (on ethical considerations regarding health databases and biobanks) of the World Medical Association and was approved (approval serial number: 201801872B0) by the institutional review board at Chang Gung Medical Foundation. Informed consent was waived due to using administrative data with de-identified (encrypted) personal information.
Design and Study Participants
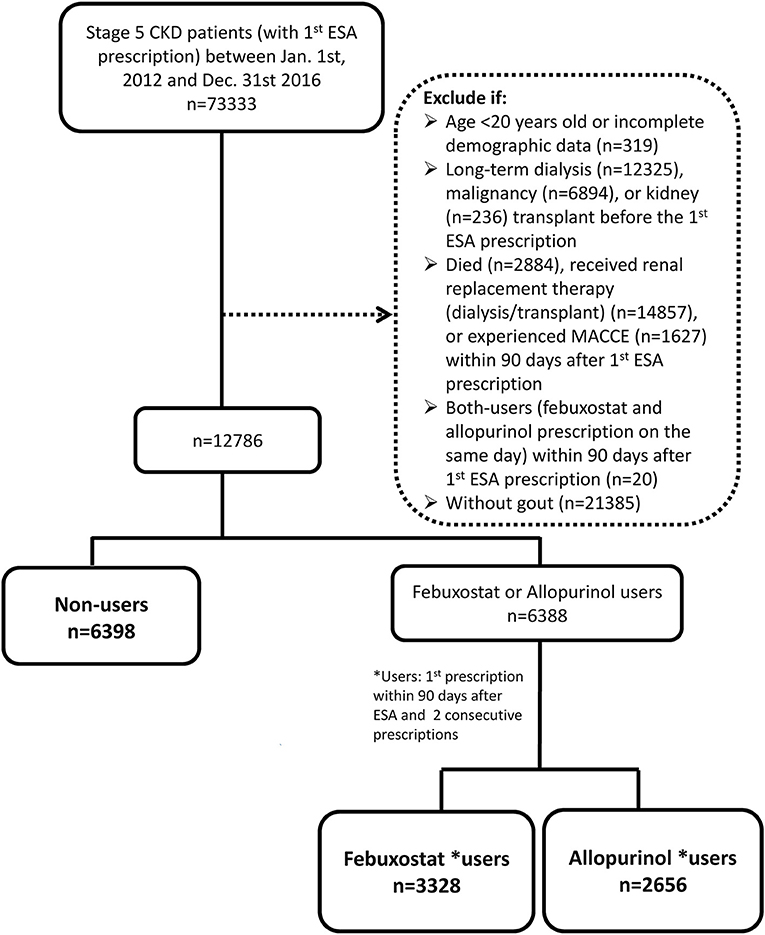
This study was a population-based retrospective cohort study. We selected patients who had at least twice been diagnosed with both CKD and gout. The diagnostic codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10 were used to define the diseases (Supplementary Table 1). Erythropoietin-stimulating agent (ESA) prescription was utilized to ascertain stage 5 CKD status, since the NHI program reimbursed its use in CKD patients with serum creatinine >6 mg/dL and hematocrit ≤28% before November 30, 2015. These reimbursement criteria guaranteed stage 5 CKD status (eGFR <15 mL/min/1.73 m2) based on the 4-variable Modification of Diet in Renal Disease Study equation and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (13, 14). Starting on December 1, 2015, the NHI altered the reimbursement criteria to stage 5 CKD patients with hemoglobin level <9 g/dL. Thus, patients who satisfied the above criteria with a first ESA prescription date between January 1, 2012 and December 31, 2016, were initially selected for this study.
We excluded patients with incomplete demographic data, those younger than 20 years (legal age based on the Civil Code), and those who had received renal replacement therapy before ESA prescription. Patients who had been diagnosed with cancer prior to ESA prescription since ESA was reimbursed for cancer chemotherapy-related anemia. Since we used prescription information within 90 days after ESA treatment to ascertain anti-hyperuricemic use, the 91st day after ESA prescription was set as the index date (15). Patients who died, required renal replacement therapy, or experienced MACCEs within 90 days after ESA prescription were also excluded (Figure 1). We were able to control for survival bias by selecting patients who survived to the 91st day after ESA prescription and following them after this exposure time window (16).
Patients with febuxostat or allopurinol prescription within 90 days after ESA prescription and at least one consecutive prescription of the same drug thereafter were categorized as febuxostat users or allopurinol users. Non-users were patients without prescription of either XOi within 90 days after ESA prescription. All analyses were conducted on an intention-to-treat basis. Other covariates, such as comorbidities, were defined as diseases with at least two outpatient diagnoses or one inpatient diagnosis within 3 years before the index date. Medication use was defined as having any prescription record after the index date.
Outcomes: Sepsis/Infection, Sepsis/Infection-Related Mortality, Major Adverse Cardiac and Cerebrovascular Events, and All-Cause Mortality
The observation period started on the 91st day after ESA prescription (the index date) and ended on the date of death or December 31, 2017 (the end date of the study), whichever came first. The outcomes of interest were sepsis/infection, sepsis/infection-related mortality, MACCEs, and all-cause mortality. The diseases included in MACCEs and sepsis/infection in this study are listed in Supplementary Table 1. The outcomes were identified by having the corresponding primary diagnosis as inpatients or emergency department visits (Supplementary Table 1) or the underlying cause of death.
Statistical Analysis
For propensity score weighting (PSW), we used stabilized weights to preserve the sample size of the original data, produce appropriate estimation of the variance of main effect, and maintain an appropriate type I error rate (17). Absolute standardized mean difference (ASMD) was applied to compare baseline characteristics between propensity-score weighted groups, with ASMD ≤0.1 indicating non-significant difference. The Kaplan-Meier Estimator was applied to generate survival probability curves, which were compared by the log-rank test. We applied the multivariate Cox proportional hazards model, which adjusted for age, sex, place of residence, income level, occupation, comorbidities, and other medication use to compare the risks of the anticipated outcomes. The proportional hazards assumption was checked and met using the log–log plot. For the sepsis/infection and MACCE outcomes, observations were censored on the date of death or the end of the study. Subgroup analyses comparing febuxostat and allopurinol use were performed for the sepsis/infection and the sepsis/infection-related mortality outcomes. All p-values were two-sided, and the significance α level was set at 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Patient Characteristics
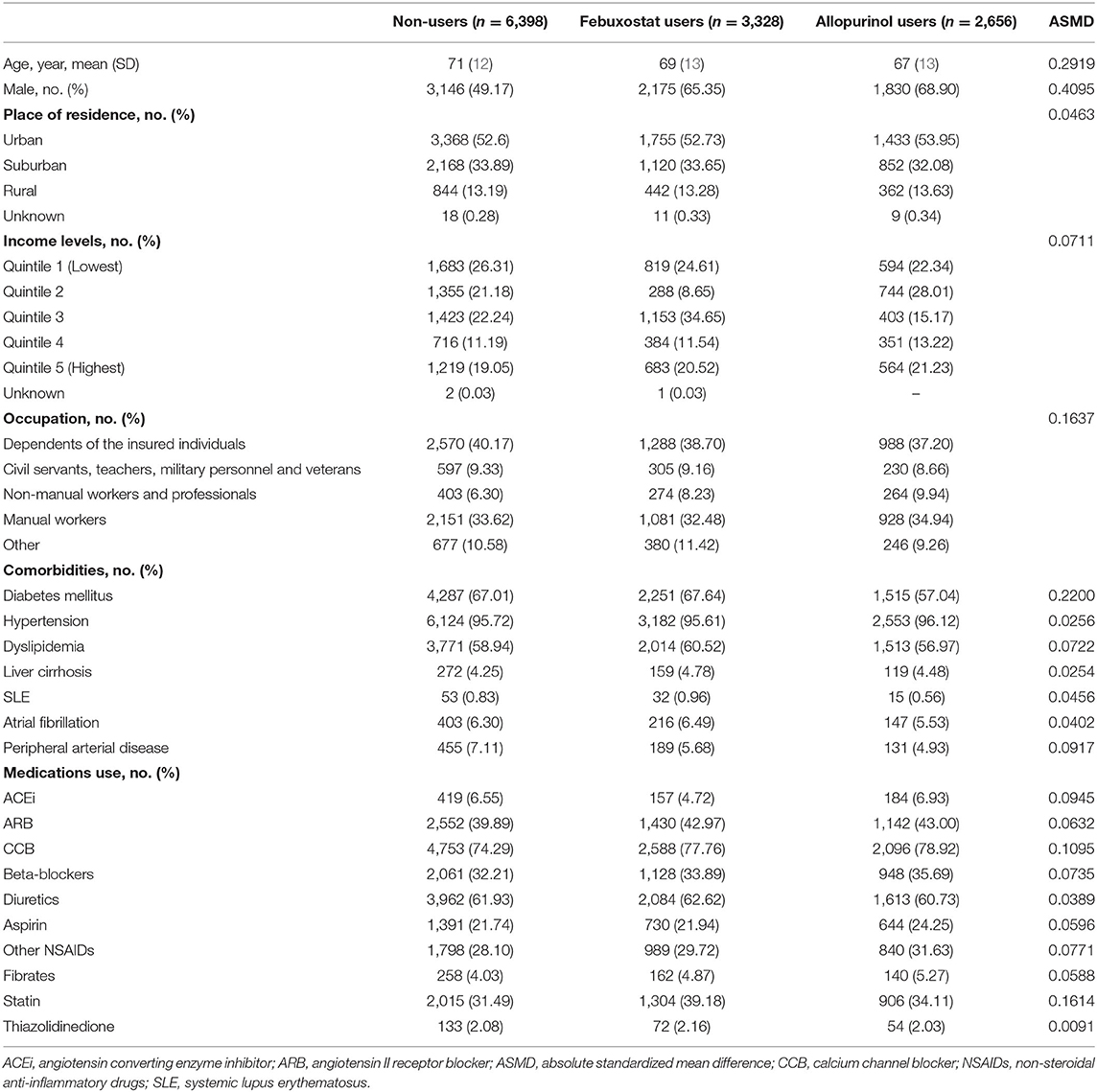
The study initially included 12,786 pre-dialysis advanced CKD patients with gout. Non-users (n = 6,398) were patients without prescription of any XOi within 90 days after ESA prescription. Patients who were prescribed the same XOi at least twice consecutively were included and divided into febuxostat users (n = 3,328) and allopurinol users (n = 2,656). The mean age of patients was 71, 69, and 67 years among non-users, febuxostat users, and allopurinol users, respectively (Table 1). Except for the non-user group, which consisted of 49.17% male, both XOi user groups were predominantly male. The most common comorbidities among all groups were hypertension, diabetes mellitus, and dyslipidemia, in the order of most to least common. Supplementary Table 2 shows the patient characteristics after propensity score weighting, which demonstrates no significant difference across all variables with the exception of place of residence.
Outcomes: Sepsis/Infection and Sepsis/Infection-Related Mortality, MACCE, and All-Cause Mortality
Four outcomes were examined during the period from 90 days after January 1, 2012 to December 31, 2017 in pre-dialysis stage 5 CKD patients. These outcomes included sepsis/infection, sepsis/infection-related death, MACCEs, and all-cause mortality. Outcome definitions were stated in Methods.
For the sepsis/infection outcome, the incidence was highest among non-users of XOis (25.67 per 100 person-years; Table 2). Compared to the reference group (non-user group), both febuxostat users and allopurinol users had lower risk of sepsis or infection [hazard ratio (HR), 0.93; 95% confidence interval (CI), 0.87–0.99; P = 0.0324 vs. HR, 0.92; 95% CI, 0.86–0.99; P = 0.0163]. Survival curves by means of the Kaplan-Meier estimator were generated and analyzed using the log-rank test. Sepsis/infection survival curves similarly demonstrated significant survival benefit for febuxostat and allopurinol users (Figure 2A).
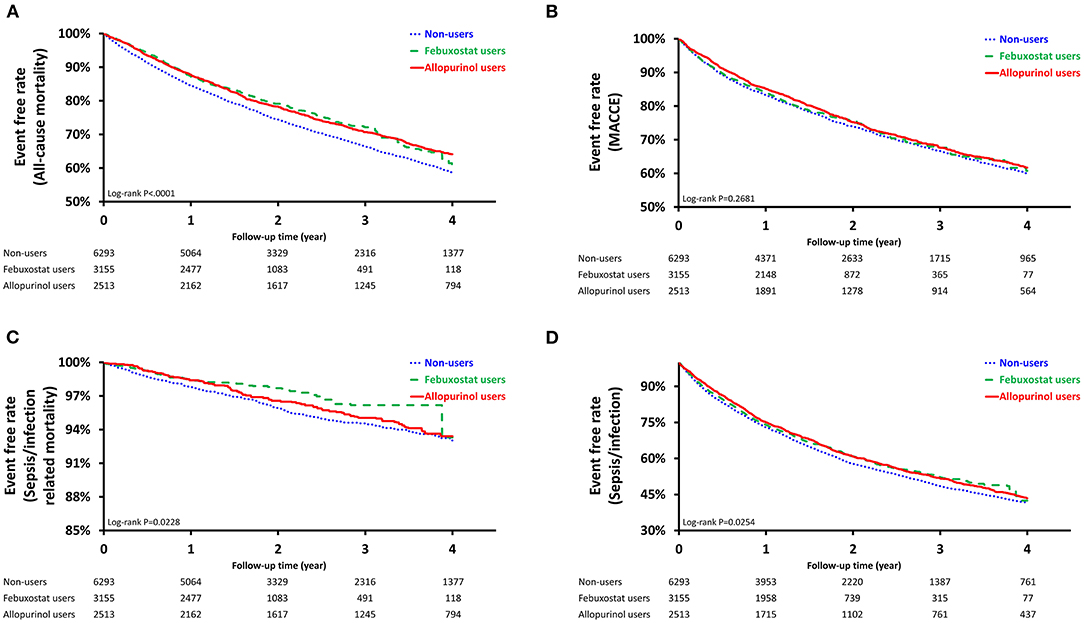
Figure 2. Survival Probability of Pre-Dialysis Stage 5 CKD Patients. Survival curves were created using the Kaplan Meier estimator. (A): Sepsis/Infection (Log-rank test, P < 0.0254); (B): Sepsis/Infection-Related Mortality (Log-rank test, P < 0.0228); (C): Major Adverse Cardiac and Cerebrovascular Events, MACCEs (Log-rank test, P < 0.2681); (D): All-Cause Mortality (Log-rank test, P < 0.0001).
The sepsis/infection-related mortality incidence was lowest among febuxostat users both before and after PSW (1.3 per 100 person-years vs. 1.33 per 100 person-years; Table 2). Though both febuxostat users and allopurinol users had lower risk of sepsis or infection-related mortality, only the former exhibited significant risk reduction after PSW (HR, 0.68; 95% CI, 0.52–0.87; P = 0.0027 vs. HR, 0.89; 95% CI, 0.71–1.10; P = 0.2834). Significant differences in survival among non-users, febuxostat users, and allopurinol users were similarly demonstrated after propensity-score weighting (P = 0.0228; Figure 2B).
Before PSW, allopurinol users were associated with the lowest incidence of MACCEs (12.34 per 100 person-years; Table 2) and lower risk of MACCE compared to non-users (HR, 0.88; 95% CI, 0.81–0.95; P = 0.0020). No significant difference was observed in MACCE-free survival among the three groups after PSW (P = 0.2681; Figure 2C). Both febuxostat use and allopurinol use were associated with reduced risk of all-cause-mortality after PSW (HR, 0.82; 95% CI, 0.76–0.90; P < 0.0001 vs. HR, 0.83; 95% CI, 0.77–0.90; P < 0.0001; Table 2). After PSW, significant difference in survival among the three groups was observed (P < 0.0001; Figure 2D).
Subgroup Analysis: Sepsis/Infection and Sepsis/Infection-Related Mortality
To determine whether patients with specific comorbid conditions were associated with reduced risk of sepsis/infection and their related mortality when choosing one XOi over the other, subgroup analysis was performed (Figures 3A,B). Comparison between allopurinol and febuxostat showed no significant difference in the risk of developing sepsis or infection in all subgroups. On the other hand, for sepsis/infection-related mortality, febuxostat use was associated with significant risk reduction for those younger than 65 years of age (HR, 0.38; 95% CI, 0.16–0.94; P = 0.0366), non-diabetics (HR, 0.51; 95% CI, 0.27–0.98; P = 0.0447), NSAID non-users (HR, 0.66; 95% CI, 0.45–0.97; P = 0.0354), and statin users (HR, 0.48; 95% CI, 0.26–0.87; P = 0.0155).
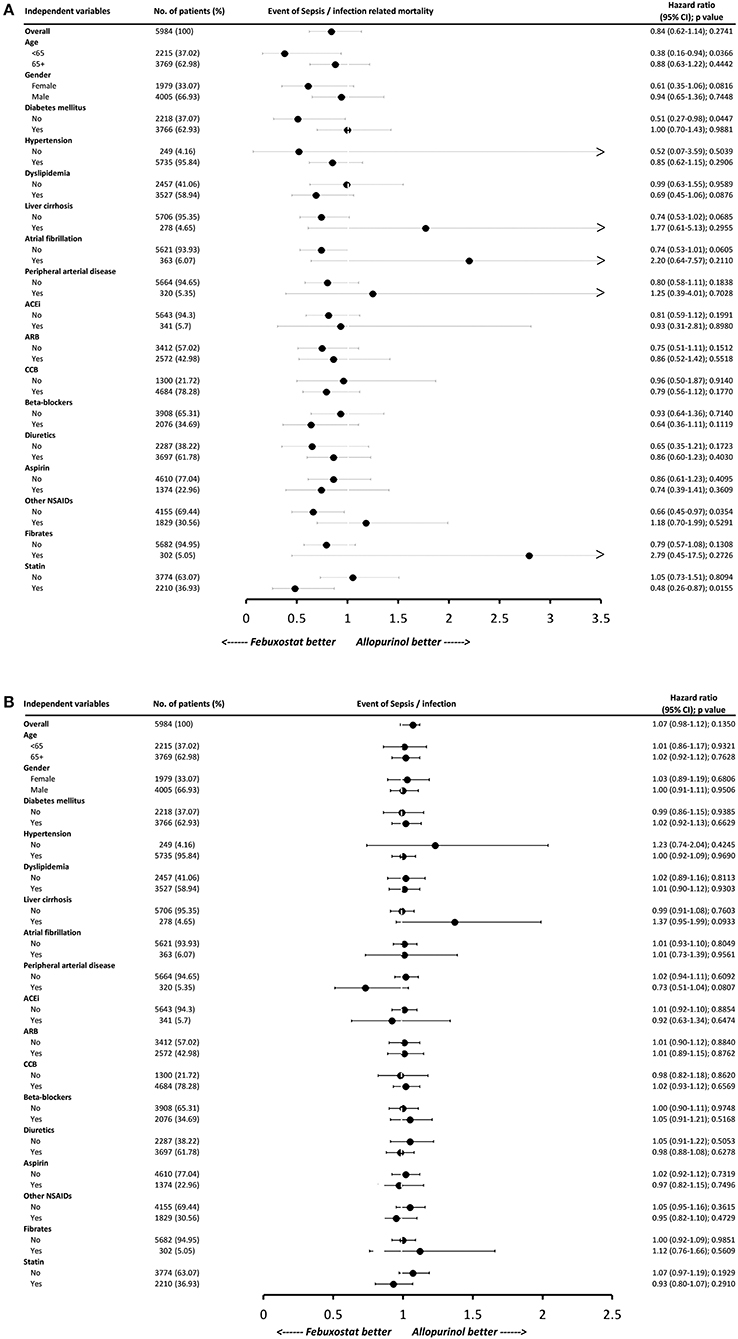
Figure 3. Subgroup analysis of (A) sepsis/infection-related mortality and (B) sepsis/infection. Hazard ratios (HRs) less than one favors febuxostat use. Each variable was adjusted for all other variables listed in Table 1. HBV indicates hepatitis B virus infection; HCV, hepatitis C virus infection; SLE, systemic lupus erythematosus; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; NSAID, non-steroidal anti-inflammatory drug.
Discussion
In our large, population-based cohort of pre-dialysis stage 5 CKD patients, xanthine oxidase inhibitor use was associated with significant reduction in risk of sepsis or infection. As for sepsis/infection-related mortality, though both XOis demonstrated risk reduction, only febuxostat showed significance. Both drugs were also associated with non-significantly decreased MACCE risk and significantly reduced all-cause mortality risk when compared to non-users. These findings suggest the overall safety of both XOis in advanced CKD.
Oxidative stress is implicated in the pathomechanism of systemic inflammatory response syndrome and sepsis-induced organ failure (18). Interestingly, uric acid has a complex role in oxidative stress as both a scavenger of free radicals (anti-oxidant) and a pro-oxidant (19). It has been previously demonstrated in a rabbit study that allopurinol, via its antioxidant effects, reduced atrial remodeling induced by diabetes mellitus-related increases in oxidative stress (20). In lipopolysaccharide-(LPS-)induced sepsis in mice, coadministration of allopurinol aggravated septic shock, leading to mortality, renal function impairment, high reactive oxygen species, and high proinflammatory interleukin levels while coadministration of febuxostat did not potentiate sepsis (7). However, studies of septic mice and rats have suggested both protective and aggravating effects of allopurinol on sepsis (7, 21). As for febuxostat, one mice study has exhibited reduced sepsis-induced liver and kidney injuries, oxidative stress, and mortality (9). Another study similarly demonstrated accelerated recovery of pulmonary endothelial barrier and improved survival in LPS-induced septic mice treated with febuxostat (8). Evidence from animal studies so far seemed to suggest febuxostat as neutral or beneficial, echoing our result that febuxostat users were associated with significant reduction of sepsis/infection-related mortality. An explanation for the non-significant risk reduction among allopurinol users may be the difficulty in achieving optimal allopurinol dose in CKD for adequate gout control (22). One study showed that patients with escalated allopurinol dose experienced an increase in all-cause mortality (23). Furthermore, underlying mechanistic differences between the two XOis may contribute to the difference but are beyond the scope of our cohort study. Whether observations from animal studies could be applied to humans still warrant investigation.
It is also possible that uric acid and xanthine oxidase inhibitors alter the immune response in the event of infection. In the context of viral infection in humans, significantly increased levels of uric acid were found in lung aspirate samples of hospitalized infants positive for RSV infection (24). Further investigation in mice demonstrated that uric acid upregulation during RSV infection exacerbated the Th2 response, while treatment with allopurinol demonstrated reduction of inflammatory infiltrates and mucus in the airways and Th2 cytokines. However, whether uric acid plays a role in the immune response against non-pulmonary or non-viral infections still warrants further investigation.
With the increase in XOi use among gouty CKD patients, potential side effects of these medications have become important. This rise in prescription is at least partially contributed to hyperuricemia's suggested role in the progression to end-stage renal disease with studies demonstrating renoprotective potential of XOis (25–28). Furthermore, hyperuricemia is widely accepted as a risk factor for cardiovascular diseases, perhaps partially due to promotion of chronic formation of neutrophil extracellular traps, thereby contributing to vascular damage (29). Ironically, cardiovascular (CV) event risk associated with XOi use has been an unresolved topic of debate. The U.S. Food and Drug Administration previously warned against increased CV risk of febuxostat based on comparison with allopurinol in a randomized controlled trial by White et al. (30). On the other hand, the FAST trial has demonstrated that febuxostat is non-inferior to allopurinol with respect to the primary cardiovascular events without an increased risk of death or serious adverse events, including infections (31), further suggesting febuxostat's relative safety. In fact, several cohort studies have found that XOi use (either allopurinol or febuxostat) is not associated with change in cardiovascular risk and even proposed reduced risk of CV events with allopurinol use (32, 33). As for patients with CKD, the FEATHER trial has found no increase in cardiovascular events with febuxostat in comparison to placebo in CKD stage 3 patients (34). In terms of overall survival, previous studies have suggested improvement with allopurinol use (35, 36). When comparing the all-cause mortality of the two XOis, febuxostat has been found as both comparable and at higher risk to allopurinol (37). However, in our previous study, febuxostat was associated with superior renoprotection without compromising survival compared to allopurinol in CKD 5 patients (38). Without a consensus, further studies focusing on the mortality risk of the different XOis in CKD 5 patients are necessary.
In the present national cohort study, the gender distribution is not matched with the control group, as both XOi user groups were predominantly male. It is possible that the different baseline gender distribution between groups does not reflect disease severity, but rather, merely differential clinician practice. Studies have shown that the prescription rate of medications may be inherently different between genders. In a United Kingdom study of 40 practices with 300,000 people, compared with men with gout, prescription of allopurinol was lower for women with gout across all age strata, except for the highest age group (39). Second, it is also plausible to infer that the non-user group is not treated with anti-hyperuricemic agents because these patients are diagnosed with gout more recently. It has been demonstrated that as compared with men with gout, women with gout were older (mean age 70 vs. 58, p < 0.001) (40), consistent with the finding that menopause increases the risk of gout and that postmenopausal hormone therapy modestly reduces gout risk (41). Therefore, it is reasonable that the slightly female-predominant non-user group consisted of more newly diagnosed gout patients, hence no febuxostat or allopurinol use yet. If this inference were true, whether these patients were associated with lower risks of the study outcomes deserved future investigation and would provide further evidence that treatment with anti-hyperurecemic agents are associated with reduced risk of our study outcomes. Third, we should consider whether gender solely could modulate the risks of study outcomes, especially those that demonstrated statistical significance in our study. Interestingly, data from a recent Coronavirus disease 2019 study has found higher mortality among men and suggested the protective role of estradiol (42). Advanced CKD patients on dialysis often suffer from genitourinary, pulmonary, septicemia, and CVC related infections, with women having higher infection rates (43). However, both genders have been reported to have higher mortality rate following severe infection or sepsis (44–47). Therefore, whether gender should be considered a reliable predictor of infection-related mortality requires further validation. In terms of all-cause mortality among CKD patients, though men are observed to have higher all-cause mortality than women at all levels of eGFR (48), our male-dominant febuxostat or allopurinol group is still associated with mild but significant reduction in all-cause mortality compared to the non-user group. Last but not least, the data presented in Table 2 were propensity-scored weighted and adjusted for possible confounding factors listed in Table 1, including gender.
Our subgroup analyses found that younger patients (<65 years of age), non-diabetics, NSAID non-users, and statin users prescribed febuxostat were associated with significant decrease in the risk of sepsis/infection-related death compared to those prescribed allopurinol. It is possible that having fewer comorbidities contributed to the reduced risk of sepsis/infection-related mortality in younger patients using febuxostat. Since studies comparing the sepsis risk of the two medication are lacking, further postulations are difficult to make based on our cohort study. In our study, febuxostat was preferred over allopurinol in non-diabetics in term of decreased risk of sepsis/infection-related death. One possible explanation is their difference in urate lowering efficacy. Becker et al. previously reported that patients using febuxostat (40 mg/day) with moderate renal impairment showed lower efficacy in diabetic than in non-diabetic gout patients, demonstrating that diabetics required higher dose to achieve comparable urate lowering effect (49). However, they observed that allopurinol had lower urate lowering efficacy compared to febuxostat regardless of diabetes status. Furthermore, diabetics and non-diabetics had similar rates of upper respiratory infection, though it is not known if a certain medication contributed more to this observation. Interestingly, studies on febuxostat and statins have largely focused on myopathy risk. While a few studies have suggested that CKD with concomitant statin or fibrate therapy may represent increased risk for febuxostat-induced rhabdomyolysis (50), Liu et al. have found that patients with severely reduced eGFR had higher risk of febuxostat-associated myopathy with or without concurrent statin or fibrate use (51). On the other hand, febuxostat but not allopurinol was recently suggested to increase the oral bioavailability of rosuvastatin. How this newly discovered drug-drug interaction contributes to the overall CV risk and other complications, such as sepsis/infection risk, is at a nascent stage requiring further investigation.
To our knowledge, this study is the first large, real-world cohort study to evaluate the sepsis and infection risk in pre-dialysis stage 5 CKD patients prescribed xanthine oxidase inhibitors. We have demonstrated that both allopurinol and febuxostat are associated with reduced risk of sepsis/infection and their related mortality without increasing the risk of MACCE or all-cause mortality. Nonetheless, several limitations are present in this study. First of all, due to the limited information provided by the NHIRD, we could not correlate our observed outcomes with changes in biochemical data, such as levels of uric acid, cytokines, or other useful markers. Second, the dosage of allopurinol and febuxostat was unknown and unable to be controlled. Third, since our study was retrospective and observational in design, no causal relationship or mechanism could be proved. Ideally, randomized controlled trials (RCTs) should be performed for more definitive conclusion.
Conclusion
The use of febuxostat and allopurinol appears to associate with decreased risk of sepsis and infection and their related death in pre-dialysis stage 5 CKD patients without compromising CV health and overall survival. Future RCTs or mechanistic studies are warranted to confirm and elucidate the comprehensive functions of xanthine oxidase inhibitors, especially in patients with compromised renal function.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board at Chang Gung Medical Foundation. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Y-SH, C-YW, and H-YY wrote the manuscript. Y-SH, C-YT, L-FC, S-HC, C-LY, M-HH, C-CL, THL, and H-YY designed the research. H-TT, THL, J-JC, and Y-TH performed the research. Y-SH, C-YW, GK, C-YH, H-LL, T-HY, Y-CC, Y-CT, C-WY, GA, and H-YY analyzed the data. H-TT and Y-TH contributed new reagents/analytical tools. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG3G1233 and CORPG3J0641).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0046) at Chang Gung Memorial Hospital for study design and monitor, data analysis and interpretation. We thank the Research Services Center for Health Information, Chang Gung University for administrative, technical and funding support. This study is based in part on data from the National Health Insurance research database provided by the National Health Insurance Administration and managed by Health and Welfare Data Science Center, Ministry of Health and Welfare. However, the interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital, National Health Insurance Administration and Ministry of Health and Welfare.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.818132/full#supplementary-material
References
1. Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. (2006) 13:199–204. doi: 10.1053/j.ackd.2006.04.004
2. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
3. Chuang C-C, Shiesh S-C, Chi C-H, Tu Y-F, Hor L-I, Shieh C-C, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. (2006) 10:R36 doi: 10.1186/cc4826
4. Yoshida H, Inaguma D, Koshi-Ito E, Ogata S, Kitagawa A, Takahashi K, et al. Extreme hyperuricemia is a risk factor for infection-related deaths in incident dialysis patients: a multicenter prospective cohort study. Renal Failure. (2020) 42:646–55. doi: 10.1080/0886022X.2020.1788582
5. Wang W, Xu D, Wang B, Yan S, Wang X, Yin Y, et al. Increased risk of cancer in relation to gout: a review of three prospective cohort studies with 50,358 subjects. Med Inflam. (2015) 2015:680853. doi: 10.1155/2015/680853
6. Luchtemberg MN, Petronilho F, Constantino L, Gelain DP, Andrades M, Ritter C, et al. Xanthine oxidase activity in patients with sepsis. Clin Biochem. (2008) 41:1186–90. doi: 10.1016/j.clinbiochem.2008.07.015
7. Ramos MFdP, Monteiro de Barros AdCM, Razvickas CV, Borges FT, Schor N. Xanthine oxidase inhibitors and sepsis. Int J Immunopathol Pharmacol. (2018) 32:2058738418772210. doi: 10.1177/2058738418772210
8. Damarla M, Johnston LF, Liu G, Gao L, Wang L, Varela L, et al. XOR inhibition with febuxostat accelerates pulmonary endothelial barrier recovery and improves survival in lipopolysaccharide-induced murine sepsis. Physiol Rep. (2017) 5:e13377. doi: 10.14814/phy2.13377
9. Ibrahim YF, Fadl RR, Ibrahim SAE, Gayyed MF, Bayoumi AMA, Refaie MMM. Protective effect of febuxostat in sepsis-induced liver and kidney injuries after cecal ligation and puncture with the impact of xanthine oxidase, interleukin 1β, and c-Jun N-terminal kinases. Human Exp Toxicol. (2020) 39:906–19. doi: 10.1177/0960327120905957
10. Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. (2010) 12:R63. doi: 10.1186/ar2978
11. Wang HE, Gamboa C, Warnock DG, Muntner P. Chronic kidney disease and risk of death from infection. Am J Nephrol. (2011) 34:330–6. doi: 10.1159/000330673
12. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol. (2019) 11:349–58. doi: 10.2147/CLEP.S196293
13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Int Med. (1999) 130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
15. Hsu TW, Liu JS, Hung SC, Kuo KL, Chang YK, Chen YC, et al. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. (2014) 174:347–54. doi: 10.1001/jamainternmed.2013.12700
16. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. (2005) 162:1016–23. doi: 10.1093/aje/kwi307
17. Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. (2010) 13:273–7. doi: 10.1111/j.1524-4733.2009.00671.x
18. Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Bri J Anaesth. (2011) 107:57–64. doi: 10.1093/bja/aer093
19. Kang D-H, Ha S-K. Uric acid puzzle: dual role as anti-oxidantand pro-oxidant. Electrolyte Blood Press. (2014) 12:1–6. doi: 10.5049/EBP.2014.12.1.1
20. Yang Y, Zhao J, Qiu J, Li J, Liang X, Zhang Z, et al. Xanthine oxidase inhibitor allopurinol prevents oxidative stress-mediated atrial remodeling in alloxan-induced diabetes mellitus rabbits. J Am Heart Assoc. (2018) 7:e008807. doi: 10.1161/JAHA.118.008807
21. Chatterjee S, Ehrenshaft M, Bhattacharjee S, Deterding LJ, Bonini MG, Corbett J, et al. Immuno-spin trapping of a post-translational carboxypeptidase B1 radical formed by a dual role of xanthine oxidase and endothelial nitric oxide synthase in acute septic mice. Free Radic Biol Med. (2009) 46:454–61. doi: 10.1016/j.freeradbiomed.2008.10.046
22. Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. (2006) 33:1646–50.
23. Coburn BW, Michaud K, Bergman DA, Mikuls TR. Allopurinol dose escalation and mortality among patients with gout. Arthr Rheumatol. (2018) 70:1298–307. doi: 10.1002/art.40486
24. Fonseca W, Malinczak C-A, Schuler CF, Best SKK, Rasky AJ, Morris SB, et al. Uric acid pathway activation during respiratory virus infection promotes Th2 immune response via innate cytokine production and ILC2 accumulation. Mucosal Immunol. (2020) 13:691–701. doi: 10.1038/s41385-020-0264-z
25. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. (2004) 44:642–50. doi: 10.1016/S0272-6386(04)00934-5
26. Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. (2010) 5:1388–93. doi: 10.2215/CJN.01580210
27. Chewcharat A, Chen Y, Thongprayoon C, Harrison AM, Mao MA, Cheungpasitporn W. Febuxostat as a renoprotective agent for treatment of hyperuricemia: a meta-analysis of randomized controlled trials. Int Med J. (2021) 51:752–62. doi: 10.1111/imj.14814
28. Lee TH, Chen JJ, Wu CY, Yang CW, Yang HY. Hyperuricemia and progression of chronic kidney disease: a review from physiology and pathogenesis to the role of urate-lowering therapy. Diagnostics. (2021) 11:1674. doi: 10.3390/diagnostics11091674
29. Arai Y, Nishinaka Y, Arai T, Mizugishi K, Adachi S, Takaori-Kondo A, et al. Uric acid induces NADPH oxidase–independent neutrophil extracellular trap formation. Blood. (2013) 122:2270. doi: 10.1182/blood.V122.21.2270.2270
30. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. (2018) 378:1200–10. doi: 10.1056/NEJMoa1710895
31. Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey CJ, Webster J, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. (2020) 396:1745–57. doi: 10.1016/S0140-6736(20)32234-0
32. Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med. (2015) 128:653.e7–16. doi: 10.1016/j.amjmed.2015.01.013
33. Bredemeier M, Lopes LM, Eisenreich MA, Hickmann S, Bongiorno GK, d'Avila R, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiov Dis. (2018) 18:24. doi: 10.1186/s12872-018-0757-9
34. Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. (2018) 72:798–810. doi: 10.1053/j.ajkd.2018.06.028
35. Dubreuil M, Zhu Y, Zhang Y, Seeger JD, Lu N, Rho YH, et al. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. (2015) 74:1368–72. doi: 10.1136/annrheumdis-2014-205269
36. Luk AJ, Levin GP, Moore EE, Zhou X-H, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology. (2009) 48:804–6. doi: 10.1093/rheumatology/kep069
37. Zhang M, Solomon DH, Desai RJ, Kang EH, Liu J, Neogi T, et al. Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol: population-based cohort study. Circulation. (2018) 138:1116–26. doi: 10.1161/CIRCULATIONAHA.118.033992
38. Hsu Y-SO, Wu IW, Chang S-H, Lee C-C, Tsai C-Y, Lin C-Y, et al. Comparative renoprotective effect of febuxostat and allopurinol in predialysis stage 5 chronic kidney disease patients: a nationwide database analysis. Clin Pharmacol Therap. (2020) 107:1159–69. doi: 10.1002/cpt.1697
39. Harris CM, Lloyd DC, Lewis J. The prevalence and prophylaxis of gout in England. J Clin Epidemiol. (1995) 48:1153–8. doi: 10.1016/0895-4356(94)00244-K
40. Harrold LR, Yood RA, Mikuls TR, Andrade SE, Davis J, Fuller J, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis. (2006) 65:1368–72. doi: 10.1136/ard.2006.051649
41. Hak AE, Curhan GC, Grodstein F, Choi HK. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis. (2010) 69:1305–9. doi: 10.1136/ard.2009.109884
42. Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne PE, Heiland M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. (2020) 18:369. doi: 10.1186/s12916-020-01851-z
43. Chang CH, Fan PC, Kuo G, Lin YS, Tsai TY, Chang SW, et al. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: a nationwide cohort study. Sci Rep. (2020) 10:2938. doi: 10.1038/s41598-020-59794-7
44. Nasir N, Jamil B, Siddiqui S, Talat N, Khan FA, Hussain R. Mortality in sepsis and its relationship with gender. Pak J Med Sci. (2015) 31:1201–6. doi: 10.12669/pjms.315.6925
45. Xu J, Tong L, Yao J, Guo Z, Lui KY, Hu X, et al. Association of sex with clinical outcome in critically ill sepsis patients: a retrospective analysis of the large clinical database MIMIC-III. Shock. (2019) 52:146–51. doi: 10.1097/SHK.0000000000001253
46. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. (2003) 348:1546–54. doi: 10.1056/NEJMoa022139
47. Pietropaoli AP, Glance LG, Oakes D, Fisher SG. Gender differences in mortality in patients with severe sepsis or septic shock. Gend Med. (2010) 7:422–37. doi: 10.1016/j.genm.2010.09.005
48. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. (2018) 14:151–64. doi: 10.1038/nrneph.2017.181
49. Becker MA, MacDonald PA, Hunt BJ, Jackson RL. Diabetes and gout: efficacy and safety of febuxostat and allopurinol. Diab Obesity Metab. (2013) 15:1049–55. doi: 10.1111/dom.12135
50. Ghosh D, McGann PM, Furlong TJ, Day RO. Febuxostat-associated rhabdomyolysis in chronic renal failure. Med J Aust. (2015) 203:107–8. doi: 10.5694/mja14.01404
Keywords: chronic kidney disease, sepsis, MACCE, febuxostat, allopurinol
Citation: Yang H-Y, Hsu Y-SO, Lee TH, Wu C-Y, Tsai C-Y, Chou L-F, Tu H-T, Huang Y-T, Chang S-H, Yen C-L, Hsieh M-H, Lee C-C, Kuo G, Hsiao C-Y, Lin H-L, Chen J-J, Yen T-H, Chen Y-C, Tian Y-C, Yang C-W and Anderson GF (2022) Reduced Risk of Sepsis and Related Mortality in Chronic Kidney Disease Patients on Xanthine Oxidase Inhibitors: A National Cohort Study. Front. Med. 8:818132. doi: 10.3389/fmed.2021.818132
Received: 19 November 2021; Accepted: 28 December 2021;
Published: 31 January 2022.
Edited by:
Gian Marco Ghiggeri, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Roberto Pontremoli, University of Genoa, ItalyGiacomo Garibotto, University of Genoa, Italy
Copyright © 2022 Yang, Hsu, Lee, Wu, Tsai, Chou, Tu, Huang, Chang, Yen, Hsieh, Lee, Kuo, Hsiao, Lin, Chen, Yen, Chen, Tian, Yang and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerard F. Anderson, Z2FuZGVyc29uJiN4MDAwNDA7amh1LmVkdQ==
 Huang-Yu Yang
Huang-Yu Yang Yun-Shiuan Olivia Hsu
Yun-Shiuan Olivia Hsu Tao Han Lee
Tao Han Lee Chao-Yi Wu5
Chao-Yi Wu5 Li-Fang Chou
Li-Fang Chou Hui-Tzu Tu
Hui-Tzu Tu Yu-Tung Huang
Yu-Tung Huang Shang-Hung Chang
Shang-Hung Chang Cheng-Chia Lee
Cheng-Chia Lee George Kuo
George Kuo Chih-Yen Hsiao
Chih-Yen Hsiao Jia-Jin Chen
Jia-Jin Chen Tzung-Hai Yen
Tzung-Hai Yen