- Department of Rheumatology, School of Medicine, Renji Hospital, Shanghai Jiao Tong University, Shanghai, China
Background: To investigate the association of D-dimer with gut inflammation in spondyloarthritis (SpA).
Methods: Sixty-five patients with SpA and 70 healthy controls were included. Demographic, clinical, and laboratory parameters were collected. The differences of clinical and laboratory parameters were compared between patients with SpA and healthy controls, and between patients with SpA, with and without gut inflammation. The associations of D-dimer with laboratory data were analyzed. The predictive value of D-dimer was obtained by a receiver operator characteristic (ROC) curve analysis. The independent risk factors for gut inflammation in SpA were investigated by binary logistic regression analysis.
Results: Patients with SpA had higher D-dimer than healthy controls (P = 0.016). D-dimer was positively correlated with platelet (PLT), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), and negatively correlated with hemoglobin (Hb). Besides, significant differences were observed in D-dimer between SpA patients with and without gut inflammation (P < 0.001). Furthermore, SpA patients with gut inflammation were more likely to have peripheral joint involvement than those without gut inflammation (P < 0.001). The AUC of D-dimer was 0.865 at cut-off value of 0.29 mg/L, with a sensitivity of 82.6%, and a specificity of 81%. Elevated D-dimer (OR = 15.451, 95% CI: 3.030–78.780, P = 0.001) was independently associated with gut inflammation in SpA.
Conclusion: D-dimer may be a potential biomarker for identifying SpA patients with gut inflammation.
Introduction
Spondyloarthritis (SpA) is a common chronic inflammatory disease, affecting the 0.2–1.61% of the population (1, 2). The SpA is currently classified as axial spondyloarthritis [axSpA, including ankylosing spondylitis (AS)] and peripheral spondyloarthritis (pSpA). The axSpA mainly affects spine or sacroiliac joints, with or without peripheral joint involvement (PJI), while pSpA refers to the condition where only the peripheral joint is involved (arthritis, enthesitis, or dactylitis) (3, 4). In addition, extra-articular manifestations are common, including anterior uveitis (AU), psoriasis, and inflammatory bowel disease (IBD), including Crohn's disease (CD), and ulcerative colitis (UC) (5).
Gut inflammation includes both clinical and subclinical gut inflammation. While the former is well known to be in the form of CD, or UC, the latter tends to be asymptomatic (6). The prevalence of clinical gut inflammation (including IBD) is in the range of 6 to 14% among patients with SpA, while the incidence of subclinical gut inflammation is up to 44-60% (7). On the other hand, SpA occurs frequently in patients with IBD, ranging from 3.7 to 13% (8, 9). Moreover, patients with SpA have an increased risk of developing IBD (10), and the gut inflammation is related to a higher disease activity of axSpA (11). Currently, accumulated evidence indicates that there exists a great amount of genetic, immunological, and pathophysiologic overlap between the SpA and gut inflammation (12, 13).
Ileo-colonoscopy and biopsy, which are regarded as gold standard tools, are currently required for the confirmation of gut inflammation (14). In fact, gut inflammation often occurs asymptomatically, and patients are unwilling to undergo the ileo-colonoscopy for its discomfort, invasiveness, and expense, with a resultant missed diagnosis and the delayed treatment. Therefore, an effective screening tool is warranted for identifying patients with SpA who are likely to have a suspected gut inflammation.
D-dimer serves as a biomarker of both fibrin formation and degradation, reflecting the activated coagulation and fibrinolysis. Besides the thrombotic disorders, cancers, and infection, D-dimer is increased in autoimmune disease (15, 16). Vascular injury and vascular thrombosis are found to be involved in IBD, resulting in an increase in D-dimer (17, 18). Moreover, the D-dimer is elevated in axSpA and is associated with the disease activity (19, 20). The current study is to investigate the association of D-dimer with gut inflammation in SpA.
Materials and Methods
Patients
A total of 65 patients in the Department of Rheumatology, Shanghai Renji Hospital, from July 2019 to July 2020, and 70 healthy controls (HC) were recruited in this study. Each patient was diagnosed with SpA, fulfilling the classification criteria of the Assessment of Spondyloarthritis International Society criteria (ASAS) (3, 4). The ASAS was used to classify the patients as affected by axial and/or peripheral SpA. All patients have no previous history of infectious diseases, diabetes, hematological diseases, arterial, or venous thrombosis. All patients have given their informed consent prior to their inclusion in the study. This study was performed in accordance with the Declaration of Helsinki and with the ethical standards of the institutional research committee. Ethical approval was obtained by Renji Hospital Ethics Committee, Shanghai Jiaotong University School of Medicine (reference number: 2017-201).
Data Collection
Clinical and laboratory data were collected. Clinical manifestations included sacroiliitis, PJI, gastrointestinal (GI) symptoms, anterior uveitis, psoriasis, and gut inflammation. PJI was defined as the presence of peripheral arthritis, enthesitis, or dactylitis. Laboratory data involved whole blood cell counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and human leukocyte antigen B27 (HLA-B27). The coagulation parameters include D-dimer, fibrinogen (FIB), prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT).
Ileo-Colonoscopic and Histologic Examination
Ileo-colonoscopy was performed among 65 patients with SpA, permitting visualization of ileum, colon, and rectum. Meanwhile, biopsy was performed when macroscopic change was found over the surface of the digestive duct. The obtained mucosal tissues were embedded in 40% paraffin, cut in 3 mm sample sections, and stained with H&E for histological evaluation. The primary diagnosis of gut inflammation was made both macroscopically and microscopically according to mucosal lesion and inflammatory cell infiltration, as well as with the previous reports (21, 22).
Statistical Analysis
Data were analyzed using IBM SPSS Statistics 25 software and GraphPad Prism 8 software. Quantitative data were expressed as median and interquartile range (IQR), and categorical variables were presented as numbers and percentages. The Mann-Whitney U test or Student's t-test was used for continuous variables, and the chi-square test or Fisher's exact test was used for categorical variables. Spearman's correlation analysis was used to assess the associations of D-dimer with other laboratory parameters. The receiver operator characteristic (ROC) curve was used to discriminate between patients who had gut inflammation from those who did not. We determined the cut-off value and calculated the areas under the curve (AUC), sensitivity, and specificity to compare the applicability of the variables. All variables with P < 0.1 on univariate analysis were selected for the multivariate logistic regression analysis [forward: likelihood ratio (LR)] to explore the factors that are independently associated with gut inflammation in patients with SpA. Results were presented as odds ratios (OR) with 95% CI. All tests were two-tailed. The value of P < 0.05 were considered statistically significant.
Results
Demographic, Laboratory, and Clinical Characteristics Between Patients With SpA and Healthy Control
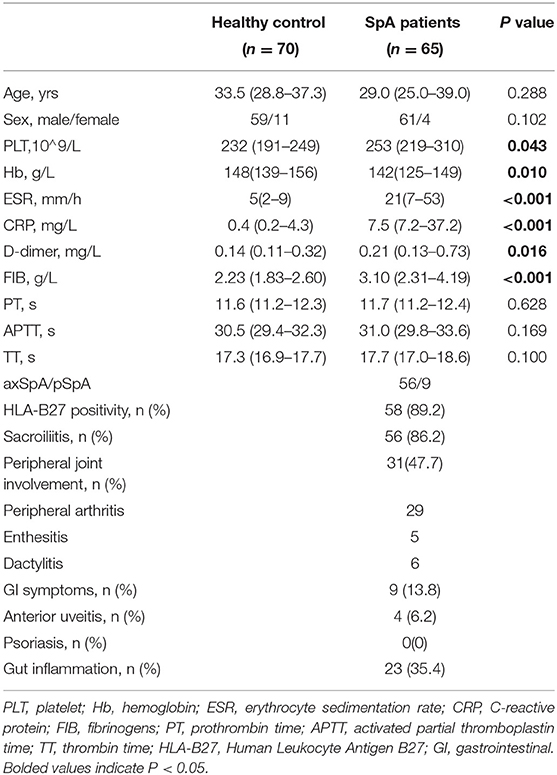
Among the 65 patients with SpA, 56 were diagnosed with axSpA (22 with PJI) and nine were diagnosed with pSpA. Thirty-two patients had PJI, 23 patients had gut inflammation, four patients had anterior uveitis, and no patient had psoriasis. Demographic, laboratory, and clinical characteristics of patients with SpA and healthy controls were listed in Table 1. D-dimer was significantly higher in patients with SpA than healthy controls (P = 0.016). Moreover, significant differences between patients with SpA and HC were observed in terms of PLT, Hb, ESR, CRP, and FIB (P < 0.05, respectively). There were no significant differences between the two groups in terms of PT, APTT, and TT (P > 0.05, respectively).
The Association of D-Dimer With Other Laboratory Parameters
Next, we analyzed the correlations between D-dimer and other laboratory parameters in patients with SpA. D-dimer was positively correlated with PLT (r = 0.564, P < 0.001), ESR (r = 0.592, P < 0.001), and CRP (r = 0.597, P < 0.001). In addition, a negative correlation was observed between D-dimer and Hb (r = −0.688, P < 0.001) (Figure 1).
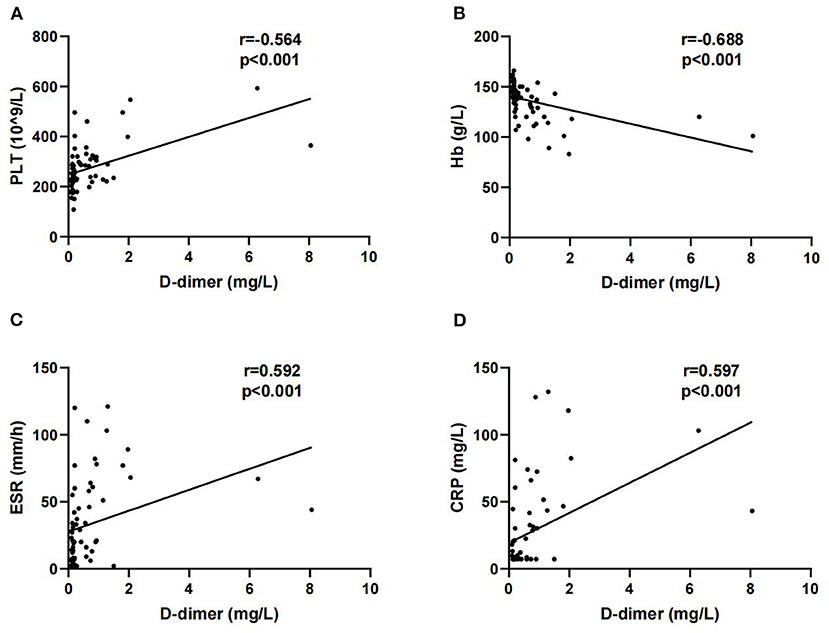
Figure 1. Correlations of D-dimer with platelet (PLT) (A), Hb (B), erythrocyte sedimentation rate (ESR) (C) and C-reactive protein (CRP) (D).
The Relationship Between D-Dimer and Clinical Features
We further analyzed the relationship between D-dimer and the clinical features. Overall, patients with pSpA had a significantly higher level of serum D-dimer than patients with axSpA [0.93 (0.42–1.89) mg/L vs. 0.19 (0.13–0.59) mg/L, P = 0.001]. Additionally, patients with SpA with PJI had a significantly higher D-dimer than those without PJI [0.73 (0.21–1.27) mg/L vs. 0.15 (0.11–0.20) mg/L, P < 0.001]. In addition, no significant difference was observed in D-dimer between patients with SpA, both with and without AU, and between patients with SpA with HLA-B27 positivity, and those with HLA-B27 negativity (P > 0.05, respectively) (Figure 2).
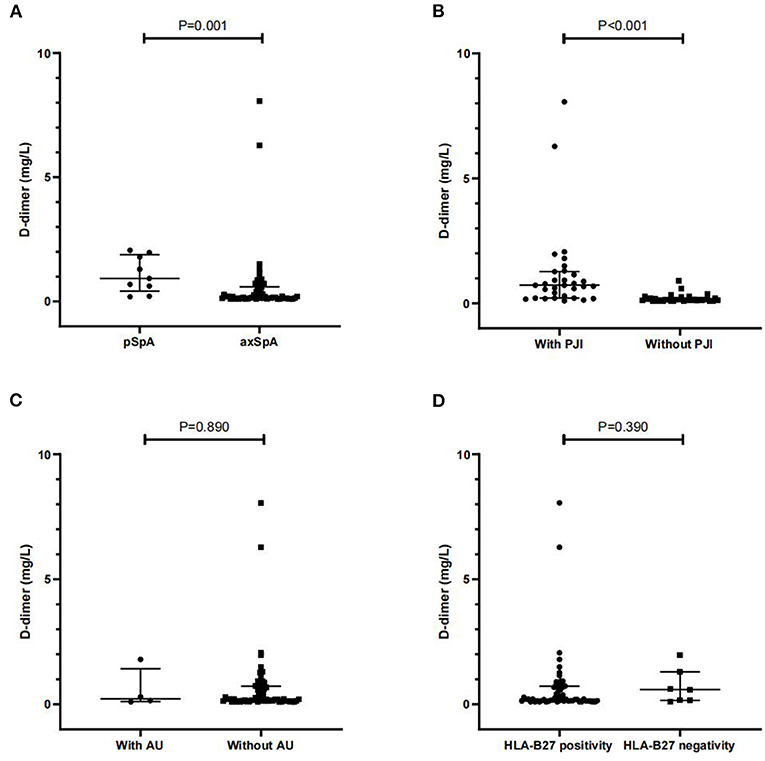
Figure 2. Comparisons of D-dimer between axial spondyloarthritis (axSpA) and pSpA patients (A), and between SpA patients with and without PJI (B), AU (C) and HLA-B27 positivity (D). PJI peripheral joint involvement; AU anterior uveitis; HLA-B27 human leukocyte antigen B27. Data are expressed as median and interquartile range (IQR).
Ileo-Colonoscopic and Histopathological Findings
Overall, 23 patients had macroscopic lesion over the surface of digestive duct, ranging from erythema, oedema, friability of the mucosa, mucosal erosion to ulcerations (Figure 3). Macroscopic lesion was detected in the terminal ileum in 12 cases, in the colon of nine patients, and in both ileum and colon of two patients were affected. Histologically, inflammatory cell infiltration was found around the gland or in the underlying submucosa in all cases, four cases had abundant neutrophil infiltration, and 10 cases had lymphocyte aggregation or lymphoid tissue formation (Figure 4). Four (6.2%) patients were diagnosed with UC and 3 (4.6%) with CD.
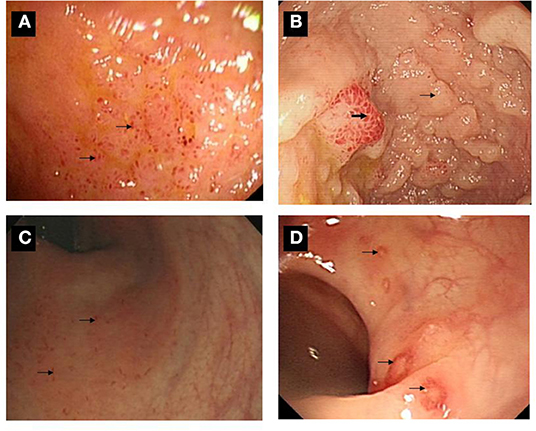
Figure 3. Ileo-colonoscopic pictures of gut inflammation among SpA patients. (A) Multiple lesions were over the surface in the terminal ileum. (B) A sign of mucosal nodularity (small arrow) with hyperemia-like changes was in terminal ileum (bold arrow). (C) Multiple small lesions were found over the surface of rectum and sigmoid colon (arrow). (D) Multiple ulcers were found over the surface of descending, sigmoid colon and rectum (arrow).
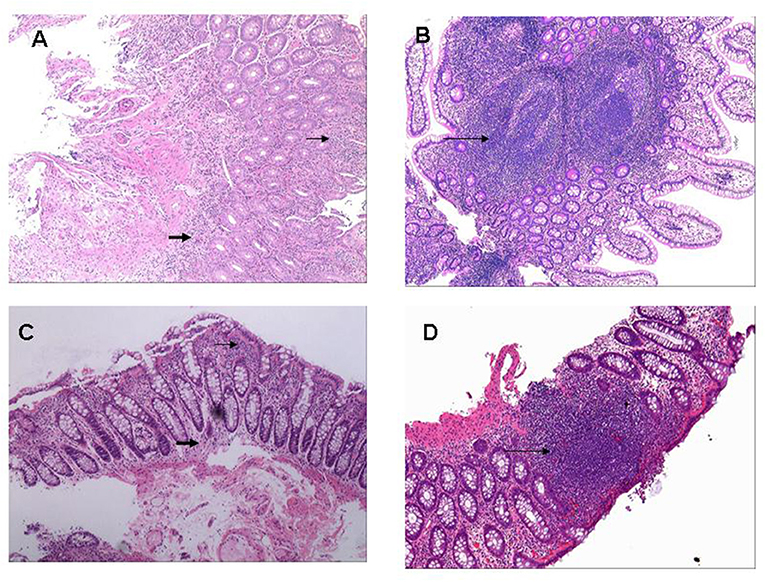
Figure 4. Histopathological pictures of gut inflammation among SpA patients. (A) Histologically infiltration of Inflammatory cells was around gland duct (small arrow) and at underlying submucosa (bold arrow) located in terminal ileum (H&E; original magnification ×10). (B) Multiple ectopic lymphoid tissues were microscopically observed in the terminal ileum (long arrow) with massive infiltration of inflammatory cells around gland duct (H&E; original magnification ×10). (C) Tissue specimen from descending colon demonstrated moderate infiltration of inflammatory cells around gland duct (small arrow) and in the underlying submucosa (bold arrow) (H&E; original magnification ×20). (D) A ectopic lymphoid tissue was microscopically observed at rectum specimen (long arrow) with massive infiltration of inflammatory cells around gland duct (H&E; original magnification ×20).
The Comparisons of Clinical and Laboratory Data Between Patients With and Without Gut Inflammation
The clinical and laboratory characteristics of patients with and without gut inflammation were listed in Table 2. There were 35.4% (23/65) patients with SpA with gut inflammation. Among these patients, gut inflammation was detected in 32.1% (18/56) patients with axSpA and 55.6% (5/9) in patients with pSpA, with no significant difference between them. However, significant difference was observed in terms of the prevalence of PJI between patients with and without gut inflammation (P < 0.001). There was no significant difference in terms of the prevalence of AU and HLA-B27 positivity between the two groups. In general, patients with SpA with gut inflammation had higher disease activity than those without gut inflammation, including PLT, ESR, and CRP (P < 0.05, respectively). Of note, among the coagulation parameters, D-dimer level was significantly higher in patients with gut inflammation than those without gut inflammation (P < 0.001), while there was no significant difference in terms of FIB, PT, APTT, and TT between them (P > 0.05, respectively). Moreover, Hb concentration was significantly lower in patients with gut inflammation (P = 0.002).
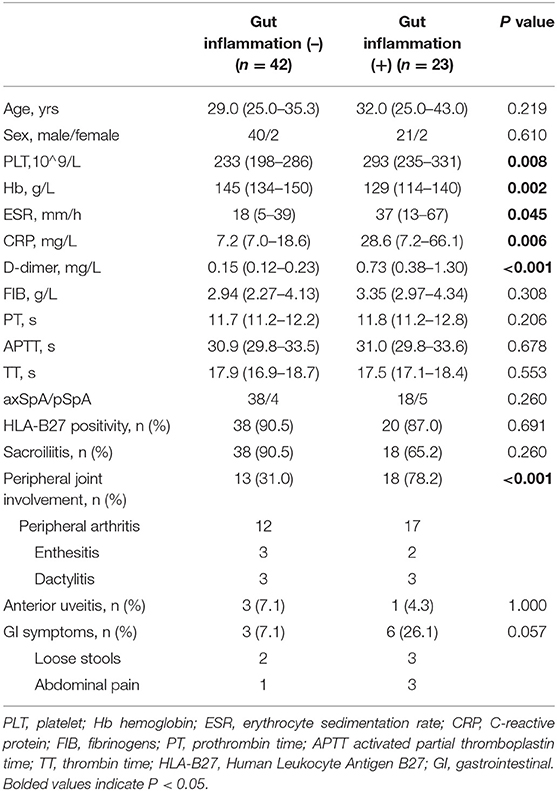
Table 2. Comparisons of demographic, laboratory, and clinical characteristics of patients with SpA between with and without gut inflammation.
ROC Analysis of Laboratory Parameters for Detecting the Gut Inflammation in SpA
We further explored the predicting efficiency of these parameters (including PLT, Hb, ESR, CRP, and D-dimer) for gut inflammation in patients with SpA. The ROC analysis was shown in Table 3 and Figure 5. Among these parameters, the AUC of D-dimer was 0.865 (95% CI: 0.777–0.954; P < 0.001), greater than other parameters. The optimum cut-off point of D-dimer was 0.29 mg/L, accompanied by a sensitivity of 82.6% and a specificity of 81%.
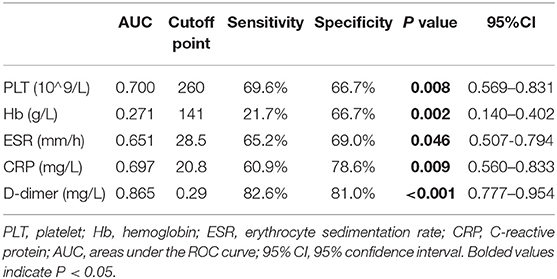
Table 3. Predictive values of the biomarkers for detecting the gut inflammation of patients with SpA.
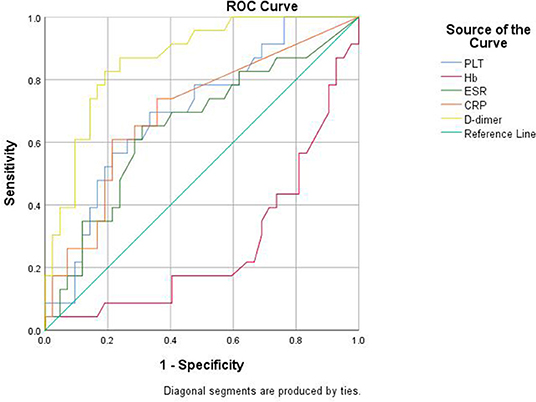
Figure 5. Receiver operator characteristic (ROC) curve analysis of the biomarkers for detecting the gut inflammation in patients with SpA.
Binary Logistic Regression Analysis of Gut Inflammation
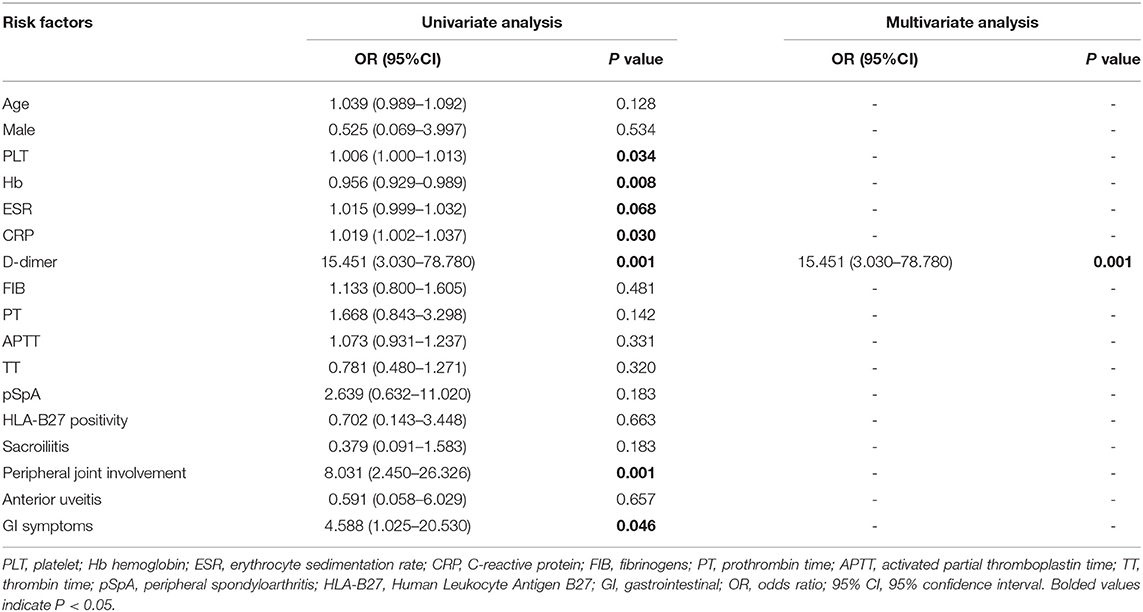
Binary logistic regression analysis was performed to investigate the factors that are independently associated with gut inflammation in SpA. The results indicated that an increased D-dimer (OR = 15.451, 95% CI: 3.030–78.780, P = 0.001) was independently associated with gut inflammation in SpA (Table 4).
Discussion
In the present study, our results demonstrated that D-dimer was correlated with inflammatory index. Moreover, D-dimer was increased in patients with SpA with PJI, as well as in patients with SpA with gut inflammation, and an elevated D-dimer was independently associated with gut inflammation in SpA. Additionally, patients with gut inflammation were more likely to have PJI than those without gut inflammation.
The D-dimer is a biomarker that reflects fibrin formation and degradation, while FIB is the precursor to fibrin, which is produced in the liver. The increased D-dimer has been reported in conditions with thrombosis (16), IBD (17, 23), rheumatoid arthritis (RA) (24, 25), and SpA (20), while FIB increases under injury, infection, or inflammation. It is reported that the activation of coagulation and fibrinolysis system was also shown in either intra-articular synovial tissues of arthritis (20) or intestinal tissues of IBD (18). Other reports suggested that the vascular injury or vasculitis might be involved in the pathogenesis of IBD (26, 27). The prevailing opinion is that coagulation system is activated excessively in the setting of inflammation. Furthermore, the mutual interaction between coagulation and inflammation may amplify the activation of both systems and may accelerate the development of disease (28). The present study demonstrated that D-dimer in patients with SpA was increased compared with healthy controls and was correlated with blood parameters (PLT, ESR, and CRP), and was consistent with the report that the coagulation system was activated in axSpA (29). Other studies also indicated that D-dimer was increased and was paralleled with the disease activity in RA (25, 30). Additionally, patients with SLE in the active group had significantly higher D-dimer level than those in the inactive and control groups (31). Also, D-dimer was significantly increased in patients with adult-onset Still's disease (AOSD) with macrophage activation syndrome (MAS), compared with those without MAS (32). In addition, we also found that the Hb concentration is negative with D-dimer level.
As for the relationship between D-dimer and different clinical features, we found that D-dimer level was significantly increased in patients with pSpA than with axSpA. Furthermore, we demonstrated that patients with PJI have higher level of D-dimer than those without PJI. In fact, the relationships between D-dimer and PJI among patients with SpA have not been reported. Nevertheless, this association can also be found in RA and in patients with juvenile idiopathic arthritis (JIA), which are both characterized by peripheral joint inflammation (25, 33).
Regarding the association of D-dimer with gut inflammation among patients with SpA, we demonstrated that these patients with SpA with gut inflammation had a higher disease activity including an increased ESR and CRP than those without gut inflammation. We also found that patients with SpA with gut inflammation had both an increased D-dimer and decreased Hb, resulted from the possible involvement of vascular injury and small vessel thrombosis in the pathogenesis (18, 26, 27). Similarly, others have shown that D-dimer level was increased in active IBD group, compared with patients with inactive IBD and healthy controls, with average of 0.326 vs. 0.229 mg/L and 0.243 vs. 0.140 mg/L, respectively (17, 34). In our study, ROC analysis demonstrated that the cut-off value of 0.29 mg/L was obtained at optimal AUC of 0.865 for identifying gut inflammation in SpA, with a sensitivity of 82.6% and a specificity of 81%.
Concerning the screening tool, few biomarkers and predictive model have been studied. Fecal calprotectin (FC) serves as a potential biomarker for small bowel inflammation, with an AUC of 0.75 (FC > 132 μg/g), accompanied by a sensitivity of 66.7%, and a specificity of 76.9% (35). Furthermore, FC could be used to detect a suspected gut inflammation and to identify patients with AS at an increased risk of developing IBD (at a baseline threshold of 266 mg/kg). Currently, the cut-off value of FC is reported to be of great variability (36–38). Also, MIRJAM D suggested that perinuclear anti-neutrophil cytoplasmic antibody (pANCA) might be a predictive indicator to perform endoscopy (39). At present, there is no simple and convenient biomarker for predicting the gut inflammation in SpA. On the other hand, a multiparametric model [including age, male gender, Bath ankylosing spondylitis disease activity index (BASDAI), and Bath ankylosing spondylitis metroloty index (BASMI)] was established for predicting microscopic gut inflammation in axSpA, with a sensitivity of 81.8% and a specificity of 78.3% (11). In our study, we did not compare the predicting efficiency between D-dimer and FC, pANCA or the aforementioned model, but the serum D-dimer test should be a simple and stable tool although it remains to be validated in a larger scale. Moreover, logistic regression analysis in our study indicated that an elevated D-dimer is independently associated with the gut inflammation in SpA. To our knowledge, this is the first description of this association of an elevated D-dimer with gut inflammation among the population with SpA.
Interestingly, our results primarily showed that PJI is associated with gut inflammation. The current classification criteria for SpA are based on the location of the involved joints instead of its pathophysiology. According to the criteria, axSpA includes the involvement of axial skeleton with or without peripheral joint inflammation. However, the criteria now began to gain debates (3, 40, 41), and the focus is whether axSpA and pSpA share a same pathogenic origin. Currently, it is generally accepted that both have overlapping biological backgrounds: genetic factors play a more closely related role in axial involvement than peripheral joint inflammation, while the peripheral joint involvement is reported to be linked with gut inflammation (42–44). Furthermore, recent advances suggested that there existed differences between them concerning immunological alterations and response to biologic disease-modifying anti-rheumatic drugs (bDMARDs) (13). Therefore, for the clinical purpose, it is important to identify potential patients with SpA with gut inflammation. We also found that it is not the axial involvement, but the PJI that is related to gut inflammation, similar to the previous findings that the patients with axSpA with subclinical gut inflammation (as assessed by FC levels) is closely linked with peripheral joint inflammation (45). Nevertheless, we did not observe that there was a significant difference in terms of the prevalence of gut inflammation between axSpA and pSpA. These results indicated that gut inflammation is more frequently seen in SpA with PJI regardless of the SpA classification. In addition, an elevated D-dimer was not only observed in patients with SpA with PJI, but also in patients with SpA with gut inflammation, suggesting there is a possible link between PJI and gut inflammation. Above all, our results suggested that PJI might be a distinct phenotype that is associated with gut inflammation.
This study has several limitations. First, our sample size is relatively small. Second, the conventional ileo-colonoscopy, instead of the capsule endoscopy (CE), was performed. The CE may detect suspected small bowel inflammation in patients with SpA with elevated D-dimer, permitting greater visualization. Third, we did not evaluate the gut inflammation, failing to explore the association between D-dimer and the disease activity of gut inflammation. Above all, larger multicenter studies should be conducted for further validation.
In conclusion, our study indicates that the elevated D-dimer is related to PJI and gut inflammation, and PJI is possibly associated with gut inflammation. Furthermore, we suggest, for the first time, that serum D-dimer may be a potential biomarker for identifying patients with SpA with suspected gut inflammation, but the determination of cutoff value is required in a larger study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Renji Hospital, Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JF and JL designed the research studies, acquired data, analyzed data, and wrote the manuscript. YL and YJ acquired data. FD and XC checked the medical charts. XC designed the research studies, revised the article, and provided the mentorship of this study. All the authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grants 81771729, 81971534).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Care Res (Hoboken). (2016) 68:1320–31. doi: 10.1002/acr.22831
2. van Tubergen A. The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol. (2015) 11:110–8. doi: 10.1038/nrrheum.2014.181
3. Zeidler H, Amor B. The Assessment in Spondyloarthritis International Society (ASAS) classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general: the spondyloarthritis concept in progress. Ann Rheum Dis. (2011) 70:1–3. doi: 10.1136/ard.2010.135889
4. Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. (2009) 68:ii1–44. doi: 10.1136/ard.2008.104018
5. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:65–73. doi: 10.1136/annrheumdis-2013-203582
6. Rizzo A, Ferrante A, Guggino G, Ciccia F. Gut inflammation in spondyloarthritis. Best Pract Res Clin Rheumatol. (2017) 31:863–76. doi: 10.1016/j.berh.2018.08.012
7. Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol. (2019) 25:2162–76. doi: 10.3748/wjg.v25.i18.2162
8. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. (2015) 21:1982–92. doi: 10.1097/MIB.0000000000000392
9. Karreman MC, Luime JJ, Hazes JMW, Weel A. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. (2017) 11:631–42. doi: 10.1093/ecco-jcc/jjw199
10. Stolwijk C, Essers I, van Tubergen A, Boonen A, Bazelier MT, De Bruin ML, et al. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann Rheum Dis. (2015) 74:1373–8. doi: 10.1136/annrheumdis-2014-205253
11. Van Praet L, Van den Bosch F, Jacques P, Carron P, Jans L, Colman R, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. (2013) 72:414–7. doi: 10.1136/annrheumdis-2012-202135
12. Luchetti MM, Ciccia F, Avellini C, Benfaremo D, Rizzo A T, Spadoni, et al. Gut epithelial impairment, microbial translocation and immune system activation in inflammatory bowel disease-associated spondyloarthritis. Rheumatology (Oxford). (2021) 60:92–102. doi: 10.1093/rheumatology/keaa164
13. Gracey E, Vereecke L, McGovern D, Fröhling M, Schett G, Danese S, et al. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol. (2020) 16:415–33. doi: 10.1038/s41584-020-0454-9
14. Bernstein CN, Riddell RH. Colonoscopy plus biopsy in the inflammatory bowel diseases. Gastrointest Endosc Clin N Am. (2000) 10:755–74. doi: 10.1016/S1052-5157(18)30107-7
15. Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. (2009) 113:2878–87. doi: 10.1182/blood-2008-06-165845
16. Weitz JI, Fredenburgh JC, Eikelboom JW. A Test in Context: D-Dimer. J Am Coll Cardiol. (2017) 70:2411–20. doi: 10.1016/j.jacc.2017.09.024
17. Alkim H, Ayaz S, Alkim C, Ulker A, Sahin B. Continuous active state of coagulation system in patients with nonthrombotic inflammatory bowel disease. Clin Appl Thromb Hemost. (2011) 17:600–4. doi: 10.1177/1076029611405034
18. Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, et al. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. (1989) 2:1057–62. doi: 10.1016/S0140-6736(89)91078-7
19. Li Y, Zhang Y, Xu W, Liang J, Luo X, Han X, et al. Activated coagulation is associated with the disease activity of axial spondyloarthritis. Int Immunopharmacol. (2020) 88:106979. doi: 10.1016/j.intimp.2020.106979
20. So AK, Varisco PA, Kemkes-Matthes B, Herkenne-Morard C, Chobaz-Péclat V, Gerster JC, et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. (2003) 1:2510–5. doi: 10.1111/j.1538-7836.2003.00462.x
21. Conrad K, Roggenbuck D, Laass MW). Diagnosis and classification of ulcerative colitis. Autoimmun Rev. (2014 13:463–6. doi: 10.1016/j.autrev.2014.01.028
22. Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn's disease. Autoimmun Rev. (2014) 13:467–71. doi: 10.1016/j.autrev.2014.01.029
23. Biancone L, Scopinaro F, Maletta M, Monteleone G, Luzza F, Banci M, et al. Circulating D dimer in inflammatory bowel disease. Ital J Gastroenterol. (1994) 26:116–20.
24. Bloom BJ, Tucker LB, Miller LC, Schaller JG. Fibrin D-dimer as a marker of disease activity in systemic onset juvenile rheumatoid arthritis. J Rheumatol. (1998) 25:1620–5.
25. Ingegnoli F, Fantini F, Favalli EG, Soldi A, Griffini S, Galbiati V, et al. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor-alpha blockade. J Autoimmun. (2008) 31:175–9. doi: 10.1016/j.jaut.2008.07.002
26. Stevens TR, Harley SL, Groom JS, Cambridge G, Leaker B, Blake DR, et al. Anti-endothelial cell antibodies in inflammatory bowel disease. Dig Dis Sci. (1993) 38:426–32. doi: 10.1007/BF01316494
27. Kruschewski M, Buhr HJ. The vasculitis in IBD is associated with the degree of inflammation. Dig Dis Sci. (2010) 55:733–8. doi: 10.1007/s10620-009-0763-y
28. Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. (2016) 118:1392–408. doi: 10.1161/CIRCRESAHA.116.306853
29. Prati C, Racadot E, Cedoz JP, Wendling D. Thrombin generation in ankylosing spondylitis. Clin Rheumatol. (2011) 30:511–4. doi: 10.1007/s10067-010-1552-x
30. Gualtierotti R, Ingegnoli F, Griffini S, Grovetti E, Meroni PL, Cugno M. Prothrombotic biomarkers in patients with rheumatoid arthritis: the beneficial effect of IL-6 receptor blockade. Clin Exp Rheumatol. (2016) 34:451−8. doi: 10.12659/MSM.935952
31. Zheng CS, Qin XJ, Ni H, Chen RY, Liu JL, Wang WH. Evaluation of disease activity of systemic lupus erythematosus by D-dimer combined with red blood cell distribution width. Clin Lab. (2021) 67. doi: 10.7754/Clin.Lab.2021.210118
32. Yang XP, Wang M, Li TF, Li W, Zhang L, Liu SY. Predictive factors and prognosis of macrophage activation syndrome associated with adult-onset Still's disease. Clin Exp Rheumatol. (2019) 37:83–88.
33. Xu Q, Lin CS. Plasma levels of D-dimer in a 5-year-old girl with systemic juvenile idiopathic arthritis: A case report and literature review. Exp Ther Med. (2016) 11:1027–30. doi: 10.3892/etm.2016.3009
34. Zezos P, Papaioannou G, Nikolaidis N, Patsiaoura K, Vassiliadis T, Mpoumponaris A, et al. Elevated markers of thrombin generation and fibrinolysis in patients with active and quiescent ulcerative colitis. Med Sci Monit. (2009) 15:Cr563–72.
35. Kopylov U, Starr M, Watts C, Dionne S, Girardin M, Seidman EG. Detection of crohn disease in patients with spondyloarthropathy: the SpACE capsule study. J Rheumatol. (2018) 45:498–505. doi: 10.3899/jrheum.161216
36. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. (2010) 341:c3369. doi: 10.1136/bmj.c3369
37. Klingberg E, Strid H, Ståhl A, Deminger A, Carlsten H, Öhman L, et al. A longitudinal study of fecal calprotectin and the development of inflammatory bowel disease in ankylosing spondylitis. Arthritis Res Ther. (2017) 19:21. doi: 10.1186/s13075-017-1223-2
38. Cremer A, Ku J, Amininejad L, Bouvry MR, Brohet F, Liefferinckx C, et al. Variability of faecal calprotectin in inflammatory bowel disease patients: an observational case-control study. J Crohns Colitis. (2019) 13:1372–9. doi: 10.1093/ecco-jcc/jjz069
39. de Vries M, van der Horst-Bruinsma I, van Hoogstraten I, van Bodegraven A, von Blomberg BM, Ratnawati H, et al. pANCA, ASCA, and OmpC antibodies in patients with ankylosing spondylitis without inflammatory bowel disease. J Rheumatol. (2010) 37:2340–4. doi: 10.3899/jrheum.100269
40. Zeidler H. Peripheral arthritis in patients classified as having nonradiographic axial spondyloarthritis in trials of anti-tumor necrosis factor agents: comment on the article by Dougados et al. Arthritis Rheumatol. (2015) 67:1138–9. doi: 10.1002/art.38978
41. Akkoc N, Khan MA. ASAS classification criteria for axial spondyloarthritis: time to modify. Clin Rheumatol. (2016) 35:1415–23. doi: 10.1007/s10067-016-3261-6
42. Cuvelier C, Barbatis C, Mielants H, De Vos M, Roels H, Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. (1987) 28:394–401. doi: 10.1136/gut.28.4.394
43. Malaty HM, Lo GH, Hou JK. Characterization and prevalence of spondyloarthritis and peripheral arthritis among patients with inflammatory bowel disease. Clin Exp Gastroenterol. (2017) 10:259–63. doi: 10.2147/CEG.S136383
44. Jacques P, Elewaut D. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. (2008) 1:364–71. doi: 10.1038/mi.2008.24
Keywords: spondyloarthritis, d-dimer, gut inflammation, peripheral joint involvement, ileo-colonoscopy
Citation: Feng J, Li J, Li Y, Jin Y, Du F and Chen X (2022) Elevated Serum D-Dimer May Reflect the Presence of Gut Inflammation in Spondyloarthritis. Front. Med. 8:816422. doi: 10.3389/fmed.2021.816422
Received: 16 November 2021; Accepted: 20 December 2021;
Published: 21 January 2022.
Edited by:
Ruxandra Schiotis, Clinical Hospital of Infectious Diseases, RomaniaReviewed by:
Hsin-Hua Chen, Taichung Veterans General Hospital, TaiwanQiang Xu, Guangzhou University of Chinese Medicine, China
Copyright © 2022 Feng, Li, Li, Jin, Du and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxiang Chen, eGlhb3hpYW5nMDcyMUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Jiaqi Feng
Jiaqi Feng Jia Li
Jia Li Yixuan Li
Yixuan Li Yuyang Jin
Yuyang Jin Fang Du
Fang Du Xiaoxiang Chen
Xiaoxiang Chen