- 1Department of Pharmacy, Guangdong Food and Drug Vocational College, Guangzhou, China
- 2Department of Chemistry, Zhengzhou University, Zhengzhou, China
Although hormone replacement therapy (HRT) use is associated with elevated endometrial cancer(EC) risk, little evidence assesses potential effect-modifiers on HRT-related EC in a long-term follow-up. In this large-scale longitudinal cohort study, we tried to evaluate the association between different HRT types/methods use and risk of EC, and reveal this risk within different body mass index (BMI) groups. In whole cohort, 677 EC occurred during mean 11.6 years follow-up. Cox proportional hazards regression was used to estimate multivariable-adjusted hazards ratios (HRs) and 95% confidence intervals (CIs) with HRT status (never, former, or current) for risk of EC incidence. Current HRT use was not significantly associated with EC risk (HR for current vs. never HRT use: 1.13; 95% CI: 0.92, 1.38) in the whole cohort, but presented a dose-response effect on increased EC risk (HR for >10-year use vs. never HRT use: 1.73; 95% CI: 1.35, 2.21). Moreover, EC risk differed in distinct regimens or subsets (all Pinteraction < 0.05). Estrogen-only use was associated with elevated EC risk (HR for current vs. never HRT use: 1.51; 95% CI: 1.12, 2.04), but women with high BMI (> 30 kg/m2) who currently use estrogen-only harbored decreased EC risk (HR: 0.56; 95% CI: 0.38, 0.82) compared to counterparts without HRT use. Estrogen-only use is associated with increased EC risk, and precise monitoring of EC development for postmenopausal women with long-term HRT use are urgently needed. BMI could serve as an important surrogate to assess this risk.
Introduction
Population aging is increasing worldwide. In 2020, the number of women aged 50 years and older were up to 69 million, and by 2050, women above 50 are projected to total to 90 million (1). The mean age of natural menopause is 49 years (2). Most perimenopausal women would suffer from a series of menopause symptoms such as hot flashes, night sweats, vaginal dryness, and low mood or anxiety, which may persist for a decade or longer (3). In addition, up to 84% of postmenopausal women experience genitourinary symptoms, such as vulvovaginal atrophy and incontinence (4). The burden of menopausal symptoms can considerably affect the personal, social, and work lives of women (3). Hormone replacement therapy (HRT) is the most effective treatment for such vasomotor and genitourinary symptoms (5). In high-income countries, there were about 600 million woman-years of HRT use in the period 1970–2019, and about 12 million users in the 2010s, of whom 6 million were in the United States and the United Kingdom alone (6, 7).
Nevertheless, there is increasing attention to possible health effects of HRT beyond alleviation of menopausal symptoms, such as secondary cancer risks. Concerns of subsequent risks of gynecological tumors are raised from postmenopausal women who take HRT (8), like cervical squamous cell carcinoma (9) and endometrial cancer (EC) (10). Endometrial cancer (EC) is the most common female gynecological malignancy with an estimated lifetime incidence of 4% (11). The most characteristic pathophysiological feature of EC is its hormone-dependence. Options of HRT typically include unopposed estrogen, combined therapy (estrogen and progesterone), and tibolone. Unopposed estrogen use increases the risk of endometrial hyperplasia (12), and risk of EC is directly associated with circulating estrogen and androgen levels (13). However, progestogens are considered as an inhibitor of carcinogenesis and endometrial tumor suppressor (14, 15). An umbrella review indicated that the level of evidence from epidemiology studies on the association between HRT and risk of EC is weak because of insufficient observations (16). The counterbalancing effect of HRT is dependent on the duration of HRT. For example, the Women's Health Initiative trial indicated that daily use of a synthetic progestin, in combination with estradiol, over 5 years significantly decreases the risk of EC (17). In addition, body mass index (BMI) might interact with the effect of HRT on EC (18, 19). Specifically, obesity-related EC risk, with a relative risk of 1.59 per 5 kg/m2 incremental increase (20), could be explained by a hyperestrogenic state, which is caused by higher rates of conversion of androgenic precursors to estradiol in adipose tissue (18, 21). Postmenopausal women on estrogen-containing HRT are in an excess estrogen state.
The aim of this study was to further investigate the effect of different types and durations of HRT on EC development, with focus on interaction between BMI and HRT on the effect of EC risk.
Methods
Participants
The longitudinal data analyzed in this study were obtained from the PLCO trial (https://cdas.cancer.gov/plco/), which is a large-scale randomized trial designed and sponsored by the National Cancer Institute (NCI) to determine the effects of screening on cancer-related mortality and secondary endpoints in men and women aged 55–74, and includes following five ClinicalTrials.gov registration numbers: NCT00002540 (prostate), NCT01696968 (lung), NCT01696981 (colorectal), NCT01696994 (ovarian), and NCT00339495 (EEMS). Approximately 155,000 participants were enrolled between November 1993 and July 2001, and cancer data were collected up to December 31, 2009. In this study, 78,209 female participants were randomly assigned to the intervention arm (n = 39,103) and the control arm (n = 39,106). We further excluded 33,006 female participants if they underwent hysterectomy before the trial started (n = 27,575), did not return baseline questionnaires (n = 3,001); had cancer history before completing supplemental questionnaire (SQX) that recorded HRT regimens (n = 5,188); or had less than 6 months follow-up after SQX completion or without follow-up data (n = 133). Finally, 45,203 women with follow-up data remained in the cohort (Supplementary Figure S1).
The study protocol of the PLCO Cancer Screening Trial was approved by the Institutional Review Board of the National Cancer Institute. All the participants provided written informed consent. The data used in this study had been approved, and the project ID was PLCO-734. Given that the PLCO trial provided de-identified information of patients, the Guangdong Food and Drug Vocational College Institutional Review Board considers PLCO data analyses to be exempt from Institutional Review Board review.
Outcome Ascertainment
Outcomes included diagnosis of EC and time to EC events. All reports of EC were followed up, and medical records were abstracted and reviewed for case ascertainment. We extracted EC cases according to International Classification of Diseases for Oncology, Third Edition (ICD-3, which was coded as C54.1).
Exposure Ascertainment
At baseline, the women were asked whether they had ever used HRT (choices included current, former, and never use). On subsequent questionnaires (SQX), if they had used HRT since the previous questionnaire, they would further indicate HRT type, method of HRT use, as well as the duration of use during that time period (choose included estrogen only, progesterone only, estrogen + progesterone combined; pills or cream; and <1 year, 1–3 years, 3–5 years, 5–10 years, and >10 years).
Covariates
The women reported their age at baseline/menarche/menopause, history of fertility, race (white, non-Hispanic Black, non-Hispanic, Hispanic Asian, and others), education level (less than high school, high school graduate or equivalent, post high school education, college education, or higher), marital status (singled, married, divorced/separated, or widowed), birth control pill use, and family history of EC. Data on BMI (kg/m2), physical activity, and income (per month) (< $20,000, $20,000–49,000, $50,000–99,000, $100,000–200,000, or > $200,000) were collected at baseline and updated on subsequent follow-up questionnaires (SQX).
Statistical Analysis
We classified eligible women into three groups according to HRT use status (never, former, and current). To present the baseline characteristics across HRT status, mean (interquartile range, IQR) for continuous variables that are normally distributed as indicated by Shapiro-Wilk normality test (all P < 0.05) and frequencies (percentages) for categorical variables were calculated. T-test and chi-square test were performed to test for differences between continuous and categorical, respectively, participant characteristics.
Cox proportional risk regression was conducted to calculate crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs), where the proportional hazard (PH) assumption was examined by Schoenfeld residual test (22). Confounding factors were screened using the strategy “change-in-estimate” (23) to assess if they were associated with both HRT use and EC risk or they changed the crude risk estimate by >10% in bivariate analyses. Time-to-EC was defined as days from DQX completion to EC diagnosis. Censoring time for EC incidence models was calculated as days from DQX completion to death or last contact occurring on or before 31 December 2009 among participants without EC.
Furthermore, cross-products of important co-variates with HRT use in the multivariable-adjusted model were tested by Likelihood ratio tests, and Pinteraction < 0.05 were considered as a cut-point of significant effect modification. Accordingly, subgroup analyses stratified by crucial variables that were mentioned by prior literature (24), such as BMI (20–25, 25–30, and >30 kg/m2), and HRT method (pills and cream), were performed.
P values reported (two-sided) to be <0.05 were considered statistically significant. All the analyses were conducted using the R software (version 4.0.1).
Results
In total, 45,203 eligible women were included in this study, 18,286 in the “never” group, 7,826 in the “former” group, and 19,091 in the “current” group. Their baseline characteristics are shown in Table 1. Compared with the women who never used HRT, the current users were younger, earlier to have menopause, better educated, and were more likely White, married, and to perform regular exercise and use birth control pills, but are less likely to have a family history of EC.
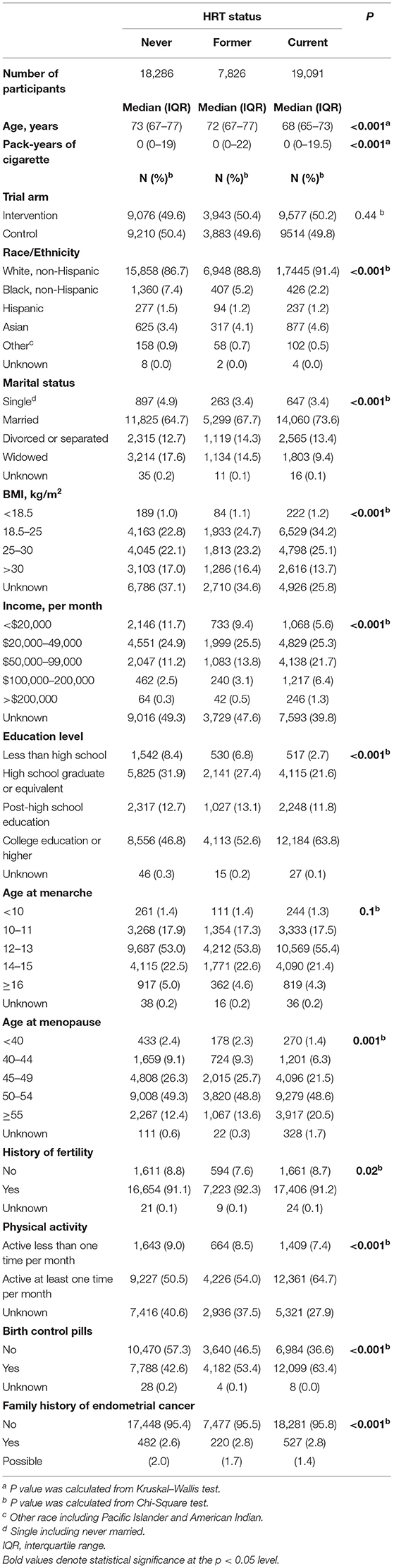
Table 1. Baseline characteristics of female subjects by status of hormone replacement therapy (HRT) in the PLCO screening trial.
A total of 677 EC events occurred during mean 11.6 years follow-up (Table 2), 267 in never group (13/10,000 person-years), 110 (12/10,000 person-years) in former group, and 300 (14/10,000 person-years) in current group. After adjustment for age at baseline, age at menopause, BMI, education level, race, physical activity, family history of EC, and birth control pills, current HRT use was significantly associated with increased EC risk (HR for current vs. never HRT use: 1.13; 95% CI: 0.92, 1.38; HR for >10-year use vs. never HRT use: 1.73; 95% CI: 1.35, 2.21, Table 2). Furthermore, EC risk differed in different HRT regimens, namely, estrogen-only, progesterone-only, and estrogen plus progesterone combined. Estrogen-only use was associated with elevated EC risk (HR for current vs. never HRT use: 1.51; 95% CI: 1.12, 2.04; HR for >10-year use vs. never HRT use: 2.92; 95% CI: 2.06, 4.14, Table 2), whereas no relationship between progesterone-only or estrogen plus progesterone combination use and risk of EC was found in status or time accumulated comparisons (Table 2).
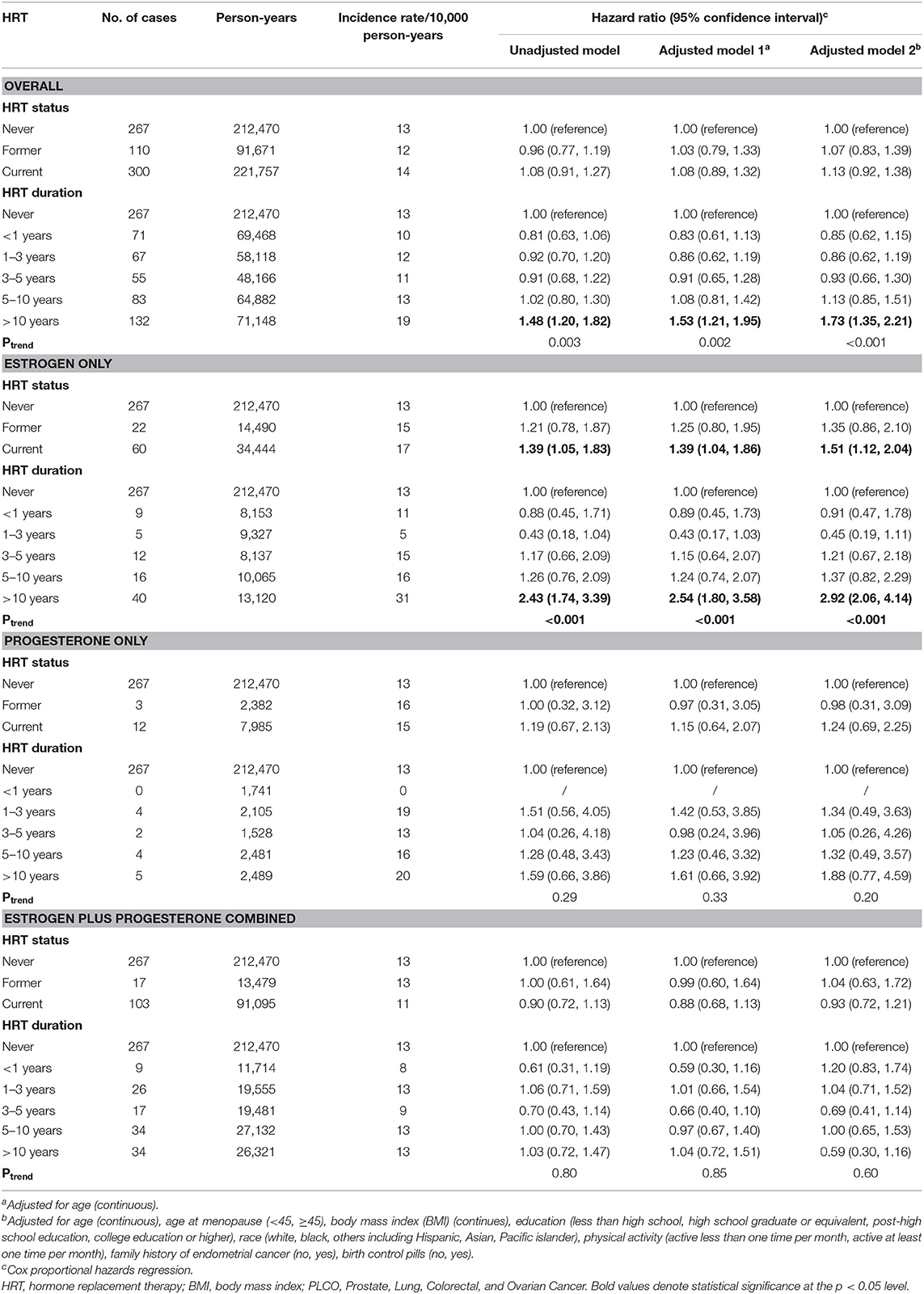
Table 2. HRs (95% CIs) for endometrial cancer incidence and death by hormone replacement therapy status in the screening arm of the PLCO screening trial: 1993–2009.
The subgroup analyses for association between estrogen-only use and EC risk, were conducted by stratifying BMI and the HRT method, which was changed across each subgroup (all Pinteraction < 0.05, Table 3). Interestingly, the increased risk of EC from the estrogen-only regimen observed before reduced among the women who used estrogen creams (HR: 1.59; 95% CI: 1.02, 2.48, Table 3) compared with those using pills (HR: 2.23; 95% CI: 1.53, 3.26, Table 3). Obese women who currently use estrogen only harbored decreased EC risk (HR: 0.56; 95% CI: 0.38, 0.82, Table 3) compared to counterparts without HRT use, but in the normal/underweighted subgroup (<25 kg/m2), and estrogen-only use was associated with increased risk of EC (HR for current vs. never HRT use: 2.75; 95% CI: 1.79, 4.24, Table 3).
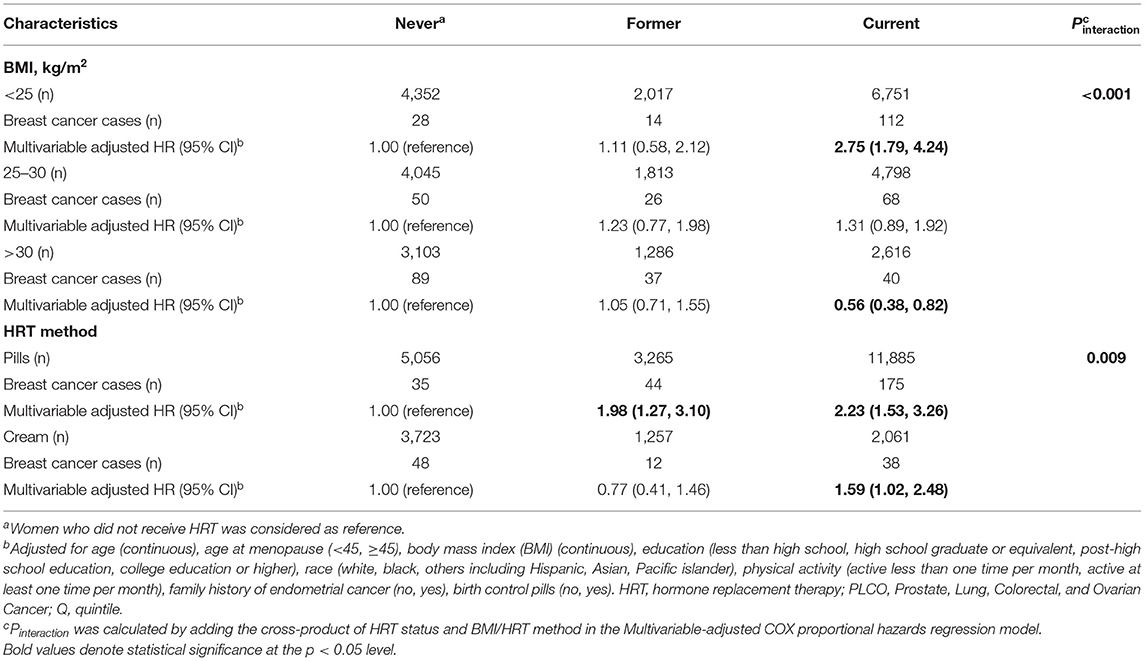
Table 3. Risk of endometrial cancer incidence from estrogen-only use stratified by body mass index, hormone replacement therapy method in the PLCO Cancer Screening Trial: 1993–2009.
Discussion
In this longitudinal study, our results found that estrogen-only use for HRT was associated with increased risk of EC development, and an obesity paradox that this association was completely reversed in obese women was confirmed. Furthermore, women with long-term use (over 10 years) of HRT, especially on the type of estrogen only, or with estrogen pills had significantly higher risk of EC.
An epidemiological study demonstrated that less than 25 and 38% of women with EC are diagnosed before menopause in Western and Asian countries, respectively (25). A meta-analysis including 28 studies (10), indicated that estrogen alone, tibolone and sequential combined therapy increase the risk of EC, even when treatment lasts less than 5 years, but continuous combined therapy might provide a lower risk than never use, and therapy for more than 10 years does not increase risk. Our study had a comparable follow-up with that of postmenopausal Breast Cancer Detection Demonstration Project (26), but did not find association between short-term use of estrogen only (<5 years) and increased risk of EC because of limited samples size and statistical power.
It is well-established that the endometrium is extremely sensitive to unopposed estrogen, either endogenous or exogenous, which can induce endometrial hyperplasia. Although simple hyperplasia is rarely directly transformed to EC, it can evolve to atypical hyperplasia, which is a precancerous lesion. Thus, progesterone is the important hormone to maintain a eutrophic endometrium, and is mainly used for women with preserved uterus. Its role is to overcome the proliferative effect of estradiol and induce differentiation of the glands, stroma, and vessels of the endometrium (27). A large amount of evidence shows that continuous-combined therapy with synthetic progestins reduces the risk of EC (17, 28–30). Combining HRT with natural progestins would increase the risk of EC when dosage is lesser and duration is longer (31, 32). In contrast, many studies support that HRT with estrogens only increases the risk of developing EC (28–30, 32, 33). This is consistent with what we found, namely, the carcinogenic effect of estrogens could be alleviated by progestins. Additionally, estrogen type and duration of use are significant modifiers on the association between estrogen use and EC risk. Long-term use of estrogens might augment the harmful effect, and short-term use of ≤ 5 years is recommended. Previous studies have also indicated that the risk was directly proportional to the duration of sequential therapy (26, 32, 34). Although both estrogen pills and cream increased the risk of EC, the effect tended to be obvious when women choose pills. It could be explained by the direct association between circulating estrogen and androgen levels and carcinogenesis of EC (13). Additionally, the reduced EC risk from estrogen-only cream compared with that administrated with pills is due to different metabolic processes (35). Therefore, doctors give greater consideration to transdermal HRT, in line with the NICE guideline (36).
Body mass index (BMI) is a risk factor for EC, and the association of BMI with cancer risk ranks highest for EC (20, 37). However, this study interestingly found an “obesity paradox” when comparing the risk between current estrogen users and women who never use estrogens. It seems that obese women bear lower HRT-related EC risk. As we know, non-exposed HRT women with high BMI are associated with increased EC risk than their underweight counterparts (16), leading to shrinking the “riskgap” in EC development between obese women with HRT exposure. Pathophysiologically, cancer cachexia and biological mechanisms such as differences in body composition and adiposity, and nutritional reserve to face anti-cancer treatments, somewhat contribute to this obesity paradox (38, 39). In the future, research should focus on the standardized incidence ratio of EC from HRT use, which is compared with the general population.
The prospective cohort design, comprehensive assessment of HRT status and types, and long follow-up period are the strengths of this study. Several limitations should also be noted. First, the PLCO trial only recruits participants aged 55–74 years. Thus, the results of this study cannot be extended to other age groups. Second, women who were diagnosed with EC before SQX completion or have invalid SQX response were excluded in this study, which might induce biases. Previous studies have indicated that most HRT-associated ECs are, thus, endometrioid adenocarcinomas (32, 33, 40), but we did not acquire the histologic subtype of EC in this PCLO project and failed to assess this relationship.
Conclusion
Long-term use (> 10 years) of estrogen-progesterone combined with HRT increased the risk of EC development. Specifically, HRT with estrogens only significantly increases the risk of EC, but there were no associations of HRT with progesterone only. The carcinogenic effect of estrogen is more obvious in pills than in cream, and among underweight women than obese women.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the National Cancer Institute. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HL designed the study and collected data. YL, HJ, and LQ analyzed the data. YL wrote the article. All authors reviewed and approved the final version of the manuscript.
Funding
This research was funded by the Characteristic Innovative Project of Guangdong Province Ordinary Institution of Higher Education (2020KTSCX259) and Science and Technology Program of Guangzhou (202102080546).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.802959/full#supplementary-material
Supplementary Figure S1. Study flowchart for identifying eligible individuals. PLCO, the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SQX, supplemental questionnaire.
Abbreviations
PLCO, the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; EC, endometrial cancer; HRT, hormone replacement therapy; SQX, supplemental questionnaire; IQR, interquartile range; HRs, hazards ratios; CIs, confidence intervals.
References
1. Division., U. N. P. World Population Prospects 2019. (2020). Available online at: https://population.un.org/wpp/DataQuery/.
2. Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol. (2014) 43:1542–62. doi: 10.1093/ije/dyu094
3. Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause—global prevalence, physiology and implications. Nat Rev Endocrinol. (2018) 14:199–215. doi: 10.1038/nrendo.2017.180
4. North American Menopause Society. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. (2020) 27:976–92. doi: 10.1097/gme.0000000000001609
5. Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2015) 100:3975–4011. doi: 10.1210/jc.2015-2236
6. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. (2015) 385:1835–42. doi: 10.1016/s0140-6736(14)61687-1
7. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. (2019) 394:1159–68. doi: 10.1016/s0140-6736(19)31709-x
8. Wachtel MS, Yang S, Dissanaike S, Margenthaler JA. Hormone replacement therapy, likely neither angel nor demon. PLoS ONE. (2015) 10:e0138556. doi: 10.1371/journal.pone.0138556
9. Vargiu V, Amar ID, Rosati A, Dinoi G, Turco LC, Capozzi VA, et al. Hormone replacement therapy and cervical cancer: a systematic review of the literature. Climacteric. (2021) 24:120–7. doi: 10.1080/13697137.2020.1826426
10. Sjögren LL, Mørch LS, Løkkegaard E. Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas. (2016) 91:25–35. doi: 10.1016/j.maturitas.2016.05.013
11. Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. (2017) 29:47–58. doi: 10.1097/gco.0000000000000338
12. Furness S, Roberts H, Marjoribanks J, Lethaby A. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev. (2012) 2012:CD000402. doi: 10.1002/14651858.CD000402.pub4
13. Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. (2004) 150:161–71. doi: 10.1530/eje.0.1500161
14. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. (2013) 34:171–208. doi: 10.1210/er.2012-1008
15. Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. (2011) 22:145–52. doi: 10.1016/j.tem.2011.01.005
16. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. (2019) 145:1719–30. doi: 10.1002/ijc.31961
17. Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, et al. Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women's Health Initiative Randomized Trial. J Natl Cancer Inst. (2016) 108:djv350. doi: 10.1093/jnci/djv350
18. Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. (2002) 11:1531–43.
19. Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. (2010) 19:3119–30. doi: 10.1158/1055-9965.EPI-10-0832
20. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. (2008) 371:569–78. doi: 10.1016/s0140-6736(08)60269-x
21. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. (2004) 4:579–91. doi: 10.1038/nrc1408
22. Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. (1980) 67:145–53. doi: 10.1093/biomet/67.1.145
23. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. (1993) 138:923–36. doi: 10.1093/oxfordjournals.aje.a116813
24. Chen JL, Luo Y, Nurmatov U, Zhang J, Critchley H. Menopausal hormone therapy and women's health: an umbrella review. PLoS Med. (2021) 18:e1003731. doi: 10.1371/journal.pmed.1003731
25. Chen Q, Tong M, Guo F, Lau S, Zhao M. Parity correlates with the timing of developing endometrial cancer, but not subtype of endometrial cancer. J Cancer. (2015) 6:1087–92. doi: 10.7150/jca.12736
26. Lacey JV, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. (2005) 14:1724–31. doi: 10.1158/1055-9965.Epi-05-0111
27. Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. (2020) 69:95–107. doi: 10.1016/j.bpobgyn.2020.05.003
28. Sponholtz TR, Palmer JR, Rosenberg LA, Hatch EE, Adams-Campbell LL, Wise LA. Exogenous hormone use and endometrial cancer in US Black Women Cancer. Epidemiol Biomarkers Prev. (2018) 27:558–65. doi: 10.1158/1055-9965.Epi-17-0722
29. Mørch LS, Kjaer SK, Keiding N, Løkkegaard E, Lidegaard Ø. The influence of hormone therapies on type I and II endometrial cancer: a nationwide cohort study. Int J Cancer. (2016) 138:1506–15. doi: 10.1002/ijc.29878
30. Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. (2005) 365:1543–51. doi: 10.1016/s0140-6736(05)66455-0
31. Allen NE, Tsilidis KK, Key TJ, Dossus L, Kaaks R, Lund E, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women in the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol. (2010) 172:1394–403. doi: 10.1093/aje/kwq300
32. Fournier A, Dossus L, Mesrine S, Vilier A, Boutron-Ruault MC, Clavel-Chapelon F, et al. Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992–2008. Am J Epidemiol. (2014) 180:508–17. doi: 10.1093/aje/kwu146
33. Odem RR, Moniz MH, Allsworth JE, Bleckman CR, Omicioli VA, Lawrence LT, et al. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol Biomarkers Prev. (2010) 19:475–83. doi: 10.1158/1055-9965.Epi-09-0712
34. Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck AR, Park Y, et al. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer. (2013) 132:417–26. doi: 10.1002/ijc.27623
35. Ligniers BD, Basdevant A, Thomas G, Thalabard JC, Mercier-Bodard CH, Conard J, et al. Biological effects of estradiol-17 beta in postmenopausal women: oral versus percutaneous administration. J Clin Endocrinol Metab. (1986) 62:536–41. doi: 10.1210/jcem-62-3-536
36. Lumsden MA, Davies M, Sarri G. Diagnosis and management of menopause: the national institute of health and care excellence (NICE) guideline. JAMA Intern Med. (2016) 176:1205–6. doi: 10.1001/jamainternmed.2016.2761
37. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. (2008) 67:253–6. doi: 10.1017/s002966510800712x
38. Dalamaga M. Interplay of adipokines and myokines in cancer pathophysiology: emerging therapeutic implications. World J Exp Med. (2013) 3:26–33. doi: 10.5493/wjem.v3.i3.26
39. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. (2016) 18:56. doi: 10.1007/s11912-016-0539-4
40. Trabert B, Coburn SB, Falk RT, Manson JE, Brinton LA, Gass ML, et al. Circulating estrogens and postmenopausal ovarian and endometrial cancer risk among current hormone users in the Women's Health Initiative Observational Study. Cancer Causes Control. (2019) 30:1201–11. doi: 10.1007/s10552-019-01233-8
Keywords: hormone replacement therapy (HRT), endometrial cancer (EC), body mass index, estrogen, progesterone
Citation: Liang Y, Jiao H, Qu L and Liu H (2022) Association Between Hormone Replacement Therapy and Development of Endometrial Cancer: Results From a Prospective US Cohort Study. Front. Med. 8:802959. doi: 10.3389/fmed.2021.802959
Received: 28 October 2021; Accepted: 09 December 2021;
Published: 17 January 2022.
Edited by:
Andrea Rosati, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Virginia Vargiu, Agostino Gemelli University Polyclinic (IRCCS), ItalyHuri Güvey, Private Parkhayat Hospital, Turkey
Baroni Alessandro, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2022 Liang, Jiao, Qu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Liu, MTc5ODYwNjYmI3gwMDA0MDtxcS5jb20=
 Ying Liang1
Ying Liang1 Hao Liu
Hao Liu