- 1Eye Unit, Department of Medicine, Surgery and Dentistry, “Scuola Medica Salernitana”, University of Salerno, Baronissi, Italy
- 2Corneal Transplant Unit, Azienda Sanitaria Locale (ASL) Napoli 1, Naples, Italy
- 3Eye Unit, “Maria SS Addolorata” Hospital, Azienda Sanitaria Locale (ASL) Salerno, Eboli, Italy
In recent years, ultrasonographic measurement of the optic nerve sheath diameter (ONSD) has been widely used to identify the presence of increased intracranial pressure (ICP). Intracranial hypertension is a life-threatening condition that can be caused by various neurological and non-neurological disorders, and it is associated to poor clinical results. Ultrasonography could be used to qualitatively and efficiently detect ICP increases, but to reach this purpose, clear cut-off values are mandatory. The aim of this review is to provide a wide overview of the most important scientific publications on optic nerve ultrasound normal values assessment published in the last 30 years. A total of 42 articles selected from PubMed medical database was included in this review. Our analysis showed that ocular ultrasonography is considered to be a valuable diagnostic tool, especially when intracranial hypertension is suspected, but unfortunately this research provided conflicting results that could be due to the different ultrasound protocols. This is mainly caused by the use of B scan alone, which presents several limitations. The use of B-scan coupled with the standardized A-scan approach could give more accurate, and reliable ultrasound evaluation, assuring higher data objectivity.
Introduction
Intracranial hypertension is a critical life-threatening condition caused by a variety of neurological and non-neurological diseases and it is associated with poor clinical outcomes and high mortality rates (1). One of the most representative sign of elevated intracranial pressure (ICP) is the presence of papilledema, characterized by disc elevation, blurred disc margins, venous congestion, hemorrhages, soft/hard exudates, and choroidal/retinal folds (1). The optic nerve is surrounded by the meninges and the space between the optic nerve sheaths and the optic nerve is connected to the subarachnoid space (2). In case of increased ICP, the subarachnoid fluid is pushed into the subarachnoid space surrounding the optic nerve, causing optic nerve sheath expansion (3).
In recent years, ultrasonographic measurement of the optic nerve sheath diameter (ONSD) has been widely used to identify the presence of increased ICP (4). Furthermore, ultrasound is a rapid, cheap, real-time, safe, and non-invasive diagnostic tool that could be really useful when the patients are critically ill, allowing a bedside appraisal. However, to perform a reliable and well-executed ocular ultrasonography, especially in the optic nerve evaluation, a well-trained and skilled operator is required, and a series of precautions in the exam execution and in the utilized probe and technique should be considered (5–7). Ultrasonography could be used to qualitatively and efficiently detect ICP increases, but to reach this purpose, clear cut-off values are mandatory, and some papers report conflicting results on the cut-off values and ultrasound protocols to identify intracranial hypertension.
Considering all the aforesaid reasons, the purpose of this review is to provide a wide overview of the most important scientific publications on optic nerve ultrasound normal values assessment published in the last 30 years, focusing on studies carried out in animal models and healthy volunteers, and to discuss the limitations of the most widely used B-scan technique.
Materials and Methods
We searched within the PubMed medical database. A preliminary general Web search using Google was also performed in order to get a larger vision and understanding of the issue. We entered search strings including terms related to ocular ultrasonography, ICP, animals, and healthy subjects. Text words were chosen based on the existing literature and/or were obtained from related bibliographies as well. Bibliographies from the initial searches were manually searched for additional inclusions too.
Only English full articles and case reports or case series concerning optic nerve ultrasound evaluation in animals and healthy people were included in this study. The earliest publication date was set at January 1990, while the search ended in August 2021.
Results
Our initial search yielded 137 results, of which 3 were excluded due to no English language. Subsequently, another 92 articles were excluded through successive reviews because they are not directly related to the discussed topic or because they were comments or observations to other articles. At last, 42 articles were included and divided into 2 subgroups: 12 articles concerned studies on animals, while 30 articles regarded studies on healthy subjects.
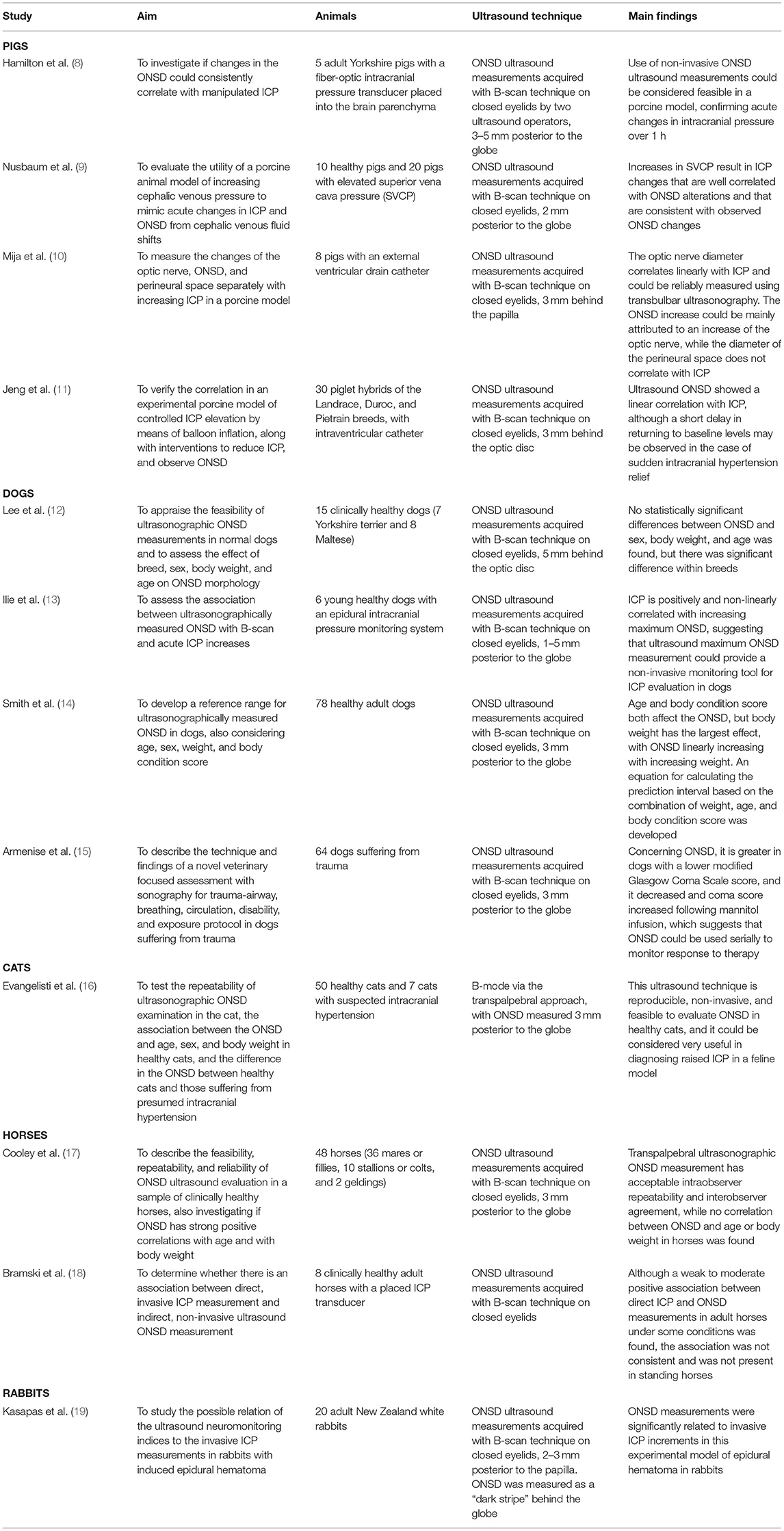
Animals
Some studies were carried out in different animal models to evaluate the reliability of ocular ultrasound in the diagnosis of intracranial hypertension. Of the twelve articles on animals selected for this review, four were performed on pigs, four on dogs, one on cats, two on horses, and one on rabbits.
The aim, the utilized ultrasound technique and the main findings of these studies are summarized in the Table 1.
Healthy Volunteers
Considering the studies carried out on healthy subjects, eleven articles tried to establish the normal ONSD reference values, also focusing on possible effects of ethnicity, age, and sex, four articles concerned ONSD ultrasound accuracy and reliability, four articles focused on the effects of different body positions on ONSD, five articles discussed the effects of oxygen variations on ONSD, four articles evaluated the effects of cervical collar application on ONSD, while two articles assessed ONSD modifications due to physical activity.
ONSD Normal Range Values
Several papers on healthy participants tried to establish ultrasound ONSD normal reference values. Some of these studies were carried out on a limited number of patients (20–22), while others considered much more significant study samples (23–30).
Garcia et al. (20) assessed 23 healthy adults with coronal C-scan ultrasound, performing the exam with open eyelids and using topical anesthesia, showing a mean ONSD of 4.8 mm for the analyzed sample, while a mean ONSD of 4.9 mm in males and a mean ONSD of 4.5 mm in females were found.
Bäuerle et al. (21) appraised 40 healthy adults (18–77 years) with the same ultrasound protocol, proposing a value of 5.8 mm as threshold, with a specificity of 80%.
In a prospective observational cohort study on 50 Australian healthy pregnant women with uncomplicated singleton pregnancies, Kane et al. (22) performed a single prenatal ultrasonographic examination on all participants, with a postnatal examination performed on a subgroup with uncomplicated deliveries. The authors detected a mean ONSD of 4.34 mm, with no clear relationship with gestation or mean arterial pressure.
Ballantyne et al. (23) evaluated 102 healthy children with B-scan ultrasonography and closed eyelids, suggesting that an ONSD of >4 mm in infants <1 year, and 4.5 mm or greater in older children, should be considered as abnormal.
Maude et al. (24) carried out a prospective observational study on 136 Bangladeshi healthy subjects, finding out that ultrasonographic ONSD has a narrow bimodal distribution independent of gender, age, and head circumference, and an ONSD value greater of 4.75 mm should be considered abnormal in this population.
Chen et al. (25) tried to determine the ONSD reference values in a cohort of 519 healthy Chinese adults using B-scan ultrasound performed on closed eyelids. They found the median and the ONSD 95% percentile to be 5.1 and 5.9 mm, respectively. Moreover, they showed that ONSD was correlated with optic nerve diameter, while it was independent of gender, age, height, and weight.
Contrariwise, in another study carried out in Chinese population with the same ultrasound protocol, Wang et al. (26) appraised 230 healthy individuals, finding out the mean ONSD to be 3.46 mm, with 95% of participants in the range 3.42–3.49 mm, and to be correlated with sex and BMI. Furthermore, this study showed that the upper ONSD limit was lower than that one observed among Caucasian and African samples, also suggesting a potential ethnic/racial differences.
Concerning these possible ethnic differences, Kim et al. (27) studied ONSD ultrasound measurements in 585 Korean healthy volunteers, stating that obvious differences between Korea and other countries were not found, with a mean ONSD in their study sample of 4.11 mm.
Goeres et al. (28) assessed ultrasound ONSD measurements in a cohort of 120 healthy adult volunteers, stating that a lack of relationship to age, height, and weight was found. However, they found a difference depending on gender, suggesting the possible need for separate reference ranges for men and women. In fact, in this study, mean ONSD for men was 3.78 mm, compared with 3.60 mm for women.
Avci et al. (29) aimed to determine the standard ONSD value in 195 healthy adults aged 65 or older with no previous diagnosis of raised ICP. They compared right and left ONSD values and ONSD differences, according to the gender and age of the patients. In this study, the authors found out that the ONSDs of both eyes did not vary with age and gender in the study group, with a mean ONSD of 4.16 mm.
Focusing on potential ONSD differences related to sex and age, Chandrapatham et al. (30) evaluated 122 healthy individuals with B-scan ultrasonography, stratifying them for sex, and dividing them into three different age groups. The authors found ONSD to increase with age and to be greater in males (range: 3.9–4.6 mm) than in females (range: 3.6–4.2 mm).
The reference range values established by these studies are summarized in the Table 2.
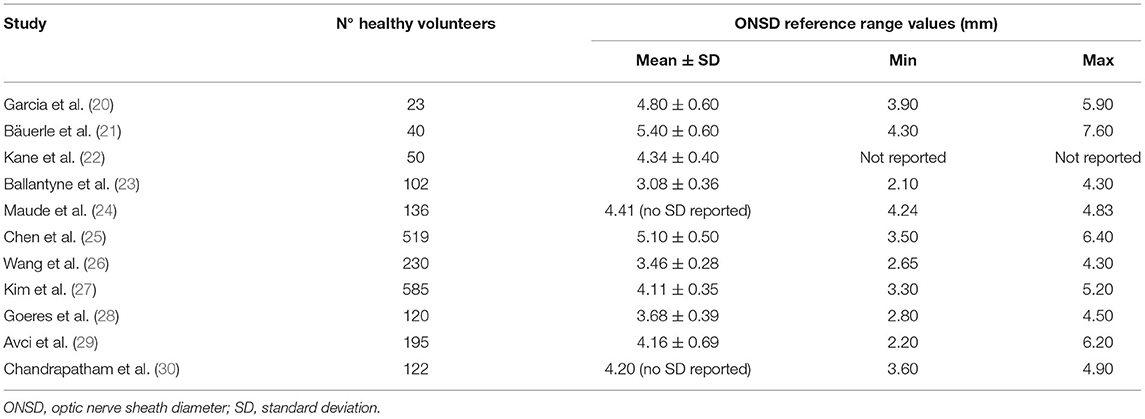
Table 2. Summary of the optic nerve sheath diameter ultrasound reference range values established in studies on healthy people.
ONSD Accuracy and Reliability
In addition, different papers tried to establish the accuracy and the reliability of ONSD ultrasound appraisal in healthy people.
Betcher et al. (31) attempted to evaluate the ability of 23 military trainees to ultrasonographically measure the ONSD in healthy volunteers after attending a very brief training session, compared to four expert emergency physicians, concluding that trainees were able to perform ONSD with an accuracy similar to ultrasound experts.
Amini et al. (32) compared the traditional ultrasound visual axis technique to coronal axis technique for evaluating the ONSD in 42 healthy volunteers, concluding that the two ultrasound approaches were similar, with the coronal one slightly faster to perform and not technically challenging.
These results are in contrast with that ones by Blehar et al. on 27 healthy adults (33), who found that visual axis measurements do not reliably correlate with coronal axis measurements, with a statistically significant ONSD increase as the nerve coursed posteriorly when measured in the visual axis.
Cimilli Ozturk et al. (34) tried to define the operator variations in the ONSD ultrasound measurement performed on 60 healthy adults, utilizing the axial and the longitudinal approaches. They concluded that, although the levels of compatibilities for most of the measurements were found at acceptable levels statistically, ONSD sonographic measurement could not be considered a highly reliable method both in longitudinal and transverse planes due to the difficulties in the demonstration of the nerve sheath borders and small deflections in the gaze of eye direction.
ONSD and Body Position
In other three studies, the effect of different positions on the ONSD values were investigated.
Maissan et al. (35) carried out a study in which ONSD was measured by ultrasound in five healthy volunteers during helicopter liftoff and acceleration in the supine position or with a raised headrest. The authors found out that ONSD increased during helicopter acceleration from baseline, while after headrest elevation (20°-25°) the ONSD did not raise during helicopter acceleration. For this reason, the authors stated that ONSD and ICP seem to increase during helicopter transportation and, by raising the headrest of the gurney before liftoff, these effects could be prevented.
Romagunolo et al. (36) evaluated ONSD potential changes between the supine, Trendelenburg's, and reverse Trendelenburg's positions in 10 healthy people, discovering no statistically significant differences.
Inversely, Özdogan et al. (37) assessed the effect of spinal immobilization at 20°, compared to that one at 0°, on the ICP via the ONSD ultrasonographic measurement of 140 healthy adults, finding out that both these procedures increased ONSD.
Analogously, Pardon et al. (38) studied the ONSD differences over 12 h in seated and 6° head-down tilt postures in 30 healthy individuals, reporting no significant difference in sitting position, while ONSD increased during the head-down tilt posture.
ONSD and Oxygen Variations
Dinsmore et al. (39) examined the dynamic ONSD changes in response to mild fluctuations in cerebral blood volume induced by changes in end-tidal carbon dioxide on 11 healthy volunteers. A single investigator repeatedly measured ONSD for 10 min at each level of carbon dioxide: normocapnia (baseline), hypercapnia (6.5 kPa), normocapnia (baseline 1), hypocapnia (3.9 kPa), and on return to normocapnia (baseline 2). There was a significant ONSD increase with hypercapnia, while on return to normocapnia ONSD rapidly reverted back to baseline values, confirming dynamic ONSD changes with corresponding changes in carbon dioxide.
Some authors also studied the correlation between ONSD variations and acute mountain sickness (AMS) in healthy patients.
Keyes et al. (40) performed ultrasound ONSD measurements on 57 healthy subjects at 1,400 m and 18 h after rapid ascent to 4,300 m, both before and after oxygen treatment. They found that mean ONSD increased in subjects with AMS at high altitude, while individual variation was high, and most ONSD values were below the clinical threshold for raised ICP.
On the other hand, Di Pasquale et al. (41) manipulated exercise duration, barometric pressure, and ambient oxygen to assess 36 healthy volunteers before, during and after 8 h exposures in normobaric normoxia (300 m elevation equivalent), normobaric hypoxia (4,400 m equivalent), and hypobaric hypoxia (4,400 m equivalent). They assessed ultrasound ONSD measurements, documenting a small but significant increase in AMS patients, suggesting mildly elevated ICP, as well as further increased ONSD with longer exercise duration.
Kanaan et al. (42) evaluated 86 healthy adults enrolled at 1,240 m, drove to 3,545 m and then hiked to and slept at 3,810 m, performing ultrasound ONSD measurements before, the evening of, and the morning after ascent. They concluded that the mean ONSD increased on ascent to high altitude compared to baseline values, but not to a statistically significant degree, while the magnitude of the ONSD difference was positively associated with AMS diagnosis.
Strapazzon et al. (43) investigated changes in oxidative stress biomarkers and reactive oxygen species (ROS) during exposure to hypobaric hypoxia in 16 lowlanders, trying to correlate ROS related cellular damage and ultrasound ONSD as an indirect ICP measurement. Baseline measurement of clinical signs and symptoms, biological samples and ultrasonography were appraised at 262 m and after passive ascent to 3,830 m. Although ONSD was found to concurrently increase, regression analysis did not infer a causal relationship between oxidative stress biomarkers and ONSD changes.
ONSD and Cervical Collar Application
Maissan et al. (44) assessed the effect of application of a rigid cervical collar in 45 healthy volunteers by measuring their ONSD with ocular ultrasonography. Application of a collar resulted in a significant ONSD increase in both eyes, suggesting that ICP could raise after this application. This could be primarily related to the restrictive ability of the cervical collar around the neck vasculature. These same findings were confirmed by other authors (45–47), suggesting that clinicians should take proactive steps to assess the actual need of cervical collar case by case basis.
ONSD and Physical Activity
At last, two papers evaluated the correlation between ONSD and physical activity.
Sadrameli et al. (48) analyzed ONSD measurements of 24 female college soccer players during the initial visit in the pre-season period and at the 3-month follow-up. The authors found an ONSD increase from 4.14 to 5.02 mm, with an average ONSD measured during the post-season follow-up showing a 21.3% increase compared to the baseline.
Conversely, Lefferts et al. (49) measured ultrasound ONSD in 20 healthy participants at rest (baseline), following a time-control condition, and following acute high-intensity resistance exercise, showing no significant changes in ONSD.
Discussion
In recent years, the use of ocular ultrasonography is spreading more and more in different medical fields, especially due to its safety and ready availability, making it easier for physicians to identify several pathological conditions (50). Specifically, this ultrasound technique is a non-invasive, non-irradiating, and cheap diagnostic tool that may be used to detect, indirectly, the presence of raised ICP. In fact, as shown in the present review, there are several scientific papers published in the literature in the last 30 years which describe the use of this ultrasound diagnostic method, also in animal models and in healthy people. However, except for one article (20), in all the other papers discussed in the present review, the authors utilized the B-mode ultrasound technique to evaluate ONSD as an indirect parameter to detect increased ICP.
Although nowadays B-scan ultrasonography could be considered highly sensitive to detect intracranial hypertension in some hospital settings, and it is often the only ready available diagnostic tool for such appraisals, it is crucial to point out some important pitfalls of this type of ocular ultrasonography, related both to how the examination is performed and to intrinsic limitations to the technique itself (51, 52). First of all, as highlighted by several papers discussed in this review, many authors tried to identify ONSD normal reference values, essential to diagnose ICP elevation. Nevertheless, these reference values were very contrasting and dissimilar in the various papers, as shown in the Table 2, with a real difficulty in determining them in an unambiguous way. This difficulty could be related to the so-called “blooming effect” which affects the B-scan ocular ultrasound (53, 54). This effect occurs when the equipment gain-setting is not standardized, in particular when performing repeated measurements over time. In fact, it is due to the absence of a standard gain and sensitivity setting: when a lesion is measured utilizing different gains, this will appear larger decreasing the gain, and smaller increasing it (55). Thus, considering the “blooming effect” and related less precise calipers location during ONSD evaluations, to examine very small structures, such as optic nerve, with B-scan ultrasound may not be objective and effective, providing potential bias and unreliable data (56).
In the early 70' Ossoinig introduced the Standardized A-scan technique (57), an ultrasound method equipped with an a 8 MHz non-focused probe, with a special S-shaped amplification, which is free of “blooming effect” and therefore permits more accurate measurements. Moreover, A scan shows easily discerned high-reflective spikes at the interface between arachnoid and subarachnoid fluid where the markers to measure such a structure can be easily placed, becoming even more important in case of follow up measurements (58–60). Furthermore, it is also important to remark how ocular ultrasonography should be performed to get more trustworthy ONSD measurements. Except for the study by Garcia et al. (20), in all the papers discussed in this review the echographic probe was positioned on the closed eyelids, making impossible to visualize the ocular globe and the patient's gaze direction. This could lead to errors during the ultrasound examination, providing less objective data (61). For this reason, the B- or A-scan probe should be usually used with open eyelids, utilizing methylcellulose and anesthetic drops, thus avoiding mistakes in the eye position visualization and making the probe orientation much more reliable (62).
In conclusion, ocular ultrasonography is certainly a powerful diagnostic device available to the physicians, especially in conditions where a potential intracranial hypertension is suspected. However, for a more complete, precise, and reliable ultrasound evaluation, B-scan ultrasound could be useful for a screening purpose, but it should always be associated with the standardized A-scan technique, to ensure greater data objectivity and accuracy provided by the examination.
Author Contributions
LV, MD, and LC analyzed the literature and wrote the original draft. PC and NR conceived the article and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The research was funded with the FARB grant from the University of Salerno.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. del Saz-Saucedo PD, Redondo-Gonzalez O, Mateu-Mateu A, Huertas-Arroyo R, Garcia-Ruiz R, Botia-Paniagua E. Sonographic assessment of the optic nerve sheath diameter in the diagnosis of idiopathic intracranial hypertension. J Neurol Sci. (2016) 361:122–7. doi: 10.1016/j.jns.2015.12.032
2. Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. (2003) 10:376–81. doi: 10.1111/j.1553-2712.2003.tb01352.x
3. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. (1996) 18:323–8. doi: 10.1007/BF01627611
4. Wang L, Feng L, Yao Y, Wang Y, Chen Y, Feng J, et al. Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PLoS ONE. (2015) 10:e0117939. doi: 10.1371/journal.pone.0117939
5. Capasso L, De Bernardo M, Vitiello L, Rosa N. Ultrasound options for measuring optic nerve sheath diameter in children. Pediatr Crit Care Med. (2021) 22:e329–30. doi: 10.1097/PCC.0000000000002676
6. De Bernardo M, Vitiello L, Capone M, Rosa N. A-scan ultrasonography and optic nerve sheath diameter evaluation in children with acute liver failure. Liver Int. (2020) 40:1504. doi: 10.1111/liv.14372
7. Cornetta P, Marotta G, De Bernardo M, Vitiello L, Rosa N. Ultrasound and optic neuritis. Am J Emerg Med. (2019) 37:1598. doi: 10.1016/j.ajem.2019.02.001
8. Hamilton DR, Sargsyan AE, Melton SL, Garcia KM, Oddo B, Kwon DS, et al. Sonography for determining the optic nerve sheath diameter with increasing intracranial pressure in a porcine model. J Ultrasound Med. (2011) 30:651–9. doi: 10.7863/jum.2011.30.5.651
9. Nusbaum DM, Clark JB, Brady KM, Kibler KK, Sutton JP, Easley RB. Intracranial pressure and optic nerve sheath diameter as cephalic venous pressure increases in swine. Aviat Space Environ Med. (2013) 84:946–51. doi: 10.3357/asem.3486.2013
10. Mija R, Zubak I, Schuetz A, Glas M, Fung C, Jakob SM, et al. Correlation of optic nerve and optic nerve sheath diameter with intracranial pressure in pigs. PLoS ONE. (2020) 15:e0228242. doi: 10.1371/journal.pone.0228242
11. Jeng BCP, de Andrade AF, Brasil S, Bor-Seng-Shu E, Belon AR, Robertis M, et al. Estimation of intracranial pressure by ultrasound of the optic nerve sheath in an animal model of intracranial hypertension. J Clin Neurosci. (2021) 86:174–9. doi: 10.1016/j.jocn.2021.01.021
12. Lee HC, Choi HJ, Choi MC, Yoon JH. Ultrasonographic measurement of optic nerve sheath diameter in normal dogs. J Vet Sci. (2003) 4:265–8.
13. Ilie LA, Thomovsky EJ, Johnson PA, Bentley RT, Heng HG, Lee HC, et al. Relationship between intracranial pressure as measured by an epidural intracranial pressure monitoring system and optic nerve sheath diameter in healthy dogs. Am J Vet Res. (2015) 76:724–31. doi: 10.2460/ajvr.76.8.724
14. Smith JJ, Fletcher DJ, Cooley SD, Thompson MS. Transpalpebral ultrasonographic measurement of the optic nerve sheath diameter in healthy dogs. J Vet Emerg Crit Care (San Antonio). (2018) 28:31–8. doi: 10.1111/vec.12677
15. Armenise A, Boysen RS, Rudloff E, Neri L, Spattini G, Storti E. Veterinary-focused assessment with sonography for trauma-airway, breathing, circulation, disability, and exposure: a prospective observational study in 64 canine trauma patients. J Small Anim Pract. (2019) 60:173–82. doi: 10.1111/jsap.12968
16. Evangelisti MA, Carta G, Burrai GP, Pinna Parpaglia ML, Cubeddu F, Ballocco I, et al. Repeatability of ultrasound examination of the optic nerve sheath diameter in the adult cat: comparison between healthy cats and cats suffering from presumed intracranial hypertension. J Feline Med Surg. (2020) 22:959–65. doi: 10.1177/1098612X19898006
17. Cooley SD, Scrivani PV, Thompson MS, Irby NL, Divers TJ, Erb HN, et al. Age, and body weight in clinically normal horses. Vet Radiol Ultrasound. (2016) 57:49–57. doi: 10.1111/vru.12300
18. Bramski JH, Reed RA, Diehl KA, Epstein KL, Ryan CA. Evaluation of transpalpebral ultrasonographic measurement of optic nerve sheath diameter for indirect assessment of intracranial pressure in anesthetized and standing healthy adult horses. J Vet Emerg Crit Care (San Antonio). (2021) 31:315–22. doi: 10.1111/vec.13061
19. Kasapas K, Diamantopoulou A, Pentilas N, Papalois A, Douzinas E, Kouraklis G, et al. Invasive and ultrasound based monitoring of the intracranial pressure in an experimental model of epidural hematoma progressing towards brain tamponade on rabbits. ScientificWorldJ. (2014) 2014:504248. doi: 10.1155/2014/504248
20. Garcia JP Jr, Garcia PT, Rosen RB, Finger PT. A 3-dimensional ultrasound C-scan imaging technique for optic nerve measurements. Ophthalmology. (2004) 111:1238–43. doi: 10.1016/j.ophtha.2003.10.026
21. Bäuerle J, Lochner P, Kaps M, Nedelmann M. Intra- and interobsever reliability of sonographic assessment of the optic nerve sheath diameter in healthy adults. J Neuroimaging. (2012) 22:42–5. doi: 10.1111/j.1552-6569.2010.00546.x
22. Kane SC, Dennis AT, Da Silva Costa F, Kornman LH, Cade TJ, Brennecke SP. Optic nerve sonography and ophthalmic artery Doppler velocimetry in healthy pregnant women: an Australian cohort study. J Clin Ultrasound. (2019) 47:531–9. doi: 10.1002/jcu.22735
23. Ballantyne J, Hollman AS, Hamilton R, Bradnam MS, Carachi R, Young DG, et al. Transorbital optic nerve sheath ultrasonography in normal children. Clin Radiol. (1999) 54:740–2. doi: 10.1016/s0009-9260(99)91176-5
24. Maude RR, Hossain MA, Hassan MU, Osbourne S, Sayeed KL, Karim MR, et al. Transorbital sonographic evaluation of normal optic nerve sheath diameter in healthy volunteers in Bangladesh. PLoS ONE. (2013) 8:e81013. doi: 10.1371/journal.pone.0081013
25. Chen H, Ding GS, Zhao YC Yu RG, Zhou JX. Ultrasound measurement of optic nerve diameter and optic nerve sheath diameter in healthy Chinese adults. BMC Neurol. (2015) 15:106. doi: 10.1186/s12883-015-0361-x
26. Wang L, Feng L, Yao Y, Deng F, Wang Y, Feng J, et al. Ultrasonographic evaluation of optic nerve sheath diameter among healthy Chinese adults. Ultrasound Med Biol. (2016) 42:683–8. doi: 10.1016/j.ultrasmedbio.2015.11.020
27. Kim DH, Jun JS, Kim R. Ultrasonographic measurement of the optic nerve sheath diameter and its association with eyeball transverse diameter in 585 healthy volunteers. Sci Rep. (2017) 7:15906. doi: 10.1038/s41598-017-16173-z
28. Goeres P, Zeiler FA, Unger B, Karakitsos D, Gillman LM. Ultrasound assessment of optic nerve sheath diameter in healthy volunteers. J Crit Care. (2016) 31:168–71. doi: 10.1016/j.jcrc.2015.10.009
29. Avci M, Kozaci N, Komut E, Komut S, Caliskan G, Tulubas G. The measurement of elderly volunteers' optic nerve sheath diameters by ocular ultrasonography. Medicina (Kaunas). (2019) 55:413. doi: 10.3390/medicina55080413
30. Chandrapatham K, Cardim D, Czosnyka M, Bertuccio A, Di Noto A, Corradi F, et al. Variability of the optic nerve sheath diameter on the basis of sex and age in a cohort of healthy volunteers. Acta Neurochir Suppl. (2021) 131:121–4. doi: 10.1007/978-3-030-59436-7_25
31. Betcher J, Becker TK, Stoyanoff P, Cranford J, Theyyunni N. Military trainees can accurately measure optic nerve sheath diameter after a brief training session. Mil Med Res. (2018) 5:42. doi: 10.1186/s40779-018-0189-y
32. Amini R, Stolz LA, Patanwala AE, Adhikari S. Coronal axis measurement of the optic nerve sheath diameter using a linear transducer. J Ultrasound Med. (2015) 34:1607–12. doi: 10.7863/ultra.15.14.09039
33. Blehar DJ, Gaspari RJ, Montoya A, Calderon R. Correlation of visual axis and coronal axis measurements of the optic nerve sheath diameter. J Ultrasound Med. (2008) 27:407–11. doi: 10.7863/jum.2008.27.3.407
34. Cimilli Ozturk T, Demir H, Yorulmaz R, Ozdemir S, Isat G, Ecmel Onur O. Assessment of intra-interobserver reliability of the sonographic optic nerve sheath diameter measurement. Kaohsiung J Med Sci. (2015) 31:432–6. doi: 10.1016/j.kjms.2015.06.004
35. Maissan IM, Verbaan LA, van den Berg M, Houmes RJ, Stolker RJ, den Hartog D. Helicopter transportation increases intracranial pressure: a proof-of-principle study. Air Med J. (2018) 37:249–52. doi: 10.1016/j.amj.2018.02.010
36. Romagnuolo L, Tayal V, Tomaszewski C, Saunders T, Norton HJ. Optic nerve sheath diameter does not change with patient position. Am J Emerg Med. (2005) 23:686–8. doi: 10.1016/j.ajem.2004.11.004
37. Özdogan S, Gökçek Ö, Katirci Y, Çorbacioglu SK, Emektar E, Çevik Y. The effects of spinal immobilization at 20° on intracranial pressure. Am J Emerg Med. (2019) 37:1327–30. doi: 10.1016/j.ajem.2018.10.010
38. Pardon LP, Cheng H, Chettry P, Patel NB. Optic nerve head morphological changes over 12 hours in seated and head-down tilt postures. Invest Ophthalmol Vis Sci. (2020) 61:21. doi: 10.1167/iovs.61.13.21
39. Dinsmore M, Han JS, Fisher JA, Chan VW, Venkatraghavan L. Effects of acute controlled changes in end-tidal carbon dioxide on the diameter of the optic nerve sheath: a transorbital ultrasonographic study in healthy volunteers. Anaesthesia. (2017) 72:618–23. doi: 10.1111/anae.13784
40. Keyes LE, Paterson R, Boatright D, Browne V, Leadbetter G, Hackett P. Optic nerve sheath diameter and acute mountain sickness. Wilderness Environ Med. (2013) 24:105–11. doi: 10.1016/j.wem.2012.11.003
41. Di Pasquale DM, Muza SR, Gunn AM Li Z, Zhang Q, Harris NS, et al. Evidence for cerebral edema, cerebral perfusion, and intracranial pressure elevations in acute mountain sickness. Brain Behav. (2016) 6:e00437. doi: 10.1002/brb3.437
42. Kanaan NC, Lipman GS, Constance BB, Holck PS, Preuss JF, Williams SR, et al. Optic nerve sheath diameter increase on ascent to high altitude: correlation with acute mountain sickness. J Ultrasound Med. (2015) 34:1677–82. doi: 10.7863/ultra.15.14.10060
43. Strapazzon G, Malacrida S, Vezzoli A, Dal Cappello T, Falla M, Lochner P, et al. Oxidative stress response to acute hypobaric hypoxia and its association with indirect measurement of increased intracranial pressure: a field study. Sci Rep. (2016) 6:32426. doi: 10.1038/srep32426
44. Maissan IM, Ketelaars R, Vlottes B, Hoeks SE, den Hartog D, Stolker RJ. Increase in intracranial pressure by application of a rigid cervical collar: a pilot study in healthy volunteers. Eur J Emerg Med. (2018) 25:e24–8. doi: 10.1097/MEJ.0000000000000490
45. Woster CM, Zwank MD, Pasquarella JR, Wewerka SS, Anderson JP, Greupner JT, et al. Placement of a cervical collar increases the optic nerve sheath diameter in healthy adults. Am J Emerg Med. (2018) 36:430–4. doi: 10.1016/j.ajem.2017.08.051
46. Sanri E, Karacabey S. The impact of head of bed elevation on optic nerve sheath diameter in cervical collar applied healthy volunteers. J Emerg Med. (2019) 56:371–7. doi: 10.1016/j.jemermed.2018.12.043
47. Ladny M, Smereka J, Ahuja S, Szarpak L, Ruetzler K, Ladny JR. Effect of 5 different cervical collars on optic nerve sheath diameter: a randomized crossover trial. Medicine (Baltimore). (2020) 99:e19740. doi: 10.1097/MD.0000000000019740
48. Sadrameli SS, Wong MS, Kabir R, Wiese JR, Podell K, Volpi JJ, et al. Changes in transcranial sonographic measurement of the optic nerve sheath diameter in non-concussed collegiate soccer players across a single season. Cureus. (2018) 10:e3090. doi: 10.7759/cureus.3090
49. Lefferts WK, Hughes WE, Heffernan KS. Effect of acute high-intensity resistance exercise on optic nerve sheath diameter and ophthalmic artery blood flow pulsatility. J Hum Hypertens. (2015) 29:744–8. doi: 10.1038/jhh.2015.12
50. Vitiello L, De Bernardo M, Guercio Nuzio S, Mandato C, Rosa N, Vajro P. Pediatric liver diseases and ocular changes: what hepatologists and ophthalmologists should know and share with each other. Dig Liver Dis. (2020) 52:1–8. doi: 10.1016/j.dld.2019.11.009
51. De Bernardo M, Vitiello L, Rosa N. Ocular ultrasonography to detect intracranial pressure in aneurysmal subarachnoid hemorrhage. Ann Clin Transl Neurol. (2020) 7:1459–60. doi: 10.1002/acn3.51116
52. De Bernardo M, Vitiello L, Rosa N. A-scan ultrasonography and optic nerve sheath diameter assessment during acute elevations in intra-abdominal pressure. Surgery. (2020) 167:1023–4. doi: 10.1016/j.surg.2020.01.008
53. De Bernardo M, Vitiello L, Rosa N. Intracranial pressure evaluation in acute liver failure. Neurocrit Care. (2019) 30:495–6. doi: 10.1007/s12028-019-00680-0
54. Iaconetta G, De Bernardo M, Rosa N. Coronal axis measurement of the optic nerve sheath diameter. J Ultrasound Med. (2017) 36:1073. doi: 10.1002/jum.14198
55. De Bernardo M, Rosa N. Comment on 'Invasive and noninvasive means of measuring intracranial pressure: a review'. Physiol Meas. (2018) 39:058001. doi: 10.1088/1361-6579/aac540
56. De Bernardo M, Vitiello L, Rosa N. Optic nerve sheath diameter ultrasonography in differentiation of ischemic and hemorrhagic strokes. Am J Emerg Med. (2019) 37:1384–5. doi: 10.1016/j.ajem.2018.12.048
57. Ossoinig KC. Standardized echography of the optic nerve. In: Till P, editor. Documenta Ophthalmologica Proceedings Series. Vol. 55. Ophthalmic Echography 13. Dordrecht: Springer, Netherlands (1990). p. 3–99
58. De Bernardo M, Vitiello L, Rosa N. Ultrasound optic nerve sheath diameter evaluation in patients undergoing robot-assisted laparoscopic pelvic surgery. J Robot Surg. (2019) 13:709–10. doi: 10.1007/s11701-019-00966-7
59. De Bernardo M, Vitiello L, Rosa N. Optic nerve evaluation in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. (2019) 40:E36. doi: 10.3174/ajnr.A6091
60. Vitiello L, De Bernardo M, Capasso L, Rosa N. Optic nerve ultrasound evaluation in acute high altitude illness. Wilderness Environ Med. (2021) 32:407–8. doi: 10.1016/j.wem.2021.04.009
61. De Bernardo M, Vitiello L, Rosa N. Optic nerve ultrasonography for evaluating increased intracranial pressure in severe preeclampsia. Int J Obstet Anesth. (2019) 38:147. doi: 10.1016/j.ijoa.2018.12.010
Keywords: intracranial hypertension, optic nerve, optic nerve sheath diameter, ONSD, ultrasonography
Citation: Vitiello L, De Bernardo M, Capasso L, Cornetta P and Rosa N (2022) Optic Nerve Ultrasound Evaluation in Animals and Normal Subjects. Front. Med. 8:797018. doi: 10.3389/fmed.2021.797018
Received: 18 October 2021; Accepted: 13 December 2021;
Published: 05 January 2022.
Edited by:
Giulio Ferrari, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Luisa Pierro, San Raffaele Hospital (IRCCS), ItalyCopyright © 2022 Vitiello, De Bernardo, Capasso, Cornetta and Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maddalena De Bernardo, bWRlYmVybmFyZG9AdW5pc2EuaXQ=
 Livio Vitiello
Livio Vitiello Maddalena De Bernardo
Maddalena De Bernardo Luigi Capasso
Luigi Capasso Palmiro Cornetta3
Palmiro Cornetta3 Nicola Rosa
Nicola Rosa