- 1Department of Emergency Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 2Department of Internal Medicine, Kaohsiung Municipal United Hospital, Kaohsiung, Taiwan
- 3Shu-Zen Junior College of Medicine and Management, Kaohsiung, Taiwan
- 4Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 5Department of Recreation and Sports Management, Tajen University, Pingtung, Taiwan
- 6Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital, Pingtung Branch, Pingtung, Taiwan
- 7Department of Physical Therapy, Shu-Zen Junior College of Medicine and Management, Kaohsiung, Taiwan
- 8Graduate Institute of Bioresources, National Pingtung University of Science and Technology, Pingtung, Taiwan
- 9Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
- 10College of Medicine, China Medical University, Taichung, Taiwan
- 11Institute of Public Health, National Yang Ming Chiao Tung University, Hsinchu, Taiwan
- 12Division of Allergy, Immunology and Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 13Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
Background: Candida infection is prevalent in patients with Sjögren's syndrome (SjS), which usually takes years to reach diagnosis. Is the link a two-way street? The role of Candida infection before SjS has not been examined clearly. This study was conducted to provide epidemiological evidence regarding the relationship between the first acquisition of Candida infection and subsequent SjS.
Methods: Totally, 23,494 individuals newly diagnosed with Candida infection were enrolled from 2000, to 2012. Controls (N = 93,976) were selected at a 1:4 ratio through propensity score matched (PSM) using the greedy algorithm. Exposure was defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
Main Outcomes and Measures: SjS was recorded in the Registry for Catastrophic Illness Patients Database (RCIPD). Cox proportional hazard model was used to analyze the association and sensitivity analyses for cross-validation.
Results: Of 117,470 individuals (106,077 [89%] women), 23,494 individuals (20.0%) had Candida infection and 104 individuals (0.1%) developed SjS. The incidence of SjS was higher in the exposed group compared with the controls (1.92 vs. 0. 98 per 10,000 person-years) with adjusted hazard ratio (aHR) 1.90 (95% CI, 1.25–2.87). The aHRs in subgroups of aged 18–30 years, oral candidiasis and depression were 4.30 (95% CI, 1.60–11.55), 4.70 (4.70–13.93) and 6.34 (2.16–18.66). Sensitivity analyses yield consistent results.
Conclusions: Residents in Taiwan with Candida infection have higher risk of SjS. For early diagnosis of SjS, clinicians are advised to take Candida infection into account in some situation.
Key Messages
- Sjögren's syndrome (SjS) is a chronic, slow-progressing disorder, with no single diagnostic test could confirm the diagnosis.
- Some infections can trigger autoimmunity and the pathogenetic process of SjS.
- This study suggests that Candida infection is associated with a higher incidence of SjS, which may be considered for the early diagnosis of SjS.
Introduction
Sjögren's syndrome (SjS) is a chronic, slow-progressing disorder with an extremely variable clinical manifestation that varies from a specific organ to a systemic autoimmune disease (1). The incidence of SjS varies widely from 3 to 11 cases per 100,000 individuals (2–5) and is highest in Asian women (6). However, the global incidence of SjS may have been underestimated, as some patients are asymptomatic or have mild symptoms and they may never get diagnosed. SjS adversely affects the quality of life of the patients; it also leads to a greater use of medical services by the patients, with an average total direct cost of US$20,000 per patient per annum in the United States (7). In spite of this, however, no test has yet been designed to definitively diagnose the SjS. Patience is the key when it comes to diagnose patients who initially report multiple instances of vague discomfort, which may delay the diagnosis of SjS by years. Many patients report numerous non-sicca features 20 years prior to the onset of typical dryness associated with the SjS (8). Hyposalivation is one of the most common symptoms of the SjS and Candida is the most prevalent yeast infection (9). Most of the previous studies focused on the prevalence and treatment of Candida infection after SjS; only a few studies examined the risk of SjS developing in patients who already have Candida infection. It has recently been shown that infections can trigger autoimmunity and begin the pathogenetic process of SjS (10). We hypothesized that Candida infection can be present before the occurrence of SjS or in the early state of undiagnosed SjS and is associated with the development of SjS. Herein, we investigate the association between SjS and Candida by using a population-based database with up to 14 years of follow-up and by analyzing the cumulative incidence, as well as by performing stratified analysis based on age, sex, and site of infection.
Methods
Data Source
The present study is a retrospective cohort study designed using the Longitudinal Health Insurance Database 2000 (LHID2000), a subset of Taiwan National Health Insurance Research Datasets (NHIRD). LHID2000 comprises data of 1,000,000 people randomly sampled from the year 2000 registry of all beneficiaries of the National Health Insurance. The NHIRD is a database of the medical claims (including demographics, diagnoses for various diseases, procedures, medication prescription, and in-patient and out-patient expenditure claims) of all National Health Insurance beneficiaries (11). No statistically significant difference was observed in the distribution of age and sex between the LHID2000 and original NHIRD databases (http://nhird.nhri.org.tw/date_cohort.htm). The health database has a characteristically large sample size of longitudinal nature and hence is suitable for non-experimental observational studies (12). Disease diagnoses were coded based on the 9th International Classification of Diseases (ICD-9). The Bureau of National Health Insurance regularly conducts quarterly expert-reviews on a random sample comprising the claims of out-patient and in-patient departments of all medical institutions of Taiwan. The Registry for Catastrophic Illness Patient Database (RCIPD) is a sub-dataset of the NHIRD, is linked with the NHIRD. Those who meet the criteria for catastrophic illnesses as defined by the Ministry of Health and Welfare, Government of Taiwan, can apply for a catastrophic illness certificate, and their information will be uploaded to the RCIPD. Patients should provide records of their diagnosis and the pathological or radiological report confirming the diagnosis, which are verified by an expert review committee. Individuals who qualify for the catastrophic illness-related category are not required to pay for their treatment.
The identification was scrambled to protect the privacy of the patients. This study was approved by the Institutional Review Board of China Medical University Hospital Research Ethics Committee [CMUH104-REC2-115(AR-4)].
Study Participants
The participants were the patients newly diagnosed with Candida infection between 2000 and 2012, as identified from the LHID2000 based on ICD-9 codes (ICD-9 code 112). The participants should have at least three out-patient department visits or any hospitalization. The index date was defined as the date on which the Candida infection was first diagnosed; this date was then assigned to the matched non-Candida subjects with identical enrolment criteria. Patients with a history of Candida infection and those with SjS before the index date were excluded. Patients with incomplete information about their age and/or sex were also not considered. The control group comprised patients who had never been diagnosed with Candida between 1997 and 2012 from the same dataset. Propensity score matching (PSM) was performed for the pairs groups (control and study group) at a 4:1 ratio based on the individual age-, sex-, index years-, covariates-matching, including hypertension (ICD-9-CM codes 401-405), diabetes mellitus (DM, ICD-9-CM code 250), hyperlipidaemia (ICD-9-CM code 272), cerebral vascular accident (ICD-9-CM code 430–438), chronic kidney disease (CKD, ICD-9-CM code 585), chronic obstructive pulmonary diseases (COPD, ICD-9-CM codes 491, 492, 496), chronic liver diseases (CLD, ICD-9-CM code 571.x), depression (ICD-9-CM codes 296.2, 296.3, 298.0, 300.4, 311), allergic rhinitis (ICD-9-CM code 477.x), urticaria (ICD-9-CM code 708.x), systemic lupus erythematosus (SLE, ICD-9-CM code 710), and rheumatoid arthritis (RA, ICD-9-CM code 714.0). The data on these comorbidities were collected during the baseline period (i.e., within 2 years before the index date). Increasing the PSM ratio increases the statistical power, and such an effect is slightly advantageous when the ratio is >4 (13). To balance the baseline characteristics and relevant covariates between the two groups, greedy algorithm on the propensity score was applied (14). For each participant in the study group, the corresponding controls were selected based on the nearest propensity scores.
Outcome and Relevant Variables
The main outcome is the diagnosis of new-onset SjS (ICD-9 code 710.2) extracted from LHID2000 and linked to RCIPD for confirmation during the follow-up period. The diagnosis was based on the classification criteria established by the European Community Study Group in 1993 (15). The ICD coding algorithm was used (16). All the participants were followed until the confirmed diagnosis of SjS, their death, or the end of 2013. The date of the determination of the first principal ICD-9 code of SjS during the follow-up period was defined as the endpoint of interest. To address potential measurable confounders, we included the following data into the regression model: sociodemographic factors (sex, age, and occupation) and baseline medical comorbidities (including hypertension, DM, hyperlipidaemia, CVA, CKD, COPD, CLD, depression, allergic rhinitis, urticaria, SLE, and RA).
Statistical Analysis
The Chi-square (χ2) test was applied to process the demographic characteristic data, including the distributions of categorical sex, age, occupation, comorbidities between the Candida group and non-Candida group to check the category variables from the two groups. Person-years were calculated based on the amount of the follow-up time required for each individual, which was the period recorded from the index date to the end point of the study. The incidence density of SjS per 10,000 person-years was calculated for both groups. To investigate the independent association of Candida infection with SjS, a multivariable Cox proportional hazard regression analysis was conducted to estimate the hazard ratio (HR) with the 95% confidence interval (CI) after adjusting for full covariates. In this study, only the variables found to be statistically significant in the univariable analysis were further examined in the multivariable model (i.e., sex, age, occupation, hypertension, hyperlipidaemia, CVA, COPD, CLD, depression, allergic rhinitis, urticaria, SLE and RA). The Kaplan–Meier method was used to describe the cumulative incidence of SjS between the two groups. Differences in the incidence between the two groups were evaluated using the log-rank test. Sensitivity analysis I (to exclude the coincidence of SLE or RA developed from the index date to 2013, the end of the study) was conducted to re-evaluate the HR of SjS after exposure to Candida infection. Sensitivity analysis II was conducted using case-control analysis to identify patients with SjS and matched controls in the same database from 2000 to 2013. The multivariable conditional logistic regression model was used in sensitivity analysis II to examine the odds ratio (OR) of the previous exposure to Candida infection. Sub-group analysis was conducted based on the age, sex, and site of Candida infection. All the data and statistics were processed and analyzed using the SAS software Version 9.4 (SAS Institute, Inc., Cary, NC). All statistical tests were 2-sided, and P < 0.05 was considered statistically significant.
Results
Demographic Characteristics and Comorbidities
Of the 117,470 individuals (106,077 [89%] women) aged over 18 years, 23,494 (20.0%) had Candida infection (2,1981 women [90%]; mean [SD] age 37.8 [16.0] years) and 104 (0.1%) developed SjS; 93,976 individuals who had no Candida infection (84,096 women [89%]; mean [SD] age 37.0 [15.7] years) were matched by age, sex, and comorbidities (Table 1). Among the 23,494 participants with Candida infection, 18,960 (80.0%) were aged 50 years or younger. PSM resulted in 93,976 matched individuals in each group. The baseline characteristics were found to be well balanced between the groups after PSM. The group with Candida infection in this study, compared with that without Candida infection, had similar proportions of concomitant SLE (89 individuals [0.4%] vs. 313 individuals [0.3%]; SMD, 0.008) and RA (380 individuals [2%] vs. 1,299 individuals [1%]; SMD, 0.019).
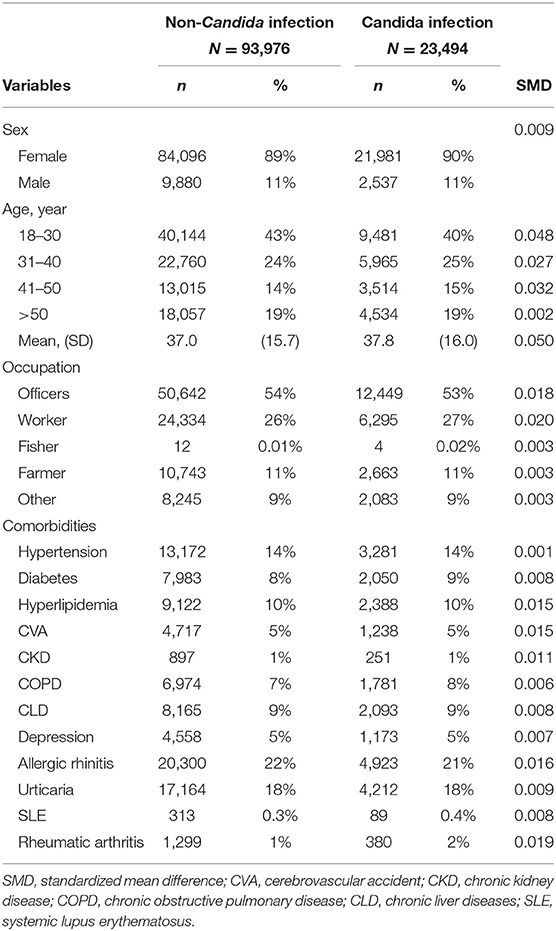
Table 1. The demographic variables and comorbidities of patients with and without Candida infection.
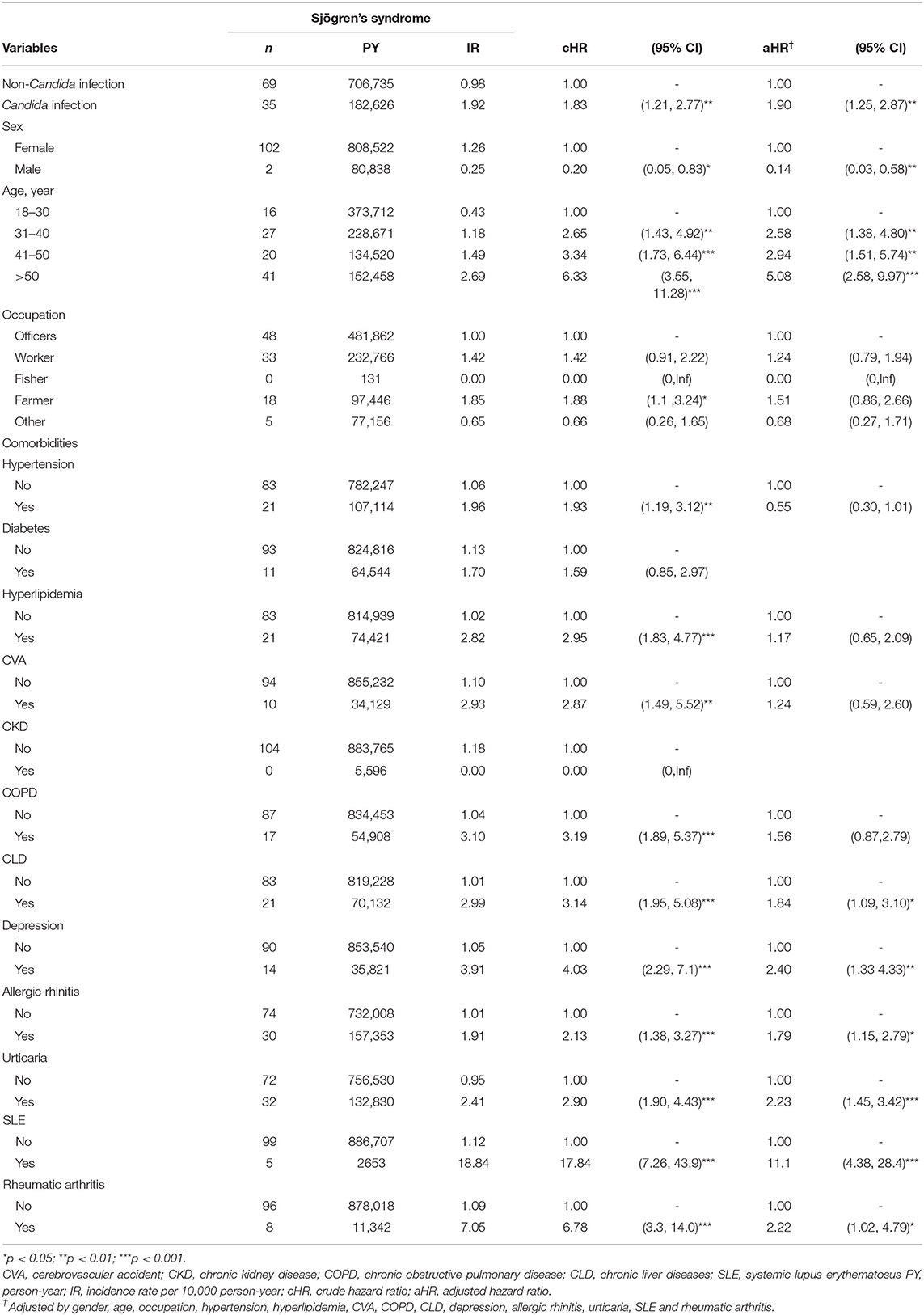
Comparison of SjS Incidence and HR Between Candida and Control Groups
Both univariable and multivariable Cox regression analyses showed that individuals with Candida infection had a higher risk of subsequent SjS compared with those without Candida infection, with an incidence rate of 1.92 per 10,000 person-years vs. 0.98 per 10,000 person-years (see Table 2). Individuals with a history of Candida infection were more likely to develop SjS (unadjusted HR, 1.83; 95 CI, 1.21–2.77). Figure 1 shows the survival curve. The cumulative incidence of SjS is higher in the Candida infection group than in the control group (P = 0.004). The results of multivariable Cox regression analysis are listed in Table 2, which reveals a positive correlation of Candida infection with the development of SjS. After adjusting for demographic variables and comorbidities at baseline, it was found that individuals with Candida infection had a 90% higher risk of developing subsequent SjS than those in the control group, with an adjusted HR of 1.90 (95% CI, 1.25–2.87). We also found that older participants were at a higher risk of developing SjS [adjusted HR (aHR) = 2.58, 95% CI, 1.38–4.80; aHR = 2.94, 95% CI, 1.51–5.74; aHR = 5.08, 95% CI, 2.58–9.97 in the age groups of 31–40, 41–50, and >50 years, respectively]. The participants with CLD, depression, allergic rhinitis, and urticaria also had a higher risk for subsequent SjS (with aHR = 1.84, 2.40, 1.79, 2.23, and 2.22, respectively; see Table 2). Patients with comorbidity and SLE had a significantly high relative risk of SjS (aHR = 11.1, 95% CI 4.38–28.4).
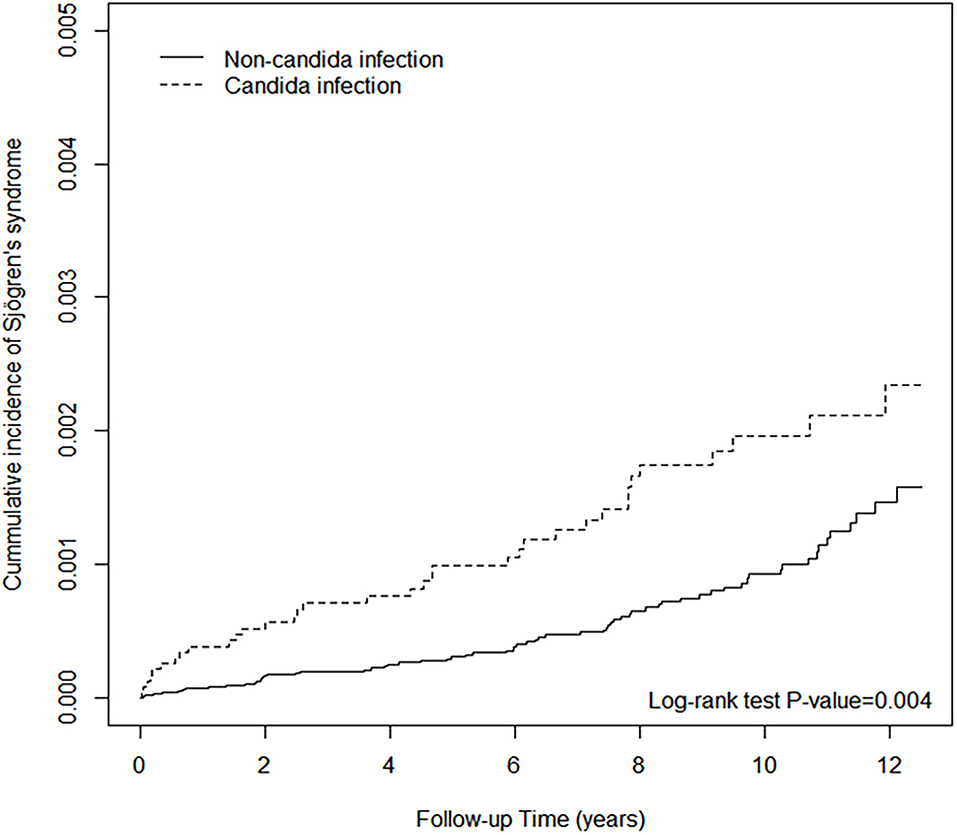
Figure 1. The cumulative incidence of Sjögren's syndrome of non-Candida infection and Candida infection.
Sensitivity Analyses
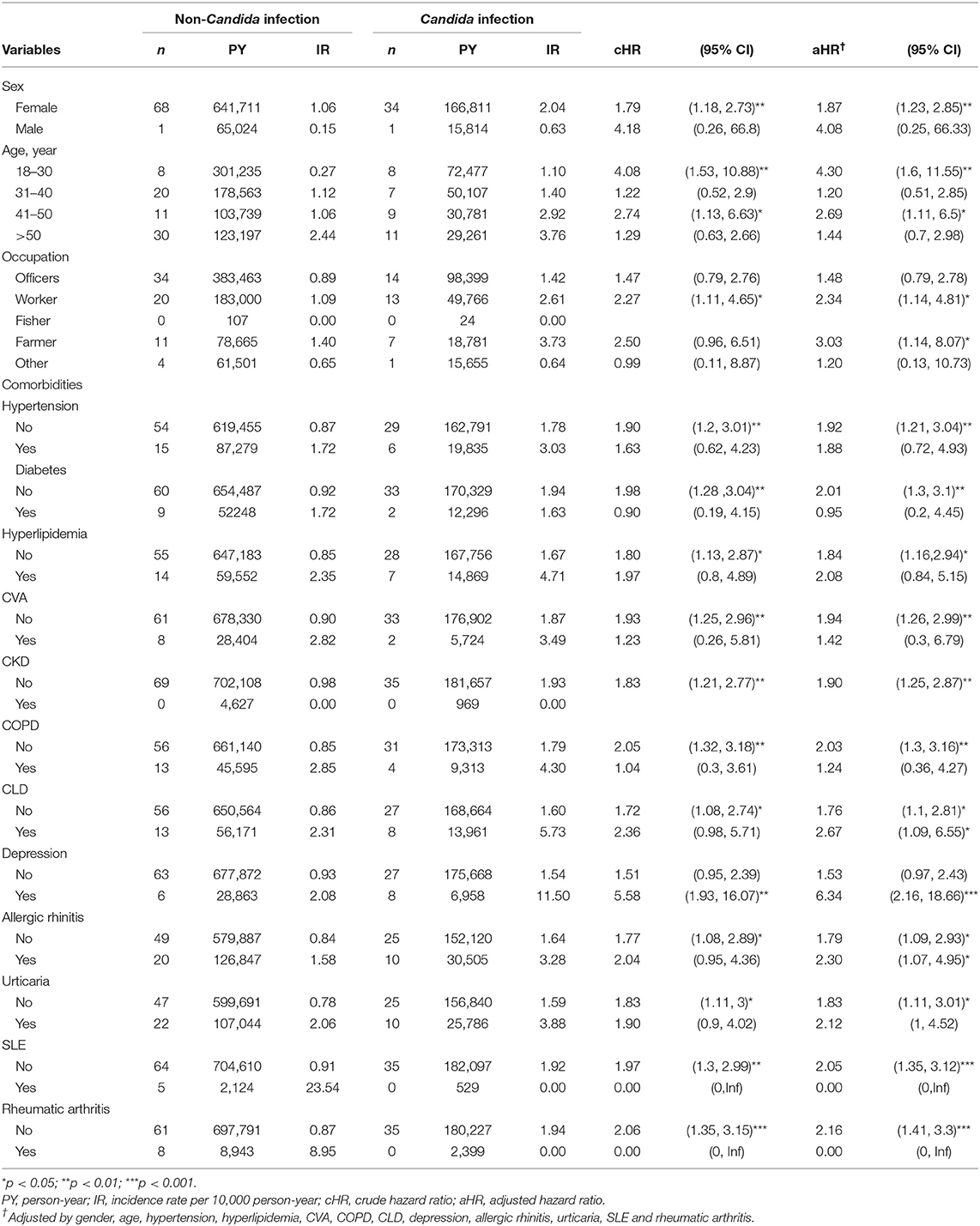
Supplementary Tables 1, 2 list a scenario to examine SjS events using the robustness of HR. We excluded patients with both SjS and SLE and RA during the follow up period (from the index date till the end of the study) to minimize the effect of secondary SjS and possible bias of collinearity. Sensitivity analysis showed the aHR = 1.95 (95% CI 1.14–3.35) for SjS with exposure to Candida infection.
Supplementary Tables 3, 4 list another scenario using case and control analysis. We identified 810 cases and 3,240 matched controls. Candida infection was associated with a 1.34-fold increase in the risk of SjS (adjusted OR (aOR) 1.34, 95% CI 1.03–1.73, P < 0.05).
Subgroup Analysis
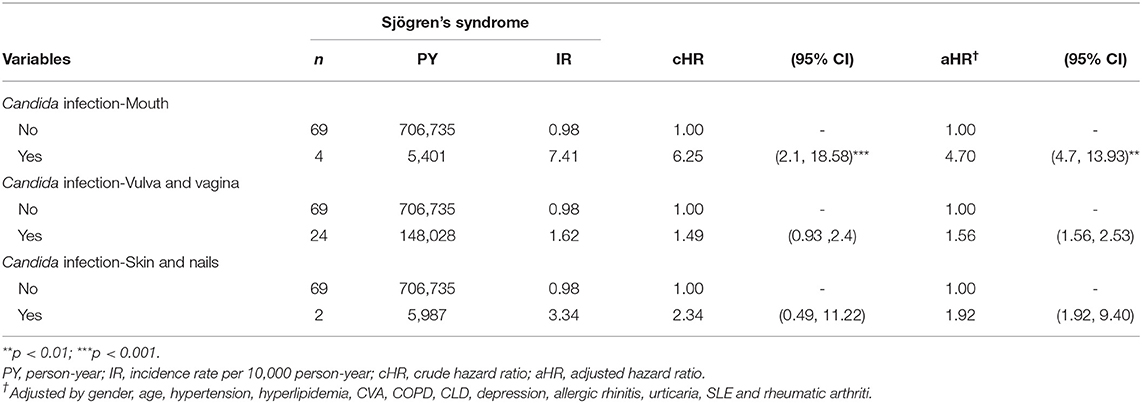
Table 3 lists the results of different stratifications. The analysis of the sex subgroup showed that women with Candida infection have a higher risk of developing SjS (aHR = 1.87, 95% CI 1.23–2.85; P < 0.01), while men with Candida infection did not show any significant association (aHR = 4.08, 95% CI 0.25–66.33). The analysis of the age subgroup revealed that, compared with the age-matched non-Candida subgroup, 18–30-year-old participants with Candida infection had a significantly higher risk of developing SjS (aHR = 4.30, 95% CI 1.60–11.55; P < 0.01). The analysis of the comorbidity subgroup showed that, in patients with depression, the presence of Candida infection is associated with a significantly increased risk of the occurrence of SjS than in those not exposed to Candida infection (aHR = 6.34, 95% CI 2.16–18.66; P < 0.001). Table 4 shows the risk of SjS associated with the occurrence of Candida infection at different sites. Patients with oral Candida infection are associated with the subsequent diagnosis of SjS (aHR = 4.70, 95% CI 4.70–13.39; P < 0.01). Patients with Candida infection at the vulva/vagina and skin/nails are also associated with the subsequent SjS (aHR = 1.56, 95% CI 1.56–2.53 and aHR = 1.92, 95% CI 1.92–9.40, respectively).
Discussion
To the best of our knowledge, this is the first retrospective observation study that used nation-wide population-based data to investigate the link between Candida infection and the risk of SjS. The results showed that patients with Candida infection have a 1.90-fold higher risk of developing SjS compared to the controls; those with oral Candida infection have a 4.70-fold increased risk of SjS. Furthermore, the positive association has been consistently observed in sensitivity analysis models.
Previous studies have reported an incidence rate of 3–11 per 100 000 person-years for SjS (2–5). Our study showed that women have a higher incidence rate of SjS (12.6 per 100,000 person-years) than men; similar results have been reported in a previous study too (17). Our study also showed that the participants with comorbidity and SLE have a significantly higher relative risk of SjS. It has been reported that women, particularly those in their menopause, comprise a greater proportion of patients with SjS (18) and that oral Candida colonization and infection is a common clinical manifestation in patients with SjS (19, 20). A previous study has shown that the sicca syndrome, fatigue, and non-specific musculoskeletal pain in patients with SjS may be mistaken for aging, and that depression in patients with pSS and systemic manifestations of SjS can sometimes occur prior to the sicca syndrome (21). In the present study, we showed that patients diagnosed with depression at baseline with a Candida infection were at a significantly higher risk of being diagnosed with SjS later. Our study results are consistent with those of previous studies. In addition, our study provides more information on the long-term cumulative incidence and role of Candida infection before the diagnosis of SjS.
The potential role of and the possible mechanism through which Candida infection triggers or amplifies the pathway to SjS is still unknown. Multiple factors are responsible for the occurrence of SjS. Using animal models, some studies have suggested that the Candida infection is an important environmental factor responsible for SjS (22). Mofors et al. reported an increased likelihood of developing SjS following a history of infections (23). Candida spp. are common commensal fungi, some of which can act as pathogens under certain conditions. Therefore, it is necessary to carefully investigate the relation between the Candida infection and SjS. Candida induces the human Th17 cell response, which plays an important physiological role in the development of SjS (24, 25). SjS patients have been shown to have significantly higher serum levels of galectin-3, which plays a possibly active role in host defense against Candida infection (26–29). The pivotal role of galectin-3 in autoimmune diseases and neutrophil phagocytosis of Candida infection (28, 30) implies that Candida infection and SjS share a common pathological pathway. Thirdly, candidalysin, a toxin secreted by Candida albicans, can damage the oral epithelial membrane and trigger a double-sided blade signal pathway that activates epithelial immunity (31). Interleukin-17 signaling is crucial for both innate and adaptive immunity to C. albicans. Interleukin-17 expression increases in the patients with SjS. The interplay between candidalysin and interleukin-17 sets a self-reinforcing loop, leading to inflammation amplification (32).
Our study has several limitations as well. First, there are at least 15 species of Candida that can infect humans, and Candida albicans is the most prevalent one. However, the NHIRD could not provide reports on microbiological cultures, and therefore, we could not identify the Candida species in detail, which prevents us from performing a more comprehensive analysis to explore the relationship between C. albicans and non-C. albicans infection and the occurrence of SjS. Second, this database cannot provide any information about the inflammatory marker either; in addition, the diagnosis of SS should not be based solely on SSA/Ro and/or SSB/La antibodies, as these antibodies can be found in healthy people too. Third, the diagnosis of Candida infection and SjS is based on the ICD-9-CM coded data and not on prospective clinical collections. A misclassification bias may also occur. The diagnosis of Candida infection depends primarily on the clinical appearance and/or the results of specimen examinations. Some patients with mild clinical symptoms of Candida infection did not seek medical advice, and hence, they may have been included in the control group. Such situations may lead to type 2 errors. However, if the Candida infection is supposed to be positively associated with the subsequent SjS, such misclassification would bias the estimated HRs toward the null. Therefore, more research should be performed by including participants from other countries and ethnic cultures as well to confirm and extrapolate our findings.
Conclusion
This study suggests that Candida infection is associated with a higher incidence of SjS thereafter, particularly in individuals with oral Candida infection, or concomitant depression. We suggested that, in some cases, Candida infection should be considered along with other criteria for the early diagnosis of SjS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study was approved by the Institutional Review Board of China Medical University Hospital Research Ethics Committee (CMUH104-REC2-115(AR-4). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-LC, M-CC, RC, and JC-CW: conceptualization. H-TY: data curation and funding acquisition. C-LC, F-CC, Y-MH, M-CC, RC, and JC-CW: formal analysis and methodology. C-LC and F-CC: investigation. C-LC and Y-MH: writing (original draft preparation). RC, JC-CW, and M-CC: writing (review and editing). All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors expressed their appreciation to the Department of Medical Education and Research and Research Center of Medical Informatics in Kaohsiung Veterans General Hospital for the comments. The authors expressed appreciation to the Department of Medical Education and Research and Research Center of Medical Informatics in Kaohsiung Municipal United Hospital for the comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.796324/full#supplementary-material
References
1. Brito-Zerón P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjögren syndrome. Nat Rev Dis Primers. (2016) 2:16047. doi: 10.1038/nrdp.2016.47
2. Weng MY, Huang YT, Liu MF, Lu TH. Incidence and mortality of treated primary Sjogren's syndrome in Taiwan: a population-based study. J Rheumatol. (2011) 38:706–8. doi: 10.3899/jrheum.100883
3. Narváez J, Sánchez-Fernández S, Seoane-Mato D, Díaz-González F, Bustabad S. Prevalence of Sjögren's syndrome in the general adult population in Spain: estimating the proportion of undiagnosed cases. Sci Rep. (2020) 10:10627. doi: 10.1038/s41598-020-67462-z
4. Maciel G, Crowson CS, Matteson EL, Cornec D. Incidence and Mortality of Physician-Diagnosed Primary Sjögren Syndrome: Time Trends Over a 40-Year Period in a Population-Based US Cohort. Mayo Clin Proc. (2017) 92:734–43. doi: 10.1016/j.mayocp.2017.01.020
5. Elfving P, Marjoniemi O, Niinisalo H, Kononoff A, Arstila L, Savolainen E, et al. Estimating the incidence of connective tissue diseases and vasculitides in a defined population in Northern Savo area in 2010. Rheumatol Int. (2016) 36:917–24. doi: 10.1007/s00296-016-3474-7
6. Izmirly PM, Buyon JP, Wan I, Belmont HM, Sahl S, Salmon JE, et al. The incidence and prevalence of adult primary Sjögren's syndrome in New York County. Arthritis Care Res (Hoboken). (2019) 71:949–60. doi: 10.1002/acr.23707
7. Birt JA, Tan Y, Mozaffarian N. Sjögren's syndrome: managed care data from a large United States population highlight real-world health care burden and lack of treatment options. Clin Exp Rheumatol. (2017) 35:98–107.
8. Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. Jama. (2013) 310:1854–5. doi: 10.1001/jama.2013.278448
9. Medeiros CCG, Dos Anjos Borges LG, Cherubini K, Salum FG, Medina da. Silva R, de Figueiredo MAZ. Oral yeast colonization in patients with primary and secondary Sjögren's syndrome. Oral Dis. (2018) 24:1367–78. doi: 10.1111/odi.12896
10. Kivity S, Arango MT, Ehrenfeld M, Tehori O, Shoenfeld Y, Anaya JM, et al. Infection and autoimmunity in Sjogren's syndrome: a clinical study and comprehensive review. J Autoimmun. (2014) 51:17–22. doi: 10.1016/j.jaut.2014.02.008
11. Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. (2008) 148:258–67. doi: 10.7326/0003-4819-148-4-200802190-00004
12. Hsing AW, Ioannidis JP. Nationwide population science: lessons from the taiwan national health insurance research database. JAMA Intern Med. (2015) 175:1527–9. doi: 10.1001/jamainternmed.2015.3540
13. Lubin JH. Some efficiency comments on group sizes in study design. Am J Epidemiol. (1980) 111:453–7. doi: 10.1093/oxfordjournals.aje.a112921
14. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. (1999) 150:327–33. doi: 10.1093/oxfordjournals.aje.a010011
15. Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. (1993) 36:340–7. doi: 10.1002/art.1780360309
16. Hsu CY, Hung KC, Lin MS, Ko CH, Lin YS, Chen TH, et al. The effect of pilocarpine on dental caries in patients with primary Sjögren's syndrome: a database prospective cohort study. Arthritis Res Ther. (2019) 21:251. doi: 10.1186/s13075-019-2031-7
17. Brandt JE, Priori R, Valesini G, Fairweather D. Sex differences in Sjögren's syndrome: a comprehensive review of immune mechanisms. Biol Sex Differ. (2015) 6:19. doi: 10.1186/s13293-015-0037-7
18. Yang L, Wei W, He X, Xie Y, Kamal MA Li J. Influence of hormones on Sjögren's syndrome. Curr Pharm Des. (2018) 24:4167–76. doi: 10.2174/1381612824666181010153536
19. Ergun S, Cekici A, Topcuoglu N, Migliari DA, Külekçi G, Tanyeri H, et al. Oral status and Candida colonization in patients with Sjögren's Syndrome. Med Oral Patol Oral Cir Bucal. (2010) 15:e310–5. doi: 10.4317/medoral.15.e310
20. Yan Z, Young AL, Hua H, Xu Y. Multiple oral Candida infections in patients with Sjogren's syndrome – prevalence and clinical and drug susceptibility profiles. J Rheumatol. (2011) 38:2428–31. doi: 10.3899/jrheum.100819
21. Brito-Zerón P, Theander E, Baldini C, Seror R, Retamozo S, Quartuccio L, et al. Early diagnosis of primary Sjögren's syndrome: EULAR-SS task force clinical recommendations. Expert Rev Clin Immunol. (2016) 12:137–56. doi: 10.1586/1744666X.2016.1109449
22. Bedoya SK, Lam B, Lau K, Larkin J. Th17 cells in immunity and autoimmunity. Clin Dev Immunol. (2013) 2013:986789. doi: 10.1155/2013/986789
23. Mofors J, Arkema EV, Björk A, Westermark L, Kvarnström M, Forsblad-d'Elia H, et al. Infections increase the risk of developing Sjögren's syndrome. J Intern Med. (2019) 285:670–80. doi: 10.1111/joim.12888
24. Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell. (2019) 176:1340–55. e15. doi: 10.1016/j.cell.2019.01.041
25. Verstappen GM, Corneth OB, Bootsma H, Kroese FG. Th17 cells in primary Sjögren's syndrome: pathogenicity and plasticity. J Autoimmun. (2018) 87:16–25. doi: 10.1016/j.jaut.2017.11.003
26. Karnell JL, Mahmoud TI, Herbst R, Ettinger R. Discerning the kinetics of autoimmune manifestations in a model of Sjögren's syndrome. Mol Immunol. (2014) 62:277–82. doi: 10.1016/j.molimm.2014.05.006
27. Verma P, Laforce-Nesbitt SS, Tucker R, Mao Q, De Paepe ME, Bliss JM. Galectin-3 expression and effect of supplementation in neonatal mice with disseminated Candida albicans infection. Pediatr Res. (2019) 85:527–32. doi: 10.1038/s41390-019-0279-x
28. Linden JR, Kunkel D, Laforce-Nesbitt SS, Bliss JM. The role of galectin-3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils. Cell Microbiol. (2013) 15:1127–42. doi: 10.1111/cmi.12103
29. Linden JR, De Paepe ME, Laforce-Nesbitt SS, Bliss JM. Galectin-3 plays an important role in protection against disseminated candidiasis. Med Mycol. (2013) 51:641–51. doi: 10.3109/13693786.2013.770607
30. de Oliveira FL, Gatto M, Bassi N, Luisetto R, Ghirardello A, Punzi L, et al. Galectin-3 in autoimmunity and autoimmune diseases. Exp Biol Med. (2015) 240:1019–28. doi: 10.1177/1535370215593826
31. Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. (2016) 532:64–8. doi: 10.1038/nature17625
Keywords: Candida infection, population-based cohort study, Sjögren's syndrome, NHIRD, EPI-epidemiology
Citation: Chen C-L, Chang F-C, Hung Y-M, Chou M-C, Yip H-T, Chang R and Wei JC-C (2022) Candida Infection as an Early Sign of Subsequent Sjögren's Syndrome: A Population-Based Matched Cohort Study. Front. Med. 8:796324. doi: 10.3389/fmed.2021.796324
Received: 16 October 2021; Accepted: 12 November 2021;
Published: 21 January 2022.
Edited by:
Huji Xu, Tsinghua University, ChinaReviewed by:
Antje Mueller, University of Lübeck, GermanyEliane Pedra Dias, Fluminense Federal University, Brazil
Copyright © 2022 Chen, Chang, Hung, Chou, Yip, Chang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renin Chang, cmhhcHNvZHkxODgxQGdtYWlsLmNvbQ==; Mei-Chia Chou, bWVlaWNoaWFAZ21haWwuY29t; James Cheng-Chung Wei, d2VpMzIyOEBnbWFpbC5jb20=
 Chia-Lun Chen
Chia-Lun Chen Fang-Cherng Chang1
Fang-Cherng Chang1 Yao-Min Hung
Yao-Min Hung Mei-Chia Chou
Mei-Chia Chou Hei-Tung Yip
Hei-Tung Yip Renin Chang
Renin Chang James Cheng-Chung Wei
James Cheng-Chung Wei