- 1Department of Critical Care Medicine, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Anesthesiology, Southwest Medicine University, Luzhou, China
- 3Surgical Department, Chengdu Second People's Hospital, Chengdu, China
- 4Health Inspection and Quarantine, Chengdu Medical College, Chengdu, China
- 5Sichuan Provincial Key Laboratory for Disease Gene Study, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
If not cured promptly, tissue ischemia and hypoxia can cause serious consequences or even threaten the life of the patient. Hemoglobin-based oxygen carrier-201 (HBOC-201), bovine hemoglobin polymerized by glutaraldehyde and stored in a modified Ringer's lactic acid solution, has been investigated as a blood substitute for clinical use. HBOC-201 was approved in South Africa in 2001 to treat patients with low hemoglobin (Hb) levels when red blood cells (RBCs) are contraindicated, rejected, or unavailable. By promoting oxygen diffusion and convective oxygen delivery, HBOC-201 may act as a direct oxygen donor and increase oxygen transfer between RBCs and between RBCs and tissues. Therefore, HBOC-201 is gradually finding applications in treating various ischemic and hypoxic diseases including traumatic hemorrhagic shock, hemolysis, myocardial infarction, cardiopulmonary bypass, perioperative period, organ transplantation, etc. However, side effects such as vasoconstriction and elevated methemoglobin caused by HBOC-201 are major concerns in clinical applications because Hbs are not encapsulated by cell membranes. This study summarizes preclinical and clinical studies of HBOC-201 applied in various clinical scenarios, outlines the relevant mechanisms, highlights potential side effects and solutions, and discusses the application prospects. Randomized trials with large samples need to be further studied to better validate the efficacy, safety, and tolerability of HBOC-201 to the extent where patient-specific treatment strategies would be developed for various clinical scenarios to improve clinical outcomes.
Introduction
Tissue ischemia and hypoxia cause increased anaerobic metabolism, ion imbalance, mitochondrial uncoupling, activation of endothelial cells and various cell death programs, and pro-inflammatory immune responses (1, 2). Timely resolution of ischemia and hypoxia in cells, tissues, and organs is beneficial in restoring the cellular demand for energy metabolism and oxygen. In clinical practices, the increase of red blood cells (RBCs) and hemoglobin (Hb) in the blood is promoted mainly by the use of erythropoiesis-stimulating agents (ESAs) (3), intravenous iron (4), vitamin B12 (5), and folic acid (6) to improve tissue oxygenation. In the case of life-threatening diseases requiring blood transfusion, allogeneic blood transfusion is the most common and closest physiological option to improve the blood volume, Hb, and hypoxia of the patient. However, blood has some potential disadvantages including immune phenomena, blood infection, cross-matching, and scarcity. Therefore, the search for ideal blood substitutes to provide oxygen to ischemic and hypoxic tissues and organs to maintain normal cellular energy metabolism is an ongoing quest.
Hemoglobin-based oxygen carrier-201 (HBOC-201) [hemoglobin glutamer-250 (bovine); Hemopure, HbO2 Therapeutics LLC, Souderton PA 18964, USA] is a solution of purified, glutaraldehyde-polymerized, stroma-free, bovine Hb (7). HBOC-201 has been on the market in South Africa since 2001 and is approved for surgical patients with acute anemia (8). However, in 2008, the meta-analysis of cell-free hemoglobin-based blood substitutes and risk of myocardial infarction (MI) and death by Natanson et al. and related editorials have caused widespread controversy over the safety of clinical patients using HBOC-21 (9–12). The meta-analysis included 13 randomized controlled trials and concluded that the use of HBOCs was associated with a significantly increased risk of death and MI based on analysis of available data (9). The Food and Drug Administration (FDA), hence, suspended all the HBOC trials in the United States. However, related editorials suggested that the conclusions of this meta-study based on mixed studies at different levels must be treated with caution (10–12). They argued that the imminent risk of death due to low Hb outweighs the risk of HBOCs, so indiscriminately requiring suspension of all the HBOC trials could be fatal to these patients. The appeal submitted by the company that produces HBOC-201 to the Drug Control Board was finally successful in 2010 and the company officially resumed the registration. HBOC-201 is an investigational product that is currently only available through Expanded Access (EA). The EA program has been conducted in the United States since 2014, primarily in patients who have refused blood transfusions due to their religious beliefs (13). It is also currently approved for veterinary use in the United States and the European Union (14). HBOC-201 serves as a temporary oxygen bridge to improve local and systemic oxygenation and as a substitute for effective resuscitation fluids and RBCs in emergencies (15, 16). HBOC-201 can be used in various ischemic and hypoxic clinical scenarios such as traumatic hemorrhagic shock, hemolysis, MI, cardiopulmonary bypass, perioperative period, and organ transplantation. Compared with various resuscitation fluids, HBOC-201 is fluid as a volume expander and retains the ability to bind and release oxygen. When RBCs are not available, HBOC-201 can also replace RBCs as an oxygen bridge to improve tissue oxygenation in vivo until enough RBCs are produced to meet the needs of life. The main adverse effects currently reported with HBOC-201 include vasoconstriction, MI, and increased methemoglobin, which can be managed with close clinical monitoring and appropriate therapeutic measures (17, 18).
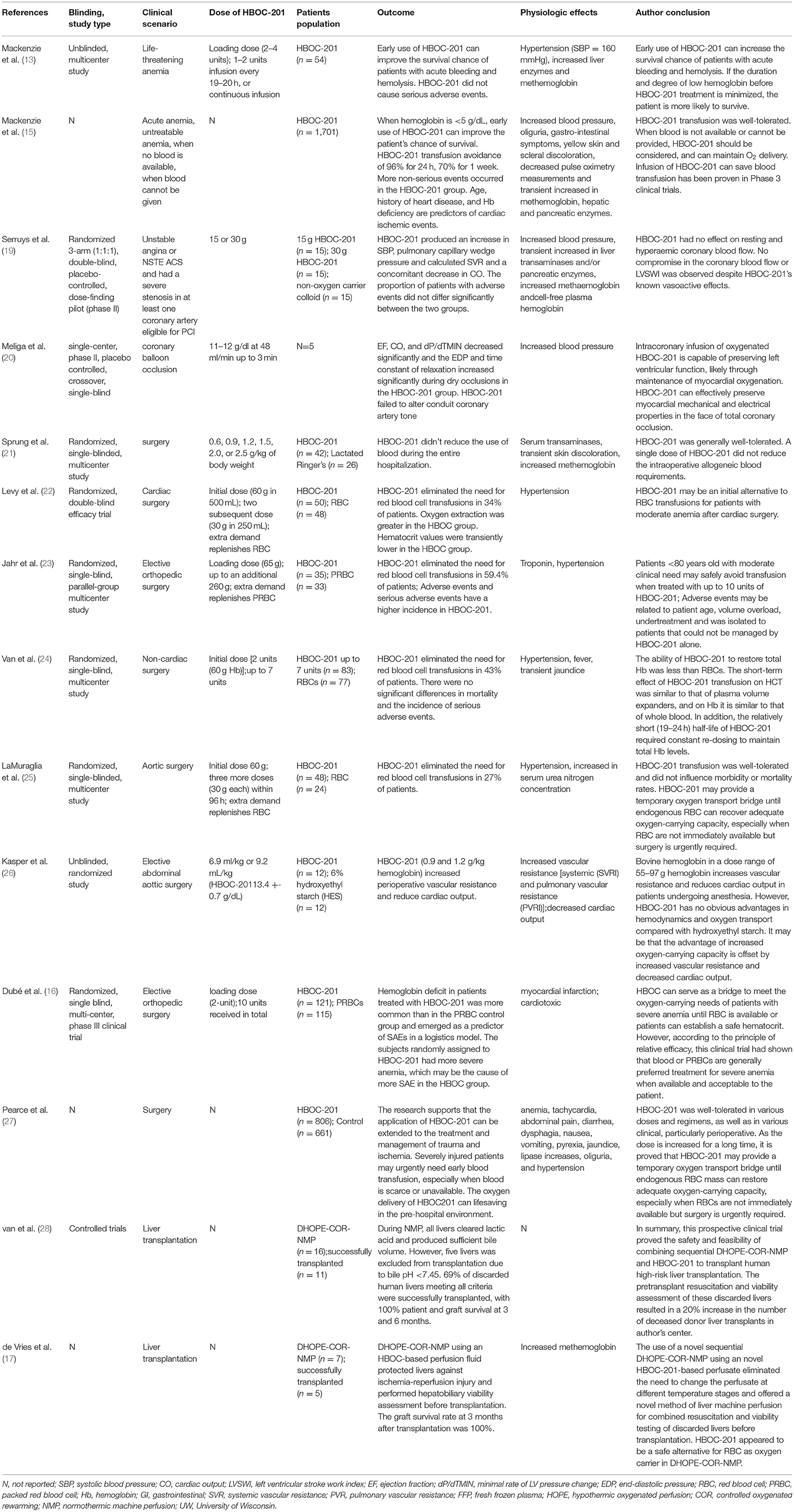
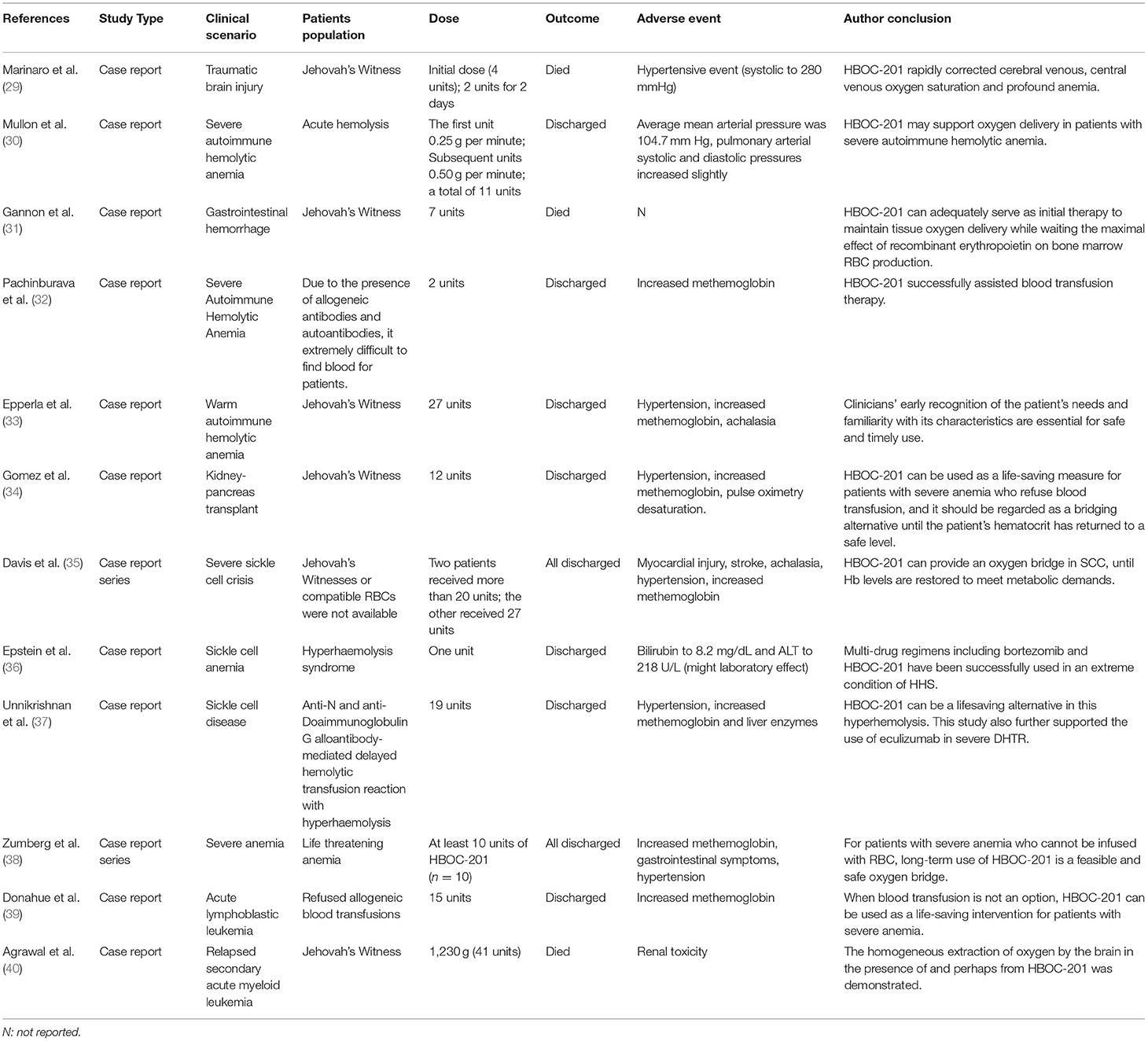
The purpose of this study is to summarize the properties of HBOC-201, the application of HBOC-201-related preclinical and clinical studies in various clinical scenarios, adverse events (AEs) and corresponding solutions, and prospects for its application. In this study, all the HBOC-201-related preclinical studies and clinical studies searched in Pubmed were included. From the selected studies, we further extracted information on HBOC-201-related clinical trials (Table 1) and case reports (Table 2). By reviewing the relevant preclinical and clinical studies of HBOC-201, clinical decision-makers are familiar with the characteristics and indications of HBOC-201 that could benefit patients in emergencies.
Properties of HBOC-201
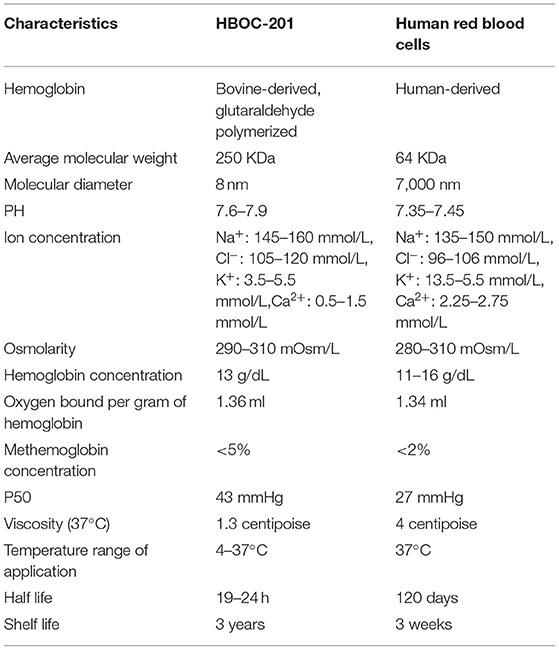
Hemoglobin-based oxygen carrier-201 is derived from bovine RBCs. Free Hbs are then purified by chromatography and cross-linked with glutaraldehyde to increase its stability and molecular size. Unlike human RBCs, HBOC-201 has no cell membrane, no blood type, no cross-matching requirement, no risks of viral infection, a long-term storage capability, timely availability, and other characteristics (Table 3). The molecular structure of HBOC-201 is similar to that of human Hb and the molecular diameter of HBOC-201 (8 nm) is much smaller than the RBC diameter (7,000 nm), which can pass through narrow blood vessels where RBCs cannot pass and penetrate the subendothelial space (41). Since HBOC-201 has a lower oxygen affinity than human Hb, it increases the rate of oxygen uptake. As the Hb dissociation curve shifts to the right, the release of oxygen to the tissue is promoted (42). HBOC-201 use chloride instead of 2,3-diphosphoglycerate (2,3-DPG) as on-loading and off-loading of oxygen (43). HBOC-201 has lower concentrations of organic phosphates, which leads to facilitated oxygen unloading in ischemic tissue (the acid Bohr effect) and increase Hb binding of carbon dioxide in the deoxygenated state (the Haldane effect) (44). The physiological effect of 1 g of bovine Hb in HBOC-201 is equivalent to 3 g of human Hb. Analysis of oxygen binding and release properties of Hb by Gregory et al. showed that HBOC-201 could theoretically deliver more oxygen to tissues than RBCs under physiological conditions (16). The results of Thomas et al. also suggested that an increase in extracellular Hb concentration increased oxygen transport efficiency for both the uptake and release (42). The plasma clearance rate of HBOC-201 follows first-order pharmacokinetics, regardless of whether it is a single dose or multiple doses, which intravascular half-life is 19 h (45). The metabolism of HBOC-201 in men and women seems to be similar and metabolized by the mononuclear phagocyte system (MPS) to bilirubin. Ashenden et al. reported that after healthy male subjects were infused with HBOC-201, the blood oxygen-carrying capacity was improved (46). HBOC-201 has been shown to be effective in increasing oxygen extraction and delivery to peripheral and central organs and improving tissue oxygenation in studies related to isovolumetric hemodilution (47–51). Although HBOC-201 can induce the production of immunoglobulin G (IgG) antibodies in humans, it will not lyse RBCs and its efficacy is not affected by specific IgG antibodies (52). Immunoglobulin E (IgE) anti-HBOC-201 was not detected in the serum samples of patients who received repeated HBOC-201 treatment (53). Therefore, repeated infusions of HBOC-201 appear to be safe for patients and do not increase the risk of type 1 hypersensitivity.
Trauma and Hemorrhagic Shock
After trauma, HS can lead to insufficient perfusion of vital organs, cell hypoxia, and organ dysfunction. HBOC-201 can provide sufficient oxygen, reduce the risk of disease transmission, and longer shelf-life. Its effect on neutrophil activation and immune function is similar to other resuscitation fluids, suggesting that it may be safe as a resuscitation fluid in patients with HS (54, 55). Compared with various resuscitation fluids, HBOC-201 can restore tissue oxygenation, maintain blood pressure, reduce the need for blood transfusion in the hospital, reverse anaerobic metabolism, and even improve survival rate (56–59). Even for low-volume resuscitation, HBOC-201 still can improve intracranial pressure and brain oxygen (60–62), improve hemodynamics (63), avoid blood transfusions (64), and improve survival rate (65). Although some preclinical studies have elevated aspartate aminotransferase in the early stage of HBOC-201 infusion, they tend to be normal afterward (63). Incidentally, the usage of HBOC-201 during HS resuscitation, combined with physiological parameters and hypotension to guide fluid volume, will reduce the risk of hyperinfusion and increase the potential benefits. The low-volume strategy for HS resuscitation can maximize the beneficial effects of HBOC-201, while minimizing any potentially harmful effects.
Trauma, bleeding, and fluid resuscitation will cause the consumption and dilution of coagulation factors, adversely affecting the coagulation of trauma patients. Arnaud et al. in a preclinical study showed that transfusion of HBOC-201 successfully resuscitated and avoided blood transfusion in animals, but it caused dilutional coagulopathy (lower hematocrit, platelets, and fibrinogen) (66). HBOC-201 combined with other coagulation substances such as freeze-dried plasma (FDP) (67) or recombinant coagulation factor VIIa (rfVIIa) that had little effect on functional coagulation indicators and had no significant survival benefit (68). However, in the combination of a bleeding model with the Folts model, due to the interaction of many factors such as platelet count, hematocrit, and platelet aggregation, HBOC-201 can reduce thrombosis (69).
In a porcine model with controlled bleeding (40%) and soft-tissue injury, infusion of HBOC-201 not only improved tissue perfusion, stabilized hemodynamics, reduced fluid requirements, and mortality (70), but also increased strong ion difference (SID) and electrochemistry to promote alkalosis (71), which might be beneficial for therapy in high lactate conditions. In the more severe porcine model with rectus abdominus crush (64), the benefit from HBOC-201 might become more pronounced. In a porcine model of uncontrollable bleeding caused by liver injury, HBOC-201 stabilized tissue oxygenation, stabilized hemodynamic and metabolic parameters, and improved survival (59, 66, 72–74). Although Gurney et al. reported that HBOC-201 increased systemic and pulmonary artery pressure (59) and Johnson et al. found that aspartate aminotransferase, lactate dehydrogenase, alkaline phosphatase, and mild renal papillary damage in the HBOC-201 group were higher than those of 6% hetastarch (HEX) (74) and these AEs were mild. In the abovementioned severe HS porcine model, selective aortic arch perfusion (SAAP) and HBOC-201 not only quickly restored survival of cardiovascular function after cardiac arrest (75), but also effectively induced return of spontaneous circulation (ROSC) and provided the critical oxygenation and perfusion support necessary for successful resuscitation (76, 77).
Compared with other resuscitation fluids, HBOC-201 can improve the mean arterial pressure (MAP), cerebral perfusion pressure (CPP), brain tissue oxygen tension [PbtO(2)] and reduce the secondary damage, and the need for blood transfusion in the traumatic brain injury (TBI) model (78–80). In preclinical studies of TBI, infusion of HBOC-201 not only rapidly restored the self-regulatory mechanisms of cardiovascular system, but also improved cerebral blood flow (CBF), CPP, PbtO(2), and cerebral vasoreactivity to hypercapnia (CVH) (78, 81–83). However, the decrease in nitric oxide (NO) caused by infusion of HBOC-201 may cause constriction of the cerebral vasculature. Cerebral metabolic demand for oxygen is critical in the case of TBI and oxygen supply is increased to minimize ongoing neuronal damage and, thus, reduce morbidity and mortality, so cerebral vasoconstriction may have a devastating effect on patients. Since HBOC-201 causes slight vasoreactivity and has the ability to carry and release oxygen efficiently, it may further improve CBF, CPP, and PbtO(2). In the porcine multiple injury model of TBI bleeding and aortic tear injury, the fluid resuscitation of HBOC-201 is not better than that of Lactated Ringer's (LR) solution (82). The possible reason is that in the case of high-pressure aortic injury, the boosting effect of HBOC-201 may lead to increased bleeding and it may also be related to the severity of shock at the beginning of resuscitation. Therefore, when considering the resuscitation strategy, it is necessary to consider the different injuries of high-pressure blood vessels and low-pressure solid organs and the different severity of shock. In future studies of traumatic HS, how to balance the benefits of HBOC-201 infusion with the potential side effects and how to personalize emergency treatment assistance to patients need to be thoroughly investigated in further studies. Jonathan et al. first used HBOC-201 in a patient with severe TBI, a Jehovah's witness who refused a blood transfusion (29). The patient was transfused with 6 units of HBOC-201 (180 g) and non-invasive cerebral oximetric devices were used to monitor the brain tissue oxygen saturation of the patient. The results of this study showed that not only brain tissue oxygen saturation, central venous oxygen saturation, and hemodynamic variables increase significantly after HBOC-201 administration, but total Hb increased from 3.2 to more than 7 g/dl. The patient developed severe hypertension 12 h after the last HBOC-201 injection, followed by massive cerebral edema and death. The possible cause was massive reperfusion injury from delayed repayment of cerebral oxygen debt in a severely ischemic brain, but it cannot yet be excluded that HBOC-201 itself directly caused massive edema and brain herniation. There is no doubt that HBOC-201 rapidly corrected cerebral venous and central venous oxygen saturation, but further applications in patients with TBI and controlled research need to be explored in the future.
Anemia
Hemoglobin-based oxygen carrier-201 has been allowed by the FDA for use under the expanded access in Jehovah's Witnesses patients who require blood transfusion. HBOC-201 has no cell membrane and lacks cell surface antigens, making HBOC-201 an ideal substitute for blood to save lives when blood is unavailable or blood transfusion is refused for patients with severe autoimmune hemolytic anemia (30, 32, 33, 36, 37). Epperla et al. demonstrated that early recognition of patient needs and familiarity with the characteristics of HBOC-201 by clinicians are essential for the safe and timely use of HBOC-201 to save the lives of the patients (33). Davis et al. reported three cases of critically ill patients with sickle cell disease with multiorgan failure who refused RBCs or were not able to use RBCs and whose lives were ultimately saved by the administration of HBOC-201 therapy (35). This study demonstrated that HBOC-201 could provide an oxygen bridge in treating patients with sickle cell crisis (SCC) until Hb levels restored to meet metabolic demands. A Jehovah's Witness patient with acute lymphoblastic leukemia (ALL) developed severe anemia (Hb as low as 3.1 g/dl) during chemotherapy. During the infusion of HBOC-201, Hb of the patient remained between 3.6 and 5.3 g/dl without any ischemia or organ dysfunction (39). Gomez et al. reported for the first time that kidney–pancreas double solid organ transplant recipients whose Hb dropped to a critical level (2 g/dl) and Hb increased to 6.8 g/dl after infusion of HBOC-201 (34). Gannon et al. demonstrated that a combination of HBOC-201 and high-dose recombinant human erythropoietin (rHuEpo) for life-threatening anemia (Hb, 3.5 g/dl) improved survival of the patient and a significant increase in Hb to 7.6 g/dl (31). This study demonstrated that HBOC-201 can provide adequate tissue oxygen supply until marrow RBC recovery and HBOC-201 combined with high-dose rHuEpo also increase hematocrit and survival in patients with severe symptomatic anemia. Therefore, HBOC-201 is a viable and safe oxygen bridge for patients who cannot infuse RBCs or for whom RBCs are not feasible, hence saving the life of the patient in a life-threatening situation. Furthermore, the existing data supported that although HBOC-201 can cause common side effects such as methemoglobin, gastrointestinal symptoms, and hypertension when the cumulative dose reaches 10 units, it is generally a feasible and safe “oxygen bridge” (13). This study showed that early use of HBOC-201 increased the chances of survival in patients with acute bleeding and hemolysis. Zumberg et al. reported on the treatment of 10 patients with life-threatening anemia and poor prognosis with at least 10 units of HBOC-201 and all of them survived and discharged without long-term complications (38). The results of these cases supported that long-term and high-dose administration of HBOC-201 is a feasible and safe treatment modality for early and proactive correction of life-threatening severe anemia until adequate intrinsic Hb is restored, in cases where blood transfusion is not an option. Agrawal et al. reported on a Jehovah's Witness who presented with chemotherapy-induced anemia following a relapse of secondary acute myeloid leukemia, documenting the highest dose and longest duration of experience with HBOC-201 treatment to date (40). Over a period of 18 days, the patient was infused with 1,230 g of HBOC-201. This study revealed by PET scan that the bilaterally similar and homogeneous extraction of oxygen in the presence of soluble HBOC-201 by the brain tissue. However, nephrotoxicity of HBOC-201 during the infusion of the patient cannot be excluded and, therefore, HBOC-201 may not be useful as the sole oxygenating component of anemia treatment during myelosuppressive therapy. These studies support the safety and feasibility of HBOC-201 in patients with anemia, but the consequences of AEs that occur to patients are worthy of attention. Future randomized, multicenter, and large sample size clinical trials are needed to obtain further high-quality evidence to validate the efficacy, safety, and tolerability of HBOC-201.
Myocardial Infarction
Since the diameter of HBOC-201 is smaller than that of RBCs, it is easily transported through plasma to places where RBCs cannot reach. Therefore, its characteristic of carrying and releasing oxygen makes HBOC-201 more beneficial than whole blood in emergencies. Infusion of HBOC-201 30 min before myocardial ischemia, the infarct size/area at risk (Inf/AAR) was significantly reduced (84). George et al. also reported a similar study that infusion of HBOC-201 after 15 min after ischemia can also significantly reduce Inf/AAR and improve myocardial survival (85). Bloodless reperfusion is a promising strategy that restores oxygen delivery and delays the re-exposure of ischemic myocardium to blood cells, plasma proteins, and other blood-borne inflammatory substances. Intracoronary preoxygenated HBOC-201 can reduce changes in infarct size and stroke volume, improve aerobic metabolism, systolic shortening (SS), and stroke volume in swine during coronary artery occlusion (CAO) in a dose- and temperature-dependent manner (86). Preoxygenated HBOC-201 can match the oxygen delivery of blood in almost equal amounts. The study by Garciá-Ruiz et al. found that the preoxygenated HBOC-201 solution did not lead to increase in infarct size (IS) or deterioration of long-term ventricular function, but it is still feasible and safe (87). The infarct size in the MI model is larger than other studies, mainly due to the use of intravenous anesthetics to avoid the known infarct-limiting effects of inhaled gas anesthetics. With the introduction of percutaneous coronary intervention (PCI), the morbidity, mortality, and disability rates of acute coronary syndromes (ACSs) have been greatly reduced, but the selection of the best drug to limit myocardial injury occurs during ischemia and early reperfusion remains challenging. Since HBOCs have the ability to deliver oxygen, they have been considered for the treatment of ACS. In a randomized, double-blind study, Serruys et al. made the first attempt to introduce HBOC-201 in ACS treatment, examining the safety and tolerability of HBOC up to 230 ml in low-to-moderate risk cardiac patients scheduled for elective PCI (19). The results showed that although intravenous HBOC-201 had side effects such as cell-free plasma Hb and increased blood pressure, no impaired coronary blood flow or left ventricular stroke work index (LVSWI) was observed in patients, nor did it affect the autoregulation of coronary blood flow. In a single-center, phase II, placebo-controlled, crossover, single-blind study, intracoronary infusion of preoxygenated HBOC-201 in five patients undergoing elective PCI improved myocardial “oxygenation” and maintained left ventricular function during brief coronary occlusion (20). This study demonstrates that HBOC-201 can be effective as an oxygen bridge to preserve myocardial mechanical and electrical properties in the face of total coronary occlusion, prolonging the “golden” time period in patients with PCI and improving myocardial oxygenation in patients with MI. More complex patient populations, including ST-elevation MI (STEMI), can benefit from oxygenated HBOC-201 in the future that remains to be further explored. Moreover, higher quality evidence is needed to further collect and validate the efficacy, safety, and tolerability of HBOC-201 in patients with MI.
Cardiopulmonary Bypass
Cerebral ischemia may occur during extracorporeal circulation, especially at low flow. Neuronal death due to cerebral ischemia can cause a series of irreversible complications. Jeffrey et al. first verified that in a porcine model of normothermic low-flow cardiopulmonary bypass (CPB), compared with donor whole-blood priming, adding HBOC-201 to the pump-priming solution during CPB seems to improve cerebral oxygenation by minimizing overall end-organ ischemia (88). Cerebral oxygenation was maintained in the HBOC-201 group, even during the decrease in blood flow. Although myocardial impairment and elevated troponin levels following HBOC-201 treatment need to be further evaluated before clinical application, this study supported the possible benefits provided by HBOC-201 during CPB. Extracorporeal membrane oxygenation (ECMO) is an accepted treatment for resuscitating critically ill neonates and children with high predicted mortality. Since the blood volume of children is so small relative to the initiation volume of the ECMO circuit, an extracorporeal circuit containing a large volume of blood is required to initiate the ECMO circuit. The donor RBCs in ECMO circuits carry the risk of viral infection, immune response, and blood from multiple donors. Given these concerns, the use of an effective blood substitute during ECMO would be beneficial for the management of ECMO in child patients. In models of ECMO of piglets with healthy or acute respiratory distress syndrome (ARDS), the results of Gregory et al. and Henderson et al. demonstrated that although the hematocrit of the HBOC-201 group was significantly lower than the blood group and the blood pressure and methemoglobin concentration in the HBOC-201 group were slightly increased, HBOC-201-primed ECMO was well-tolerated, maintained hemodynamic stability, and provided good oxygen delivery (89, 90). HBOC-201-primed ECMO appears to be very promising and feasible. HBOC-201 not only has a long half-life, but also eliminates the risk of infection of the patient, immune response, and exposure to multiple donors. However, the initial dosing, the circuit transfusion support, the potential concerns with respect to methemoglobin and vasoconstriction, and the impact on platelet and coagulation factor depletion still warrant further investigation.
Perioperative Period
Perioperative preoperative sampling, surgery-related blood loss, and hemodilution result in acute postoperative anemia, which can lead to a reduction in oxygen-carrying capacity. In addition, some emergency surgeries do not have sufficient time and physiologic reserves to donate autologous blood in advance; therefore, many patients require allogeneic RBC transfusions during the perioperative period to maintain cellular energy metabolic needs. During the 1970's and 1980's, many concerns began to be expressed about the safety of blood transfusions. However, a large number of clinical studies have shown that patients after HBOC-201 infusion had good tolerance in different dosing regimens and various clinical conditions, especially in the perioperative period (15, 21, 27, 53). In addition, the improvement of hemodynamics after administration of HBOC-201 may be attributed to its highly colloidal intravascular expansion properties. Although HBOC-201 cannot significantly increase and maintain THb, it can meet the oxygen-carrying needs of patients with severe anemia until RBC is available or patients can establish a safe hematocrit (16). Moreover, in some studies, HBOC-201 can eliminate the need for any allogeneic RBC transfusion for some patients during the perioperative period (22–24). However, insufficient treatment, effective treatment delay, and volume overload were the reasons why the incidence of AE and serious AE (SAE) in the HBOC-201 group was significantly higher than that in PRBC (23).
A multicenter, randomized clinical study showed that HBOC-201 reduced the intraoperative requirements for allogeneic blood transfusion during aortic reconstructive surgery, but led to an increased requirement for allogeneic transfusion later during the hospitalization and ultimately did not reduce the need for allogeneic blood (25). This study indicated that HBOC-201 might be most effective in eliminating need of the patient for emergency transfusions without reducing the total RBC transfusion requirement. In addition, this study discovered that HBOC-201 was associated with a transient increase in systemic blood pressure, but this slight systemic blood pressure increase produced by HBOC-201 may be an advantage for patients with vascular disease who often require temporary blood pressure support. In a study of elective abdominal aortic surgery (26), HBOC-201 provided no significant benefit to hydroxyethyl starch (HES) because the advantage of increased oxygen-carrying capacity was offset by increased vascular resistance and reduced cardiac output. In a limited safety analysis, it can be shown that HBOC-201 is acceptable in the stable trauma, hypotension, and younger age population, especially when safe blood transfusions are not available quickly (91). Overall, in cases where patients require emergency surgery or safe blood transfusions are not rapidly available, HBOC-201 provides a temporary oxygen transport bridge until endogenous RBC mass can be restored to adequate oxygen-carrying capacity or allogeneic transfusion is available.
Organ Transplantation
For dynamic preservation of isolated organs, the earliest clinical series used is hypothermic machine perfusion (HMP) without oxygenation; however, recent studies have shown that solid organ damage can be mitigated by providing oxygen dissolved in the perfusate (92–94). Subsequent clinical applications for MP employed a single roller pump device utilizing RBCs-based perfusate under normothermic conditions, but it also has several potential drawbacks including the fact that RBCs are a relatively scarce human blood product that may induce immune reactions or cause infections, RBC hemolysis, and logistical difficulties associated with using cross-matched blood (95, 96).
Hemoglobin-based oxygen carrier-201 is a polymerized bovine with low immunogenicity and similar oxygen-carrying capacity to human Hb at normothermic temperatures (21). Compared with cold static preservation (CSP) or blood perfused, the subnormothermic MP (SNMP)/HBOC-201 system significantly improved graft function and provided effective oxygenation to the tissue (97, 98). Laing et al. presented the first experience using HBOC-201 in discarded high-risk human livers of normothermic MP (NMP) (99). The vascular blood flow parameters and lactate clearance rate of the liver perfused with HBOC-201 and RBCs were similar and the HBOC group could extract more oxygen. Compared with RBCs + fresh frozen plasma (FFP) perfused livers, livers perfused with HBOC-201-based perfusion solution had significantly higher hepatic ATP content, cumulative bile production, and portal and arterial flows (100). HBOC-201 was first used in the study of discarded human kidneys NMP, which was similar to PRBC with respect to vascular flow, oxygen consumption, and reconstitution of tissue ATP (101). HBOC-201-ex-vivo normothermic limb perfusion (EVNLP) is identical to RBC-EVNLP, retaining muscle contractility and mitochondrial structure (102). The above study showed that HBOC-201 can be used as an alternative oxygen carrier to RBC in livers, kidneys, and isolated limbs NMP. However, White et al. found that RBC + Plasma can minimize injury and provide superior preservation of myocardial function during ex vivo heart perfusion (EVHP) compared with HBOC-201-based perfusion solution (100). The reason may be that the experimental conditions of this study promoted the spontaneous oxidation and the production of reactive oxygen species (ROS) of HBOC-201 and the methemoglobin gradually increased.
Red blood cell cannot be used during HMP because the stiffness of the erythrocyte lipid membranes increases and hemolysis occurs at low temperatures, thus prompting the need for an alternative oxygen carrier, especially when combined with hypothermia and NMP (17). Mahboub et al. reported better renal function recovery in a rat donation after circulatory death (DCD) kidney model after rewarming with HBOC-201 compared to rewarming without an oxygen carrier (103). Although continuous cold-to-warm (HBOC-201) protocol is similar to an interrupted hypothermic oxygenated perfusion (HOPE) [University of Wisconsin (UW) solution] + NMP (HBOC-201) protocol in terms of increasing ATP synthesis and reducing tissue expression of oxidative tissue damage markers, the use of a single perfusate eliminated unnecessary ischemia time required for perfusate exchange (103). HBOC-201-based perfusate was used in two clinical studies of the dual hypothermic oxygenated machine perfusion (DHOPE)-controlled oxygenated rewarming (COR)-NMP trial. After being assessed, most livers that met all the criteria were transplanted (17, 28). Totto B et al. increased the number of transplantable livers by 20% by using a combination of sequences DHOPE-COR-NMP and HBOC-201 to resuscitate and evaluate high-risk donor livers (28). Livers of suboptimal quality that are not repaired and functionally assessed post-transplant will increase the risk of primary non-function and early allograft dysfunction in patients. Combining HBOC-201 with the MP platform is feasible and provides novel and safe perfusion protocols for expanding the transplanted organ pool.
Adverse Events and Resolutions
Hemoglobin-based oxygen carrier-201 was originally developed for use as a blood substitute. Based on the characteristics of HBOC-201, it can increase convective oxygen delivery by increasing the oxygen-carrying capacity of blood and promote the release and diffusive transport of oxygen in the microcirculation, thereby promoting tissue oxygenation. Thus, the application of HBOC-201 can also be extended to various ischemic and hypoxic diseases such as traumatic HS, anemia, surgical settings, MI, CPB, and organ transplantation. The efficacy, safety, and tolerability of HBOC-201 in various clinical scenarios have been shown to be promising in published preclinical and clinical studies. The efficacy, safety, and tolerability of HBOC-201 in various clinical scenarios are promising in published preclinical and clinical studies. Unlike natural Hb, the Hb molecules in HBOC-201 have been chemically altered to minimize such toxicity theoretically. However, several AEs occurring during the application of HBOC-201 require widespread attention, with vasoconstriction and increased blood pressure being of great concern and the highest incidence of AEs. The high affinity of HBOC-201 for NO and the lower shear stress lead to a decrease in circulating NO, which may result in systemic vasoconstriction, decreased blood flow, increased pro-inflammatory mediators and vasoconstrictor factors, and platelet inactivation, ultimately leading to increased MI and mortality in patients (104). The vasoconstriction caused by HBOC-201 may also be attributed to increased release of endothelin (105), stimulation of α-adrenergic receptors (106), and delivery of high oxygen concentrations. HBOC-201-induced vasoconstriction and blood pressure can be modulated by reducing the dose, changing the solvent, and adding a vasodilator. In a porcine model of HS, HBOC-201 suspended in the hypertonic saline (HTS) solution significantly decreased systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR), increased mean pulmonary artery pressure (MPAP) and cardiac output (107), and reduced the high inflammatory response (108). Inhaling NO before intravenous infusion of HBOC-201 can prevent systemic vasoconstriction without causing methemoglobin (18). Sodium nitrite (NaNO2) also temporarily reduced systemic and pulmonary blood pressure increase from HBOC-201 in a dose-dependent manner (109). Three vasodilators, sodium nitroprusside (SNP), sodium nitrite and nitroglycerin, can all attenuate HBOC-mediated vasoconstriction, with nitroglycerin being the least reactive with HBOC-201 in methemoglobin formation (110). SNP can effectively and safely reduce HBOC-201-related systemic, but not pulmonary vasoactivity (111). Therefore, among the three nitrovasodilators, nitroglycerin seems to be the most promising drug for reducing hypertension caused by HBOCs.
Increased methemoglobin is another common side effect of HBOC-201 application. Unlike RBCs, HBOC-201 lacks the nicotinamide adenine dinucleotide (NADH)-dependent enzyme (methemoglobin reductase). Excessive methemoglobin will lead to a decrease in the ability to release oxygen, which will reduce the amount of oxygen delivered to the tissues. Iron ions mediate the oxidative damage of endothelial cells, leading to increased microvascular permeability. Methemoglobin concentrations below 10% do not significantly alter the mode of oxygen delivery to organs (112). Juraj et al. found that the percentage of methemoglobin was delayed to increase after HBOC-201 infusion, so the methemoglobin load was not caused by HBOC-201 infusion, but was gradually generated by oxidation of plasma Hb. Therefore, close monitoring of the changes in methemoglobin in patients and timely management are beneficial to minimize the damage caused by high concentrations of methemoglobin. The methemoglobinemia resulting from HBOC-201 infusion in the patient in the case report by Athira et al. responded well to vitamin C and showed no major toxicity secondary to HBOC-201 (37). In addition, Colin et al. showed that the increase in methemoglobin was independent of the dose of HBOC-201 and did not affect patient survival and that patients responded to injectable methylene blue infusions (13). The percentage of methemoglobin can be corrected or reduced by adding additional HBOC-201, glutathione, or vitamin C to the perfusion fluid during the perfusion of isolated organs (17). Since vitamin C affects the pH and osmolarity of the perfusate, addition to the perfusate to reduce methemoglobin is not recommended.
In addition to the two common AEs mentioned above, AEs recorded with HBOC-201 include plasma volume expansion, gastrointestinal discomfort, blood urea nitrogen values, liver and pancreatic enzyme dysregulation, yellow skin, and sclera discoloration, etc. The use of diuretics may improve plasma volume expansion in patients and anticholinergics may treat gastrointestinal discomfort. The increase in blood urea nitrogen may be due to the high protein load associated with the infusion of HBOC-201. A transient increase in hepatic transaminase and pancreatic enzyme concentrations is not usually associated with hepatic or pancreatic dysfunction (19). This may be due to an increase in metabolic load after absorption, distribution, metabolism, and excretion of HBOC-201 involving the hepatopancreatic system (19), resulting in upregulation of enzyme activity or it may be due to very slight damage to hepatocytes and pancreatic cells during the catabolism of HBOC-201. The mechanisms of elevated hepatic and pancreatic enzymes and the clinical significance need to be explored more specifically and in detail in future clinical studies. The skin discoloration and serum discoloration caused by HBOC-201 will not cause problems in pulse oximetry monitoring. The above-mentioned AEs are transient, but close observation and documentation of HBOC-201 in the clinical setting are still required to allow clinical decision-makers to respond promptly with active intervention.
Conclusion
Various clinical scenarios of ischemia and hypoxia need to be corrected in time to reduce the sequelae and mortality of patients. HBOC-201 has many potential advantages including being easily available, not affected by storage, and the ability to transport oxygen more efficiently than Hb in RBCs, which is currently a more desirable oxygen carrier. A large amount of evidence shows that HBOC-201 can be used as a temporary oxygen bridge, not only can improve the oxygenation of tissues and organs in emergency situations until the RBCs in vivo recover enough oxygen-carrying capacity, but also can provide oxygen to ischemic and hypoxic tissues or/and organs to mitigate ischemia-reperfusion injury. Based on the safety considerations of HBOC-201, clinicians would pay attention to monitoring and recording AEs related to cardiovascular, methemoglobin, clinically relevant liver enzymes and pancreatic enzymes, kidney, gastrointestinal disorders, skin and sclera color, etc. Due to different clinical scenarios, the application of HBOC-201 dosage and protocols would be further explored in the future and individualized treatment of patients will be carried out to maximize potential benefits and minimize adverse consequences. In addition, multicenter randomized clinical trials with large sample sizes would be implemented to obtain high-quality evidence to further verify the efficacy, safety, and tolerability of HBOC-201.
Author Contributions
MC, DZ, and XH conceived the idea, coordinated the staff involved in this study, and wrote the first draft. YZ, HHe, RY, and LP revised manuscript critically for important intellectual content. HHu, YR, QQ, XY, TY, and LM were involved in the data collection and checked the data. DZ and XH were responsible for final edits and general revisions. All the authors have read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81470667), the Department of Science and Technology of Sichuan Province (20YFS0435), and the Science and Technology Bureau of Chengdu (YF06-00070-SN, YF05-00198-SN).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gao XY, Zhu SZ, Xiang W, Huang KB, Hu YF, Gu Y, et al. Prolonged hypothermia exposure diminishes neuroprotection for severe ischemic-hypoxic primary neurons. Cryobiology. (2016) 72:141–7. doi: 10.1016/j.cryobiol.2016.01.003
2. Kalisvaart M, de Haan JE, Polak WG, Ijzermans NMJ, Gommers D, Metselaar HJ, et al. Onset of donor warm ischemia time in donation after circulatory death liver transplantation: hypotension or hypoxia? Liver Transpl. (2018) 24:1001–10. doi: 10.1002/lt.25287
3. Steensma DP, Fenaux P, Van Eygen K, Raza A, Santini V, Germing U, et al. Imetelstat achieves meaningful and durable transfusion independence in high transfusion-burden patients with lower-risk myelodysplastic syndromes in a phase II study. J Clin Oncol. (2021) 39:48–56. doi: 10.1200/JCO.20.01895
4. Bergamaschi G, Livraghi A, Aronico N, Barteselli C, Bonadeo E, Del Rio V, et al. Impact of in-hospital intravenous iron supplementation on red blood cell transfusions: experience from an Internal Medicine Unit. Blood Transfus. (2020) 167:20. doi: 10.2450/2020.00167-20
5. Agarwal AK. Practical approach to the diagnosis and treatment of anemia associated with CKD in elderly. J Am Med Direct Assoc. (2006) 7(9Suppl.):5. doi: 10.1016/j.jamda.2006.09.005
6. Cronkite E, Bullis J, Honikel L. Partial amelioration of AZT-induced macrocytic anemia in the mouse by folic acid. Stem Cells. (1993) 11:393–7. doi: 10.1002/stem.5530110506
7. Hughes GS, Francome SF, Antal EJ, Adams WJ, Locker PK, Yancey EP, et al. Hematologic effects of a novel hemoglobin-based oxygen carrier in normal male and female subjects. J Lab Clin Med. (1995) 126:444–51. doi: 10.1097/00003246-199501001-00454
8. Mer M, Hodgson E, Wallis L, Jacobson B, Levien L, Snyman J, et al. Hemoglobin glutamer-250 (bovine) in South Africa: consensus usage guidelines from clinician experts who have treated patients. Transfusion. (2016) 56:2631–6. doi: 10.1111/trf.13726
9. Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. J Am Med Assoc. (2008) 299:2304–12. doi: 10.1001/jama.299.19.jrv80007
10. Levien LJ, Hodgson RE, James MFM. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. J Am Med Assoc. (2008) 300:1295. doi: 10.1001/jama.300.11.1295-a
11. Sauaia A, Moore EE, Banerjee A. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. J Am Med Assoc. (2008) 300:1297a. doi: 10.1001/jama.300.11.1297-a
12. Sarani B, Gracias V. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. J Am Med Assoc. (2008) 300:1297b. doi: 10.1001/jama.300.11.1297-b
13. Mackenzie CF, Moon-Massat PF, Shander A, Javidroozi M, Greenburg AG. When blood is not an option: factors affecting survival after the use of a hemoglobin-based oxygen carrier in 54 patients with life-threatening anemia. Anesth Analg. (2010) 110:685–93. doi: 10.1213/ANE.0b013e3181cd473b
14. Jahr JS, Moallempour M, Lim JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (Biopure Corporation). Expert Opin Biol Ther. (2008) 8:1425–33. doi: 10.1517/14712598.8.9.1425
15. Mackenzie CF, Dubé GP, Pitman A, Zafirelis M. Users guide to pitfalls and lessons learned about HBOC-201 during clinical trials, expanded access, and clinical use in 1,701 patients. Shock. (2019) 52(1SSuppl.1):92–9. doi: 10.1097/SHK.0000000000001038
16. Dubé GP, Pitman AN, Mackenzie CF. Relative efficacies of HBOC-201 and polyheme to increase oxygen transport compared to blood and crystalloids. Shock. (2019) 52(1SSuppl.1):100–7. doi: 10.1097/SHK.0000000000001058
17. de Vries Y, Matton APM, Nijsten MWN, Werner MJM, van den Berg AP, de Boer MT, et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transplant. (2019) 19:1202–11. doi: 10.1111/ajt.15228
18. Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. (2008) 117:1982–90. doi: 10.1161/CIRCULATIONAHA.107.729137
19. Serruys PW, Vranckx P, Slagboom T, Regar E, Meliga E, de Winter RJ, et al. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. EuroIntervention. (2008) 3:600–9. doi: 10.4244/EIJV3I5A108
20. Meliga E, Vranckx P, Regar E, Kint PP, Duncker DJ, Serruys PW. Proof-of-concept trial to evaluate haemoglobin based oxygen therapeutics in elective percutaneous coronary revascularisation. Rationale, protocol design and haemodynamic results. EuroIntervention. (2008) 4:EIJV4I1A17. doi: 10.4244/EIJV4I1A17
21. Sprung J, Kindscher JD, Wahr JA, Levy JH, Monk TG, Moritz MW, et al. The use of bovine hemoglobin glutamer-250 (Hemopure) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesthesia Analgesia. (2002) 94:6. doi: 10.1097/00000539-200204000-00006
22. Levy JH, Goodnough LT, Greilich PE, Parr GVS, Stewart RW, Gratz I, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. (2002) 124:35–42. doi: 10.1067/mtc.2002.121505
23. Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. (2008) 64:1484–97. doi: 10.1097/TA.0b013e318173a93f
24. Van Hemelrijck J, Levien LJ, Veeckman L, Pitman A, Zafirelis Z, Standl T, et al. safety and efficacy evaluation of hemoglobin-based oxygen carrier HBOC-201 in a randomized, multicenter red blood cell controlled trial in noncardiac surgery patients. Anesth Analg. (2014) 119:766–76. doi: 10.1213/ANE.0000000000000305
25. LaMuraglia GM, O'Hara PJ, Baker WH, Naslund TC, Norris EJ Li J, et al. The reduction of the allogenic transfusion requirement in aortic surgery with a hemoglobin-based solution. J Vasc Surg. (2000) 31:299–308. doi: 10.1016/S0741-5214(00)90161-7
26. Kasper SM, Grüne F, Walter M, Amr N, Erasmi H, Buzello W. The effects of increased doses of bovine hemoglobin on hemodynamics and oxygen transport in patients undergoing preoperative hemodilution for elective abdominal aortic surgery. Anesth Analg. (1998) 87:284–91. doi: 10.1213/00000539-199808000-00009
27. Pearce LB, Gawryl MS, Rentko VT, Moon-Massat PF, Rausch CW. Chapter 37 -HBOC-201 (Hemoglobin Glutamer-250 (Bovine), Hemopure®): Clinical Studies. In: Blood Substitutes. Oxford: Academic Press (2006). p. 437–50. doi: 10.1016/B978-012759760-7/5004
28. van Leeuwen OB, de Vries Y, Fujiyoshi M, Nijsten MWN, Ubbink R, Pelgrim GJ, et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- and normothermic machine perfusion: a prospective clinical trial. Ann Surg. (2019) 270:906–14. doi: 10.1097/SLA.0000000000003540
29. Marinaro J, Smith J, Tawil I, Billstrand M, Crookston KP. HBOC-201 use in traumatic brain injury: case report and review of literature. Transfusion. (2009) 49:2054–9. doi: 10.1111/j.1537-2995.2009.02235.x
30. Mullon J, Giacoppe G, Clagett C, McCune D, Dillard T. Transfusions of polymerized bovine hemoglobin in a patient with severe autoimmune hemolytic anemia. N Engl J Med. (2000) 342:1638–43. doi: 10.1056/NEJM200006013422204
31. Gannon CJ, Napolitano LM. Severe anemia after gastrointestinal hemorrhage in a Jehovah's Witness: new treatment strategies. Crit Care Med. (2002) 30:1893–5. doi: 10.1097/00003246-200208000-00036
32. Pachinburavan M, Marik PE. Bovine blood and neuromuscular paralysis as a bridge to recovery in a patient with severe autoimmune hemolytic anemia. Clin Transl Sci. (2008) 1:172–3. doi: 10.1111/j.1752-8062.2008.00006.x
33. Epperla N, Strouse C, VanSandt AM, Foy P. Difficult to swallow: warm autoimmune hemolytic anemia in a Jehovah's Witness treated with hemoglobin concentrate complicated by achalasia. Transfusion. (2016) 56:1801–6. doi: 10.1111/trf.13607
34. Gomez MF, Aljure O, Ciancio G, Lynn M. Hemoglobin-based oxygen carrier rescues double-transplant patient from life-threatening anemia. Am J Transplant. (2017) 17:1941–4. doi: 10.1111/ajt.14226
35. Davis JM, El-Haj N, Shah NN, Schwartz G, Block M, Wall J, et al. Use of the blood substitute HBOC-201 in critically ill patients during sickle crisis: a three-case series. Transfusion. (2018) 58:132–7. doi: 10.1111/trf.14386
36. Epstein SS, Hadley TJ. Successful management of the potentially fatal hyperhaemolysis syndrome of sickle cell anaemia with a regimen including bortezomib and Hemopure. J Clin Pharm Ther. (2019) 44:815–8. doi: 10.1111/jcpt.12998
37. Unnikrishnan A, Pelletier JPR, Bari S, Zumberg M, Shahmohamadi A, Spiess BD, et al. Anti-N and anti-Do immunoglobulin G alloantibody-mediated delayed hemolytic transfusion reaction with hyperhemolysis in sickle cell disease treated with eculizumab and HBOC-201: case report and review of the literature. Transfusion. (2019) 59:1907–10. doi: 10.1111/trf.15198
38. Zumberg M, Gorlin J, Griffiths EA, Schwartz G, Fletcher BS, Walsh K, et al. A case study of 10 patients administered HBOC-201 in high doses over a prolonged period: outcomes during severe anemia when transfusion is not an option. Transfusion. (2020) 60:932–9. doi: 10.1111/trf.15778
39. Donahue LL, Shapira I, Shander A, Kolitz J, Allen S, Greenburg G. Management of acute anemia in a Jehovah's Witness patient with acute lymphoblastic leukemia with polymerized bovine hemoglobin-based oxygen carrier: a case report and review of literature. Transfusion. (2010) 50:1561–7. doi: 10.1111/j.1537-2995.2010.02603.x
40. Agrawal YP, Freedman M, Szczepiorkowski ZM. Long-term transfusion of polymerized bovine hemoglobin in a Jehovah's Witness following chemotherapy for myeloid leukemia: a case report. Transfusion. (2005) 45:1735–8. doi: 10.1111/j.1537-2995.2005.00599.x
41. Spahn DR, Kocian R. Artificial O2 carriers: status in 2005. Curr Pharm Des. (2005) 11:4099–114. doi: 10.2174/138161205774913354
42. Page TC, Light WR, McKay CB, Hellums JD. Oxygen transport by erythrocyte/hemoglobin solution mixtures in an in vitro capillary as a model of hemoglobin-based oxygen carrier performance. Microvasc Res. (1998) 55:54–64. doi: 10.1006/mvre.1997.2055
43. Bunn HF. Differences in the interaction of 2,3-diphosphoglycerate with certain mammalian hemoglobins. Science. (1971) 172:1049–50. doi: 10.1126/science.172.3987.1049
44. Tenenhouse HS, Scriver CR. Orthophosphate transport in the erythrocyte of normal subjects and of patients with X-linked hypophosphatemia. J Clin Invest. (1975) 55:644–54. doi: 10.1172/JCI107972
45. Anbari KK, Garino JP, Mackenzie CF. Hemoglobin substitutes. Eur Spine J. (2004) 13(Suppl.1):S76–82. doi: 10.1007/s00586-004-0737-x
46. Ashenden MJ, Schumacher YO, Sharpe K, Varlet-Marie E, Audran M. Effects of Hemopure on maximal oxygen uptake and endurance performance in healthy humans. Int J Sports Med. (2007) 28:381–5. doi: 10.1055/s-2006-924365
47. Standl T, Horn P, Wilhelm S, Greim C, Freitag M, Freitag U, et al. Bovine haemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. Can J Anaesthesia. (1996) 43:714–23. doi: 10.1007/BF03017957
48. Mongan PD, Moon-Massat PF, Rentko V, Mihok S, Dragovich A, Sharma P. Regional blood flow after serial normovolemic exchange transfusion with HBOC-201 (Hemopure) in anesthetized swine. J Trauma. (2009) 67:51–60. doi: 10.1097/TA.0b013e3181838030
49. Standl T, Freitag M, Burmeister MA, Horn EP, Wilhelm S, Am Esch JS. Hemoglobin-based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in skeletal muscle of anemic dogs than do stored red blood cells. J Vasc Surg. (2003) 37:859–65. doi: 10.1067/mva.2003.127
50. Freitag M, Standl TG, Gottschalk A, Burmeister MA, Rempf C, Horn EP, et al. Enhanced central organ oxygenation after application of bovine cell-free hemoglobin HBOC-201. Can J Anaesthesia. (2005) 52:904–14. doi: 10.1007/BF03022050
51. Muir WW, Ilangovan G, Zweier JL, Moon-Massat PF, Rentko VT. Vital organ tissue oxygenation after serial normovolemic exchange transfusion with HBOC-201 in anesthetized swine. Shock. (2011) 35:597–603. doi: 10.1097/SHK.0b013e31821366f6
52. Hamilton RG, Kickler TS. Bovine hemoglobin (glutamer-250, Hemopure)-specific immunoglobulin G antibody cross-reacts with human hemoglobin but does not lyse red blood cells in vitro. Transfusion. (2007) 47:723–8. doi: 10.1111/j.1537-2995.2007.01176.x
53. Standl T, Burmeister MA, Horn EP, Wilhelm S, Knoefel WT, Schulte am Esch J. Bovine haemoglobin-based oxygen carrier for patients undergoing haemodilution before liver resection. Br J Anaesthesia. (1998) 80:189–94. doi: 10.1093/bja/80.2.189
54. Ortegon DP, Dixon PS, Crow KK, Mueller DL, Kerby JD. The effect of the bovine hemoglobin oxygen therapeutic HBOC-201 on human neutrophil activation in vitro. J Trauma. (2003) 55:52921. doi: 10.1097/01.TA.0000085722.52921.6D
55. Dong F, Hall CH, Golech SA, Philbin NB, Rice JP, Gurney J, et al. Immune effects of resuscitation with HBOC-201, a hemoglobin-based oxygen carrier, in swine with moderately severe hemorrhagic shock from controlled hemorrhage. Shock. (2006) 25:50–5. doi: 10.1097/01.shk.0000187982.56030.94
56. Sampson JB, Davis MR, Mueller DL, Kashyap VS, Jenkins DH, Kerby JD, et al. comparison of the hemoglobin-based oxygen carrier HBOC-201 to other low-volume resuscitation fluids in a model of controlled hemorrhagic shock. J Trauma. (2003) 55:747–54. doi: 10.1097/01.TA.0000084519.47163.77
57. Philbin N, Handrigan M, Rice J, McNickle K, McGwin G, Williams R, et al. Resuscitation following severe, controlled hemorrhage associated with a 24 h delay to surgical intervention in swine using a hemoglobin based oxygen carrier as an oxygen bridge to definitive care. Resuscitation. (2007) 74:332–43. doi: 10.1016/j.resuscitation.2006.12.018
58. McNeil CJ, Smith LD, Jenkins LD, York MG, Josephs MJ. Hypotensive resuscitation using a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) leads to reversal of anaerobic metabolism. J Trauma. (2001) 50:1063–75. doi: 10.1097/00005373-200106000-00015
59. Gurney J, Philbin N, Rice J, Arnaud F, Dong F, Wulster-Radcliffe M, et al. A hemoglobin based oxygen carrier, bovine polymerized hemoglobin (HBOC-201) versus Hetastarch (HEX) in an uncontrolled liver injury hemorrhagic shock swine model with delayed evacuation. J Trauma. (2004) 57:726–38. doi: 10.1097/01.TA.0000147520.84792.B4
60. Lee SK, Morabito D, Hemphill JC, Erickson V, Holcroft JJ, Derugin N, et al. Small-volume resuscitation with HBOC-201: effects on cardiovascular parameters and brain tissue oxygen tension in an out-of-hospital model of hemorrhage in swine. Acad Emerg Med. (2002) 9:969–76. doi: 10.1197/aemj.9.10.969
61. Dudkiewicz M, Harpaul TA, Proctor KG. Hemoglobin-based oxygen carrying compound-201 as salvage therapy for severe neuro- and polytrauma (Injury Severity Score = 27-41). Crit Care Med. (2008) 36:2838–48. doi: 10.1097/CCM.0b013e318186f6b3
62. York GB, Eggers JS, Smith DL, Jenkins DH, McNeil JD, Mueller D, et al. Low-volume resuscitation with a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) provides adequate tissue oxygenation for survival in a porcine model of controlled hemorrhage. J Trauma. (2003) 55:873–85. doi: 10.1097/01.TA.0000092681.17874.6F
63. Knudson MM, Lee S, Erickson V, Morabito D, Derugin N, Manley GT. Tissue oxygen monitoring during hemorrhagic shock and resuscitation: a comparison of lactated Ringer's solution, hypertonic saline dextran, and HBOC-201. J Trauma. (2003) 54:242–52. doi: 10.1097/01.TA.0000037776.28201.75
64. Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, Flournoy WS, et al. Bovine polymerized hemoglobin versus Hextend resuscitation in a swine model of severe controlled hemorrhagic shock with delay to definitive care. Shock. (2006) 26:302–10. doi: 10.1097/01.shk.0000226338.48033.c2
65. Fitzpatrick CM, Biggs KL, Atkins BZ, Quance-Fitch FJ, Dixon PS, Savage SA, et al. Prolonged low-volume resuscitation with HBOC-201 in a large-animal survival model of controlled hemorrhage. J Trauma. (2005) 59:88. doi: 10.1097/01.ta.0000174730.62338.88
66. Arnaud F, Handrigan M, Hammett M, Philbin N, Rice J, Dong F, et al. Coagulation patterns following haemoglobin-based oxygen carrier resuscitation in severe uncontrolled haemorrhagic shock in swine. Transfusion Med. (2006) 16:290–302. doi: 10.1111/j.1365-3148.2006.00678.x
67. Meledeo MA, Peltier GC, McIntosh CS, Taylor AS, Bynum JA, Pusateri AE, et al. Freeze-dried plasma mitigates the dilution effects of a hemoglobin-based oxygen carrier (HBOC-201) in a model of resuscitation for hemorrhage and hemodilution. J Trauma Acute Care Surg. (2019) 87(1SSuppl.1):S83–90. doi: 10.1097/TA.0000000000002317
68. Malkevich NV, Dong F, Vandermolen CA, Philbin NB, Rice JP, Scultetus A, et al. Innate immune response after resuscitation with hemoglobin-based oxygen carrier and recombinant factor VIIA in uncontrolled hemorrhagic shock in a swine model. J Trauma. (2008) 64:1498–510. doi: 10.1097/TA.0b013e3181454a05
69. Marret E, Bonnin P, Mazoyer E, Riou B, Jacobs T, Coriat P, et al. The effects of a polymerized bovine-derived hemoglobin solution in a rabbit model of arterial thrombosis and bleeding. Anesthesia Analgesia. (2004) 98:73625. doi: 10.1213/01.ANE.0000099366.73625.DD
70. Philbin N, Rice J, Gurney J, McGwin G, Arnaud F, Dong F, et al. A hemoglobin-based oxygen carrier, bovine polymerized hemoglobin (HBOC-201) versus hetastarch (HEX) in a moderate severity hemorrhagic shock swine model with delayed evacuation. Resuscitation. (2005) 66:367–78. doi: 10.1016/j.resuscitation.2005.03.019
71. Kaplan LJ, Philbin N, Arnaud F, Rice J, Dong F, Freilich D. Resuscitation from hemorrhagic shock: fluid selection and infusion strategy drives unmeasured ion genesis. J Trauma. (2006) 61:4e. doi: 10.1097/01.ta.0000222578.85413.4e
72. Manning JE, Katz LM, Brownstein MR, Pearce LB, Gawryl MS, Baker CC. Bovine hemoglobin-based oxygen carrier (HBOC-201) for resuscitation of uncontrolled, exsanguinating liver injury in swine. Carolina Resuscitation Research Group. Shock. (2000) 13:152–9. doi: 10.1097/00024382-200013020-00010
73. Katz LM, Manning JE, McCurdy S, Pearce LB, Gawryl MS, Wang Y, et al. HBOC-201 improves survival in a swine model of hemorrhagic shock and liver injury. Resuscitation. (2002) 54:77–87. doi: 10.1016/S0300-9572(02)00053-9
74. Johnson T, Arnaud F, Dong F, Philbin N, Rice J, Asher L, et al. Bovine polymerized hemoglobin (hemoglobin-based oxygen carrier-201) resuscitation in three swine models of hemorrhagic shock with militarily relevant delayed evacuation–effects on histopathology and organ function. Crit Care Med. (2006) 34:1464–74. doi: 10.1097/01.CCM.0000215824.85190.89
75. Manning JE, Katz LM, Pearce LB, Batson DN, McCurdy SL, Gawryl MS, et al. Selective aortic arch perfusion with hemoglobin-based oxygen carrier-201 for resuscitation from exsanguinating cardiac arrest in swine. Crit Care Med. (2001) 29:2067–74. doi: 10.1097/00003246-200111000-00005
76. Manning JE, Batson DN, Gansman TW, Murphy CA, Perretta SG, Norfleet EA. Selective aortic arch perfusion using serial infusions of perflubron emulsion. Acad Emerg Med. (1997) 4:883–90. doi: 10.1111/j.1553-2712.1997.tb03814.x
77. Hoops HE, Manning JE, Graham TL, McCully BH, McCurdy SL, Ross JD. Selective aortic arch perfusion with fresh whole blood or HBOC-201 reverses hemorrhage-induced traumatic cardiac arrest in a lethal model of noncompressible torso hemorrhage. J Trauma Acute Care Surg. (2019) 87:263–73. doi: 10.1097/TA.0000000000002315
78. Rosenthal G, Morabito D, Cohen M, Roeytenberg A, Derugin N, Panter SS, et al. Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: laboratory investigation. J Neurosurg. (2008) 108:575–87. doi: 10.3171/JNS/2008/108/3/0575
79. Stern S, Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, et al. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock. (2009) 31:64–79. doi: 10.1097/SHK.0b013e3181778dc3
80. Rice J, Philbin N, Handrigan M, Hall C, McGwin G, Ahlers S, et al. Vasoactivity of bovine polymerized hemoglobin (HBOC-201) in swine with traumatic hemorrhagic shock with and without brain injury. J Trauma. (2006) 61:1085–99. doi: 10.1097/01.ta.0000236640.62893.fa
81. Teranishi K, Scultetus A, Haque A, Stern S, Philbin N, Rice J, et al. Traumatic brain injury and severe uncontrolled haemorrhage with short delay pre-hospital resuscitation in a swine model. Injury. (2012) 43:585–93. doi: 10.1016/j.injury.2010.09.042
82. White NJ, Wang X, Bradbury N, Moon-Massat PF, Freilich D, Auker C, et al. Fluid resuscitation of uncontrolled hemorrhage using a hemoglobin-based oxygen carrier: effect of traumatic brain injury. Shock. (2013) 39:210–9. doi: 10.1097/SHK.0b013e31827fd62b
83. Kerby JD, Sainz JG, Zhang F, Hutchings A, Sprague S, Farrokhi FR, et al. Resuscitation from hemorrhagic shock with HBOC-201 in the setting of traumatic brain injury. Shock. (2007) 27:652–6. doi: 10.1097/01.shk.0000248584.10400.dc
84. Caswell JE, Strange MB, Rimmer DM, Gibson MF, Cole P, Lefer DJ. A novel hemoglobin-based blood substitute protects against myocardial reperfusion injury. Am J Physiol. (2005) 288:H1796–801. doi: 10.1152/ajpheart.00905.2004
85. George I, Yi GH, Schulman AR, Morrow BT, Cheng Y, Gu A, et al. A polymerized bovine hemoglobin oxygen carrier preserves regional myocardial function and reduces infarct size after acute myocardial ischemia. Am J Physiol. (2006) 291:H1126–37. doi: 10.1152/ajpheart.00076.2006
86. Te Lintel Hekkert M, Dubé GP, Regar E, de Boer M, Vranckx P, van der Giessen WJ, et al. Preoxygenated hemoglobin-based oxygen carrier HBOC-201 annihilates myocardial ischemia during brief coronary artery occlusion in pigs. Am J Physiol. (2010) 298:H1103–13. doi: 10.1152/ajpheart.00667.2009
87. García-Ruiz JM, Galán-Arriola C, Fernández-Jiménez R, Aguero J, Sánchez-González J, García-Alvarez A, et al. Bloodless reperfusion with the oxygen carrier HBOC-201 in acute myocardial infarction: a novel platform for cardioprotective probes delivery. Basic Res Cardiol. (2017) 112:17. doi: 10.1007/s00395-017-0605-6
88. McNeil JD, Propper B, Walker J, Holguin L, Evans L, Lee K, et al. A bovine hemoglobin-based oxygen carrier as pump prime for cardiopulmonary bypass: reduced systemic lactic acidosis and improved cerebral oxygen metabolism during low flow in a porcine model. J Thorac Cardiovasc Surg. (2011) 142:411–7. doi: 10.1016/j.jtcvs.2010.11.017
89. York GB, DiGeronimo RJ, Wilson BJ, Cofer BR, Breuer CK, Josephs JD, et al. Extracorporeal membrane oxygenation in piglets using a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201). J Pediatr Surg. (2002) 37:1387–92. doi: 10.1053/jpsu.2002.35374
90. Henderson CL, Anderson CM, Sorrells DL, Wilson BJ, Dick EJ, DiGeronimo RJ. The use of a hemoglobin-based oxygen-carrying solution (HBOC-201) for extracorporeal membrane oxygenation in a porcine model with acute respiratory distress syndrome. Pediatr Crit Care Med. (2004) 5:384–90. doi: 10.1097/01.PCC.0000123544.46047.BA
91. Freilich D, Pearce LB, Pitman A, Greenburg G, Berzins M, Bebris L, et al. HBOC-201 vasoactivity in a phase III clinical trial in orthopedic surgery subjects–extrapolation of potential risk for acute trauma trials. J Trauma. (2009) 66:365–76. doi: 10.1097/TA.0b013e3181820d5c
92. Schlegel A, de Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. (2013) 58:278–86. doi: 10.1016/j.jhep.2012.10.004
93. Muralidharan V, Christophi C. Hyperbaric oxygen therapy and liver transplantation. HPB. (2007) 9:174–82. doi: 10.1080/13651820601175926
94. Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg. (2015) 262:1473. doi: 10.1097/SLA.0000000000001473
95. Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MTPR, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. (2016) 16:1779–87. doi: 10.1111/ajt.13708
96. Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. (1996) 334:1685–90. doi: 10.1056/NEJM199606273342601
97. Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. (2015) 15:381–94. doi: 10.1111/ajt.12991
98. Bhattacharjee RN, Patel SVB, Sun Q, Jiang L, Richard-Mohamed M, Ruthirakanthan A, et al. Renal protection against ischemia reperfusion injury: hemoglobin-based oxygen carrier-201 versus blood as an oxygen carrier in ex vivo subnormothermic machine perfusion. Transplantation. (2020) 104:482–9. doi: 10.1097/TP.0000000000002967
99. Laing RW, Bhogal RH, Wallace L, Boteon Y, Neil DAH, Smith A, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation. (2017) 101:2746–56. doi: 10.1097/TP.0000000000001821
100. Matton APM, Burlage LC, van Rijn R, de Vries Y, Karangwa SA, Nijsten MW, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. (2018) 24:528–38. doi: 10.1002/lt.25005
101. Aburawi MM, Fontan FM, Karimian N, Eymard C, Cronin S, Pendexter C, et al. Synthetic hemoglobin-based oxygen carriers are an acceptable alternative for packed red blood cells in normothermic kidney perfusion. Am J Transplant. (2019) 19:2814–24. doi: 10.1111/ajt.15375
102. Said SA, Ordeñana CX, Rezaei M, Figueroa BA, Dasarathy S, Brunengraber H, et al. Ex-vivo normothermic limb perfusion with a hemoglobin-based oxygen carrier perfusate. Military Med. (2020) 185(Suppl.1):110–20. doi: 10.1093/milmed/usz314
103. Mahboub P, Aburawi M, Karimian N, Lin F, Karabacak M, Fontan F, et al. The efficacy of HBOC-201 in ex situ gradual rewarming kidney perfusion in a rat model. Artif Organs. (2020) 44:81–90. doi: 10.1111/aor.13534
104. Loscalzo J. Nitric oxide binding and the adverse effects of cell-free hemoglobins: what makes us different from earthworms. J Lab Clin Med. (1997) 129:580–3. doi: 10.1016/S0022-2143(97)90191-8
105. Schultz SC, Grady B, Cole F, Hamilton I, Burhop K, Malcolm DS, et al. role for endothelin and nitric oxide in the pressor response to diaspirin cross-linked hemoglobin. J Lab Clin Med. (1993) 122:301–8.
106. Gulati A, Rebello S. Role of adrenergic mechanisms in the pressor effect of diaspirin cross-linked hemoglobin. J Lab Clin Med. (1994) 124:125–33.
107. Rivera-Chavez FA, Huerta S, Brown R, York GB, Minei JP. Resuscitation from hemorrhagic shock comparing standard hemoglobin-based oxygen carrier (HBOC)-201 versus 75% hypertonic HBOC-201. J Trauma. (2007) 63:1113–9. doi: 10.1097/TA.0b013e3181561157
108. Rivera-Chávez FA, Lu A, Liu MM, Abdalla A, Minei JP. Hypertonic HBOC-201 decreases neutrophil activation after hemorrhagic shock. J Invest Surg. (2014) 27:14–20. doi: 10.3109/08941939.2013.826756
109. Moon-Massat P, Scultetus A, Arnaud F, Brown A, Haque A, Saha B, et al. The effect HBOC-201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury. (2012) 43:638–47. doi: 10.1016/j.injury.2010.10.013
110. Fonseca V, Avizinis J, Moon-Massat P, Freilich D, Kim HW, Hai C-M. Differential sensitivities of pulmonary and coronary arteries to hemoglobin-based oxygen carriers and nitrovasodilators: study in a bovine ex vivo model of vascular strips. Vascul Pharmacol. (2010) 52:215–23. doi: 10.1016/j.vph.2009.12.005
111. Arnaud F, Scultetus AH, Haque A, Saha B, Kim B, Auker C, et al. Sodium nitroprusside ameliorates systemic but not pulmonary HBOC-201-induced vasoconstriction: an exploratory study in a swine controlled haemorrhage model. Resuscitation. (2012) 83:1038–45. doi: 10.1016/j.resuscitation.2012.01.018
Keywords: HBOC-201, red blood cell, oxygen-carrying capacities, oxygen bridge, clinical settings
Citation: Cao M, Zhao Y, He H, Yue R, Pan L, Hu H, Ren Y, Qin Q, Yi X, Yin T, Ma L, Zhang D and Huang X (2021) New Applications of HBOC-201: A 25-Year Review of the Literature. Front. Med. 8:794561. doi: 10.3389/fmed.2021.794561
Received: 13 October 2021; Accepted: 05 November 2021;
Published: 08 December 2021.
Edited by:
Björn Tampe, University Medical Center Göttingen, GermanyReviewed by:
Hiromi Sakai, Nara Medical University, JapanThorsten Perl, University of Göttingen, Germany
Copyright © 2021 Cao, Zhao, He, Yue, Pan, Hu, Ren, Qin, Yi, Yin, Ma, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Huang, ZHJodWFuZ3hiQDE2My5jb20=; Dingding Zhang, emhhbmdkZDI1QDEyNi5jb20=
 Min Cao
Min Cao Yong Zhao2
Yong Zhao2 Hongli He
Hongli He Ruiming Yue
Ruiming Yue