- 1Rheumatology Clinic, Department of Medicine, University of Udine, c/o Azienda Sanitaria Universitaria Friuli Centrale, Udine, Italy
- 2Department of Rheumatology and Immunology, Medical University of Graz, Graz, Austria
- 3Department of Rheumatology, Hospital of Bruneck, Bruneck, Italy
- 4Leeds Institute of Rheumatic and Musculoskeletal Medicine (LIRMM), University of Leeds, Leeds, United Kingdom
- 5Department of Medical and Biological Sciences, Institute of Dermatology, University of Udine, Udine, Italy
Psoriasis (PsO) and Psoriatic Arthritis (PsA) are chronic, immune-mediated diseases that share common etiopathogenetic pathways. Up to 30% of PsO patient may later develop PsA. In nearly 75% of cases, skin psoriatic lesions precede arthritic symptoms, typically 10 years prior to the onset of joint symptoms, while PsO diagnosis occurring after the onset of arthritis is described only in 15% of cases. Therefore, skin involvement offers to the rheumatologist a unique opportunity to study PsA in a very early phase, having a cohort of psoriatic “risk patients” that may develop the disease and may benefit from preventive treatment. Progression from PsO to PsA is often characterized by non-specific musculoskeletal symptoms, subclinical synovio-entheseal inflammation, and occasionally asymptomatic digital swelling such as painless toe dactylitis, that frequently go unnoticed, leading to diagnostic delay. The early diagnosis of PsA is crucial for initiating a treatment prior the development of significant and permanent joint damage. With the ongoing development of pharmacological treatments, early interception of PsA has become a priority, but many obstacles have been reported in daily routine. The introduction of digital technology in rheumatology may fill the gap in the physician-patient relationship, allowing more targeted monitoring of PsO patients. Digital technology includes telemedicine, virtual visits, electronic health record, wearable technology, mobile health, artificial intelligence, and machine learning. Overall, this digital revolution could lead to earlier PsA diagnosis, improved follow-up and disease control as well as maximizing the referral capacity of rheumatic centers.
Introduction
Psoriasis (PsO) is a chronic, immune-mediated disease affecting 2–3% of the Caucasian population (1). Up to one third of PsO patients will eventually progress to Psoriatic Arthritis (PsA) [from 6 to 42% (2)], a chronic, inflammatory and potentially debilitating arthropathy (3). In nearly 75% of cases, psoriatic skin lesions precede arthritic symptoms, with an onset typically 10 years prior to joint symptoms, while PsO diagnosis occurring after the onset of arthritis is described only in 15% of PsA cases (4).
Progression from PsO to PsA most commonly evolves across several clinically silent stages (5), as well as the prodromal phase characterized by a short period of arthralgia and fatigue despite not having synovitis or enthesitis on physical examination, as reported by Scher and Zabotti (5–7). This gives the rheumatologist the unique window of opportunity to study PsA in a very early phase, having a cohort of psoriatic “risk patients” that may develop the disease and may benefit from preventive treatment. Severe PsO, nail involvement, familiarity for PsA, arthralgia and subclinical inflammation detected by imaging are considered predictors of PsA development in PsO patients (4, 7–10).
Early diagnosis of PsA is crucial for initiating a treatment prior the development of significant and permanent joint damage (11). The advances in PsA treatment in the latest decades have demonstrated a positive effect on prognosis and disability particularly in the initial phase of the disease, whereas diagnostic delay is associated with poorer outcome (12, 13). It is therefore crucial to identify PsO subjects at higher risk of developing PsA, who may benefit from early diagnosis and treatment, while there is a growing interest for preventive treatment.
Unfortunately, in daily routine of various health care systems around the world, the possibilities for the creation of a predictive models of PsA development are limited, and many obstacles have been reported (14).
The transition from PsO to PsA may go unnoticed or be undervalued, leading to a diagnostic delay and a poor window of treatment opportunity (13, 15). Furthermore, the lack of an adequate number of specialists further worsens access to rheumatologic services (16, 17), not only for diagnostic purposes, but also for follow-up. The visit frequency is so crucial for the management of the rheumatic disease, that EULAR treatment recommendations delineate the timing of the scheduled visits (18). In the course of rheumatic diseases, an adequate amount of monitoring visits for the assessment of disease activity is still challenging, even if frequent patient monitoring is an integral part of the treat-to-target strategy. In fact, frequent rheumatological visits are associated with improved outcomes (19), but due to the relapsing-remitting nature of the disease, an immediate rheumatological assessment in case of an acute exacerbation would be desirable. In practice, however, this is rarely feasible, and patients may be seen by the rheumatologist only after the disappearance of symptoms.
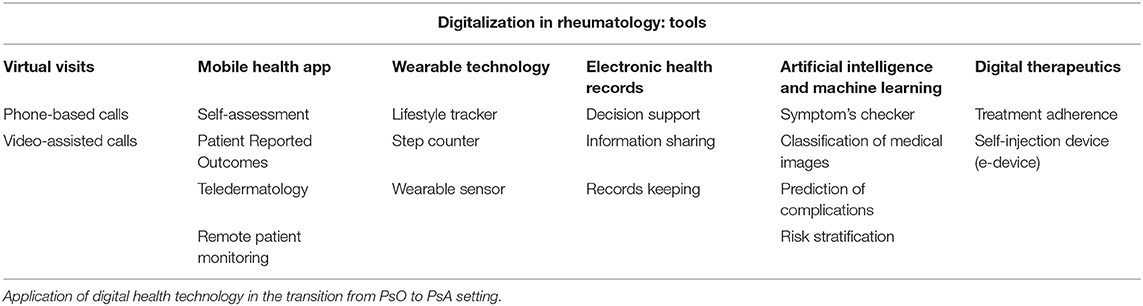
Additionally, the COVID-19 pandemic has led to radical changes in the management of rheumatic patients. The tangible risk of infection leads to a prolongation of monitoring intervals. During the first wave of the pandemic, some visits were postponed or canceled, with repercussions on patients' health, while others were replaced by telemedicine, in the form of phone calls and teleconferences (20, 21). In this scenario, the implementation of digitalization in rheumatology may represent an opportunity both for clinicians and patients (both PsO and PsA patients), as the technology may fill the gap between the demand for frequent monitoring visit and the limited resources to guarantee them. More details in Table 1.
The aim of our review is to present insights on the digital approach in rheumatology, focusing on early diagnosis and follow-up in PsA.
The Digital Approach Applied to Psoriatic Disease
In recent years, an extensive innovation in digitalization in rheumatology has occurred. The World Health Organization (WHO) defined “e-Health”, a collective term defined as “use of information and communications technology in support of health and health-related fields“ (14). This digital revolution included electronic health records, telemedicine and virtual visits, wearable technology, mobile health, artificial intelligence (AI), and machine learning.
The challenges in the development of digital health technologies are considerable, but the increased availability of these technologies offers the opportunity for improving clinical practice. These digital approaches have already been tested in rheumatology and may have great potential in the management of the transition phase of PsO to PsA. According to the author's experience, PsO patient can be stratified according to the increasing risk of developing PsA in three phases:
(i) PsO patients at lower risk of developing PsA: patients not presenting any known risk factors for progression to PsA.
(ii) PsO patients at higher risk of developing PsA in a medium/long-term: patients presenting known risk factors or predictors for progression to PsA, such as severe skin disease, nail involvement, familiarity for PsA (4, 8–10).
(iii) PsO patients at higher and imminent risk of developing PsA: patients presenting musculoskeletal complains suspicious for prodromal PsA, such as arthralgia, fatigue or Achilles' tendon pain (5, 7, 22).
A more comprehensive explanation of these group is addressed in the paragraph below (Figure 1).
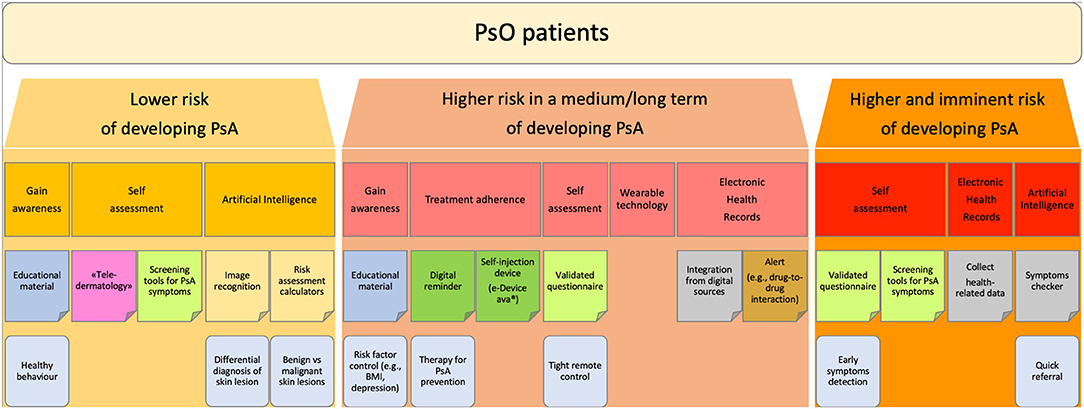
Figure 1. Flowchart: stratification of PsO patients. Proposed stratification of PsO patients according to the increasing risk of developing PsA.
The digitalization technology may offer a diversified set of digital tools which could offer new opportunities in the various PsO-to-PsA journey.
PsO Patients at Lower Risk of Developing PsA
PsO patients with a lower risk of PsA represent the majority of PsO cases. They are usually not followed by dermatologists since they often present only with mild PsO treated with topic agents prescribed by the general practitioner (23–25). These patients nevertheless have a higher risk to develop PsA as compared to the general population (1).
PsO patients have to gain awareness of their own inflammatory disease and the comorbidities related to PsO. In fact, patients affected by PsO, even in the case of minimal skin lesions, exhibit not only an increased risk of developing PsA, but also display an higher risk of cardiometabolic disease, and endocrinological disease, such as diabetes mellitus (26–28). Therefore, the creation and distribution of educational material as well as awareness-raising campaigns, aiming to inform the patient about their own disease, spreading knowledge about the risks of developing PsA, promoting healthy behavior, and encouraging them to take health-related actions are highly beneficial (29). Informative campaign has already demonstrated efficacy in the management of rheumatological disease like gout (30–32), a known independent risk factor for PsA (33).
For these cases, digitalization may provide solutions. Digital “teledermatology” tools have been tested in dermatology for the self-assessment of psoriatic lesions. As proposed by Schreier et al., a smartphone app can be used by PsO patients with the aim of reporting active psoriatic lesions through pictures, leading to similar results as a face-to-face visits in the Psoriasis Area and Severity Index (PASI) and body surface area (BSA) assessment (34, 35). The self-assessment and monitoring of psoriatic lesions done by pilot teledermatology studies (36) may open the path to a remote monitoring of PsO patients who are not frequently evaluated by dermatologists, such as patients affected by minimal psoriasis, or mild psoriatic onychopathy.
The right moment when a rheumatologist should first see a PsO patient is still unclear. For PsO patients with a lower risk of PsA development, screening tools for PsA symptoms would be helpful for dermatologists and other physicians to better define the time point for a rheumatology visit. In axial spondyloarthritis (axSpA) several screening tools have already been proposed, such as the Berlin referral tool (37), which has shown to increase the probability of axSpA from 5% in patients with unselected chronic low back to 30–45% in case the tool yielded a positive result. Furthermore, screening for axSpA can also be performed online by patients with lower back pain increasing the proportion of positive axSpA from 5% (prevalence of the general population) to 19.4% (after triage with the online tool) (38). The recognition of patient with high probability of axial disease is of particular interest, because it may reduce the diagnostic delay in the diagnosis of axial involvement in PsA (39). Similar online screening tools could also be developed for PsO patients.
In the lower-risk subgroup of PsO patients, AI may also be helpful. The general aim of these technologies in health care is to assist clinical decision-making using computerized algorithms to uncover relevant information from big data (40), but also by learning from many sources (rather than being programmed with rules) including clinical, biological, and radiological data (41). At present, some pilot studies are testing machine learning in the dermatologic field, in particular in the analysis and classification of medical images, and prediction of complications (42). Other AI-based tools such as risk assessment calculators are becoming increasingly available, mostly focusing on differentiating benign and malignant skin lesions (43). Furthermore, Aggarwal (44) applied AI-machine learning for image recognition of various dermatological diseases, including PsO, reporting promising first results.
AI and machine learning have been of great interest not only in dermatology, but also in rheumatology. Guidelines for AI studies in rheumatology have been proposed by EULAR (45), and AI is currently being researched concerning its value for diagnosis, disease prediction, risk stratification, and monitoring of rheumatic diseases (46).
PsO Patients at Higher Medium/Long-Term Risk of Developing PsA
PsO patients at higher risk of developing PsA in a medium/long-term are patients presenting with known risk factors or predictors for development to PsA. The identification of risk factors for PsA development in PsO patients is considered an unmet need in the EULAR recommendations (47).
In the years, the early diagnosis of PsA in a dermatological setting raised a lot of interest. Therefore, various screening tool for early identification of patients with PsA have been tested and validated, such as the Psoriasis Epidemiology Screening Tool (PEST) (48) and the Early ARthritis for Psoriatic patients (EARP) questionnaire (49), while no predictive models of PsA development are available. A recent systematic literature review by Zabotti et al (10) supports the predictive value of various factors such severity of cutaneous involvement, psoriatic onychopathy, imaging abnormalities of subclinical synovio-entheseal inflammation, and comorbidities such as obesity or depression. If feasible, early detection and systemic treatment of these factors might reduce the PsA development. Therefore, PsO patients need to gain awareness of their disease and learn that modifying potential risk factors, such as obesity, might decrease the probability of developing PsA. The digitalization may play a role in this educational purpose. In fact, physicians and scientific societies may support public platforms for dissemination of reliable health information.
The transition phase from PsO to PsA provides a unique opportunity for early intervention (and possibly even prevention) in a population at higher risk of developing arthritis, in which a rheumatological monitoring or disease interception with therapy directed at PsO, but also PsA could be envisaged (7). The growing number of biological drugs and other molecules that act both on skin and joints in psoriatic disease might give the possibility to prevent arthritis evolution (25). It is described in literature that a very early disease interception and the appropriate psoriasis treatment may lead to the decline in skin symptoms, pain, and subclinical inflammation, as reported in the IVEPSA study (50, 51).
The widespread use of biologic therapy available for PsO may reduce the incidence of PsA. The idea to prevent PsA by early interference with the process of psoriatic disease is both fascinating and challenging, because the feasibility of such a concept mainly depends on patient selection (i.e., those with highest risk, with awareness of the at-risk situation and with sufficient compliance), and in treatment choice given that therapy should interfere with the immunological processes that promote the transition from PsO to PsA.
Digital technology could also play a role in the assessment of treatment adherence in patients with severe PsO. Recent studies suggest that apart from a one-time education on disease and therapeutics, continuing information and support may lead to better drug-adherence. Telehealth measures have shown to be beneficial for drug-adherence in patients with osteoporosis and rheumatoid arthritis (RA) (52, 53), when delivered by members of the health care team. Digital reminders, via applications or websites, may be a more feasible alternative to phone calls or mail and could show similar efficacy, especially when well designed and integrating offers such as social support sections or gamification elements (54). Of interest is the recently developed reusable electromechanical self-injection device (e-Device ava®) for treatment administration of certolizumab (55). Tailoring self-injection devices to individual patient preference may improve adherence to treatment and help the patients in remembering the date of self-injections (55, 56). Furthermore, e-Device aims to minimize the patient needle phobia, and the concern about safety in the treatment administration, giving the patient an electronic device that provide the administration (57). Some patients reported a higher satisfaction, self-confidence, safety, and feasibility of e-Device, compared with pre-filled pen and syringes (55, 58, 59). The use of e-Device could both assess the adherence to treatment, enable patients to track their own data, perform self-assessments, and deliver questionnaires. Ideally, e-Device would be connected with smartphones and would ask patients to complete validated questionnaires (such as patient reported outcomes (PROs), health assessment questionnaires, self-evaluation) at certain time points (such as after 1, 3 and 6 months after treatment initiation), ensuring a remote follow-up. The enthusiasm toward the use of mobile and wireless technology to support the health objective is however overshadowed by the fact that most of the available smartphone applications lack quality and scientific accuracy (60–62).
In literature, there are reports about the correlation of higher patient involvement with better adherence to therapy (63). Besides, the patient's perspective of disease state and burden is increasingly recognized as fundamental for a satisfying treatment (64). PROs are subjective measures (65) that can facilitate the assessment of physical and psychological functioning. The role of PROs is to capture patient's perspective, to provide a complete picture of the disease and, when used effectively, to aid to the holistic management of PsO patients (66). Creating applications that both patients and physicians can use to collect validated outcome parameters, such as PROs, clinical and laboratory markers is a big opportunity to improve care. An obvious advantage is a deeper involvement and understanding of patients in their treatment plan. A modern program with an easy-to-use interface, may further simplify the patient visit altogether. Patients with diabetes mellitus nowadays have blood sugar sensors that send data directly to a cloud-based application that can be seen by a physician anytime by logging into a website. Diagrams such as line-charts for disease activity outcomes, with visual information when treatment changes were performed and would be a great way to facilitate a rheumatological visit and have enormous advantages for data collection for research purposes.
The use of step counters and smartwatches may also introduce health benefits. Wearable technology includes various devices (step counters, sleep monitors etc.) and some wearable sensors have already been experimented to monitor energy consumption, step count and hand mobility. Thus, they could be used by patients and clinicians for diagnosis, follow-up and self-monitoring (67, 68). The majority of studies are on RA, but this technology could be also implemented in the management of PsA and spondylarthritis patients, as well (69). The obtained data could be integrated in the patient health record, aiming to assist clinicians in the decision-making (70). At present, this technology is integrated into smartphones, smartwatches, and other devices (such as an electromechanical self-injection devices). It needs to be simple enough for patients to be used in their daily routine, thus the design usually exploits wireless or Bluetooth technology to share information between sensors and devices. In PsO, remote questionnaires may offer a tight control, promote patient education, help identify psoriasis patients at higher risk of developing arthritis, and thus increase the number of early PsA diagnoses.
In the Italian city of Udine, we created a project, called PSOART. It was designed to guarantee a tight control and follow-up of PsO patients. It contributes to the identification of subjects at increased risk of developing arthritis through the use of questionnaires and self-evaluation. The idea behind PSOART was to empower the patients: they become the main character in their journey and not only a passive spectator of their own disease. Furthermore, PSOART may reduce the diagnostic delay and maximize the “capacity” of the rheumatologic center in screening for predictors of psoriatic arthritis development. The patient periodic assessment remains crucial and the PSOART questionnaire may be beneficial in this regard.
To make PSOART easy to use and feasible, the questionnaire was digitalized for use on internet platforms and smartphone applications. The questionnaire and self-evaluation chosen were validated in the literature (such as, PSAID, BASDAI, HAQ questionnaire, Visual Analog Scale for Pain etc.) and adapted to the digital format. The patient will login to the platform and complete the self-assessment questionnaires at pre-established time points (baseline, 1, 3, 6 months etc.) over the span of 3 years and share the results with the physician (Figure 2).
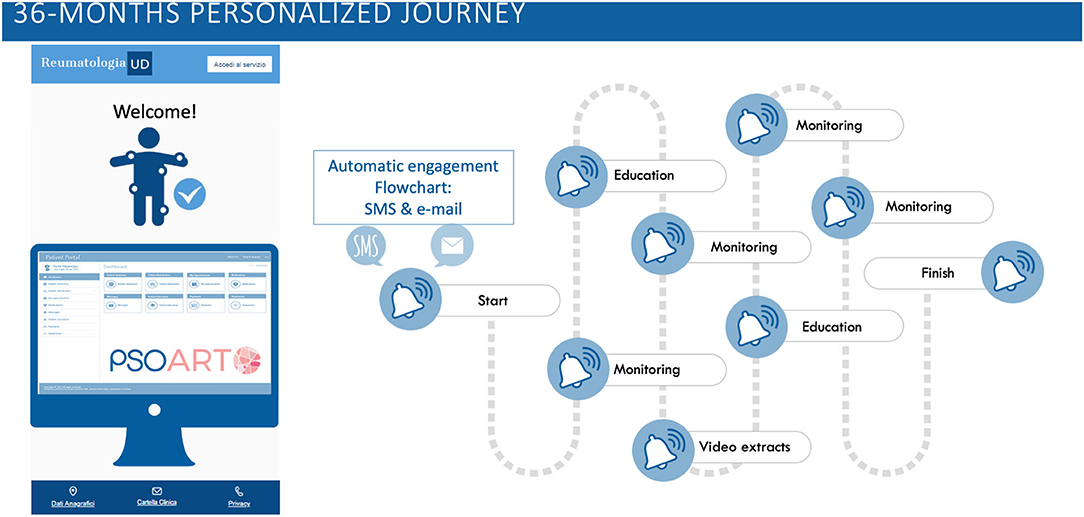
Figure 2. PSOART personalized journey. The 36-months patient-targeted journey is hereby represented. The PSOART platform uses an automatic email/sms communication system targeted on the patient. It offers encouraging goal, such as educational material and video extracts, as well as “alert” for the rheumatologic center [https://www.psoart.com].
During the patient journey, the platform will also provide educational and informational material, such as video-interviews and written articles, regarding lifestyle, tips on disease management and details about treatments. The educational material will be evaluated and approved by clinicians and aims to minimize “fake news” in rheumatology. The PSOART platform allows remote monitoring and disease interception in patients affected by PsO and seems to be well accepted by patients, as suggested by the positive comments and feedbacks. Hopefully, PSOART would not only optimize the patient follow-up, but also allow the creation of a predictive score for the development of PsA, opening the way to a possible prevention of PsA.
In addition, electronic health records (EHR), the digital version of a patient's paper chart, could ideally become the standard of practice for record keeping. EHR has the potential to help in the management of chronic and heterogeneous disease. EHR could alert dangerous drug-to-drug interactions or help with imaging assessment, depending on the disease and guidelines. In the USA, some researchers used EHR to alert clinicians that a patient using an immunosuppressive drug should receive an influenza vaccine. This intervention increased the influenza vaccination rate from 47 to 65% (71). Furthermore, EHR could integrate and organize information from other digital sources, such as wearable devices and apps, providing important opportunities to collect data for research purposes.
PsO Patients at Higher and Imminent Risk of Developing Arthritis
PsO patients at higher and imminent risk of developing PsA are patients presenting musculoskeletal complaints (e.g., arthralgia, achilles' tendon pain) or fatigue (5, 7, 22). In line with this, Eder et al., showed that arthralgia in psoriasis females is a strong predictor of PsA development (9), and Zabotti et al., described that psoriatic patients with arthralgia (PsOAr) were more prone to develop PsA compared to psoriatic patients without musculoskeletal complaints (PsO) (7). These data highlight that patient-reported symptoms in the preclinical phases of PsA may be a marker of imminent PsA development. However, the prodromal phase is difficult to define due to non-specific musculoskeletal symptoms (such as joint pain, fatigue and stiffness) that can also be caused by other disease mimicking PsA (such as concomitant osteoarthritis, fibromyalgia, or chronic pain (6, 72, 73). Therefore, remote monitoring and self-assessment cannot replace face-to-face visits. While monitoring visits using telehealth revealed promising results in patients with longstanding rheumatological diseases, the detection of definite signs of prodromal PsA requires a face-to-face visit. A clinical exam is necessary to assess PsA risk factors including tender and swollen joint counts. While tenderness can be assessed by patients themselves quite well, studies show discrepancies between the number of swollen joints assessed by patients and rheumatologists (74, 75).
Moreover, the in-person visit are viewed by some clinicians as a method for ensuring compliance to treatment and generate confidentiality of the doctor-patient relationship (76). The loss of physical contact and the difficult emotional relationship between clinicians and patients have been reported as the major limitation of telemedicine (77, 78). Upcoming EULAR points to consider on remote care do therefore reinforce that telemedicine should be seen as an additional tool, rather than a substitute for a face-to-face visit.
Digitalization may contribute to the self-assessment, allowing patients to quickly report the onset of symptoms, assumed joint swelling, and other changes (such as dactylitis). PROs for inflammatory arthritis have been developed and validated to correlate with clinical measures of disease activity (79). The assessment of web-based PROs and paper-based PROs in psoriatic arthritis proved to be comparable (80), opening the way to patient self-monitoring and in particular to a remote collection of questionnaire (81). Besides, some studies describe a good feasibility for remote monitoring of skin disease in PsO patients (36) and for patient education (82). Therefore, patients with chronic conditions may take advantage in self-management, to bring the patient in the center of his own journey. The patient role in the improvement of the health quality is crucial, in fact people living with rheumatic and musculoskeletal diseases may highlight the most important symptoms, making a difference for disease management. With this aim, digital technology in the form of programs and devices promotes healthy behavior and encourage patients to take health-related actions (29). Theoretically, the spreading and accessibility of digital technology may enable researchers to collect health-related data more frequently and may help to build-up patient networks in communities. The creation of such digital platforms, however, is still challenging (29).
Recurrent self-assessment could be crucial to deliver remote questionnaire and record data by applications. Frequent patient monitoring is an integral part of the treat-to-target strategy enabling early detection of disease flares. Telemedicine and remote monitoring may be convenient for many patients with impaired mobility and may reduce the number of visits to the rheumatologist office. The remote self-assessment would also lead to identify and screen PsO patients at risk of developing arthritis, targeting an early identification of early onset of musculoskeletal (MSK) pain such as suspected arthralgia. Subsequently, the patient could be evaluated by the rheumatologists in the setting of “telerheumatology”. The target would be to screen the MSK symptoms and differentiate mechanical from inflammatory pain. It is also necessary to take care of the axial involvement in PsA, a frequent and underdiagnosed feature (39), unlikely assessable in a remote visit. Therefore, in the setting of a suspected PsA, the rheumatologists could recommend both blood chemistry tests and imaging evaluation even before the clinical assessment, aiming to an accurate and early diagnosis in the first face-to-face medical appointment.
In the setting of patient at higher and imminent risk of developing PsA, AI-based tools (symptom checkers) are designed to collect patient symptoms, determine possible causes and direct the patient to the right specialist. In rheumatology, symptoms checkers could ensure a quick referral to the specialists and reduce diagnostic delay (14). Recently, a preliminary smartphone sensors-based measurement tool was tested in PsA (83). The smartphone gyroscope, accelerometer and camera were used to assess the arm joint function, the leg joint function, and the dactylitis and nail involvement, respectively. The authors reported that the application could distinguish some clinical features of PsA that might be helpful to early detect high risk factors for PsA development.
AI has also been tested in RA for prediction of disease progression, flares and mortality (84, 85), and in osteoarthritis for image recognition, helping in the interpretation of musculoskeletal pathologies (86). These tools are still rudimentary, nonetheless they have the potential to assist clinicians in therapeutic decisions. For an harmonization of digital apps, EULAR has recently published points to consider for the development, evaluation and implementation of mobile health applications (87), aiming to provide guidance and guidelines on important aspects of self-management in patient with rheumatic and musculoskeletal diseases (88). Although digitalization in rheumatology has great potential, attention should be paid to health equity, as the most vulnerable patients may lack the resources required for a telemedicine visit or remote self-assessment devices (89), and to the risk of medicalization when applying digital health instruments in a heterogeneous disease (90).
Conclusion
The transition phases from PsO to PsA require better characterization. Thus, a large amount of data from rheumatological services is needed to perform epidemiologic analysis. Collecting PROs via digital support provides a new tool for the monitoring of psoriatic disease. Moreover, the assessment of PROs via an online application may allow to intercept PsO patients at increased risk of transition to PsA, who otherwise would go unnoticed.
Modern models of care for PsO and PsA highlight the importance of patient involvement and self-management (91). In this scenario, integrating digital health technology will offer opportunities to complement rheumatology care, even beyond a global pandemic.
Author Contributions
IG and AZ contributed to conception and design of the study. IG wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. (2009) 361:496–509. doi: 10.1056/NEJMra0804595
2. Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. (2019) 80:251–65.e19. doi: 10.1016/j.jaad.2018.06.027
3. Ritchlin C. Psoriatic disease—from skin to bone. Nat Clin Pract Rheumatol. (2007) 3:698–706. doi: 10.1038/ncprheum0670
4. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. (2005) 64:ii14–7. doi: 10.1136/ard.2004.032482
5. Zabotti A, Tinazzi I, Aydin SZ, McGonagle D. From psoriasis to psoriatic arthritis: insights from imaging on the transition to psoriatic arthritis and implications for arthritis prevention. Curr Rheumatol Rep. (2020) 22:24. doi: 10.1007/s11926-020-00891-x
6. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. (2019) 15:153–66. doi: 10.1038/s41584-019-0175-0
7. Zabotti A, McGonagle DG, Giovannini I, Errichetti E, Zuliani F, Zanetti A, et al. Transition phase towards psoriatic arthritis: clinical and ultrasonographic characterisation of psoriatic arthralgia. RMD Open. (2019) 5:e001067. doi: 10.1136/rmdopen-2019-001067
8. Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. (2009) 61:233–9. doi: 10.1002/art.24172
9. Eder L, Polachek A, Rosen CF, Chandran V, Cook R, Gladman DD. The development of psoriatic arthritis in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms: a prospective cohort study. Arthritis Rheumatol. (2017) 69:622–9. doi: 10.1002/art.39973
10. Zabotti A, De Lucia O, Sakellariou G, Batticciotto A, Cincinelli G, Giovannini I, et al. Predictors, risk factors, and incidence rates of psoriatic arthritis development in psoriasis patients: a systematic literature review and meta-analysis. Rheumatol Ther. (2021). Available from: doi: 10.1007/s40744-021-00378-w (cited Oct 1, 2020).
11. Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. (2017) 76:21–37. doi: 10.1016/j.jaut.2016.10.009
12. Karmacharya P, Wright K, Achenbach SJ, Bekele D, Crowson CS, Ogdie A, et al. Diagnostic delay in psoriatic arthritis: a population-based study. J Rheumatol. (2021) 48:1410–6. doi: 10.3899/jrheum.201199
13. Sørensen J, Hetland ML. All departments of rheumatology in Denmark. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis. (2015) 74:e12. doi: 10.1136/annrheumdis-2013-204867
14. Fagni F, Knitza J, Krusche M, Kleyer A, Tascilar K, Simon D. Digital approaches for a reliable early diagnosis of psoriatic arthritis. Front Med. (2021) 8:1233. doi: 10.3389/fmed.2021.718922
15. Stack RJ, Nightingale P, Jinks C, Shaw K, Herron-Marx S, Horne R, et al. Delays between the onset of symptoms and first rheumatology consultation in patients with rheumatoid arthritis in the UK: an observational study. BMJ Open. (2019) 9:e024361. doi: 10.1136/bmjopen-2018-024361
16. Zink A, Schneider M. Versorgungsplanung in der Rheumatologie: Eckpunkte aus dem 2016 aktualisierten Memorandum der Deutschen Gesellschaft für Rheumatologie zur Versorgungsqualität. Aktuelle Rheumatologie. (2018) 43:390–4. doi: 10.1055/a-0573-8722
17. Unger J, Putrik P, Buttgereit F, Aletaha D, Bianchi G, Bijlsma JWJ, et al. Workforce requirements in rheumatology: a systematic literature review informing the development of a workforce prediction risk of bias tool and the EULAR points to consider. RMD Open. (2018) 4:e000756. doi: 10.1136/rmdopen-2018-000756
18. Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. (2014) 73:492–509. doi: 10.1136/annrheumdis-2013-204573
19. Ward MM. Rheumatology visit frequency and changes in functional disability and pain in patients with rheumatoid arthritis. J Rheumatol. (1997) 24:35–42.
20. Dejaco C, Alunno A, Bijlsma JW, Boonen A, Combe B, Finckh A, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis. (2021) 80:518–26. doi: 10.1136/annrheumdis-2020-218697
21. George MD, Venkatachalam S, Banerjee S, Baker JF, Merkel PA, Gavigan K, et al. Concerns, healthcare use, and treatment interruptions in patients with common autoimmune rheumatic diseases during the COVID-19 pandemic. J Rheumatol. (2021) 48:603–7. doi: 10.3899/jrheum.201017
22. Eder L, Haddad A, Rosen CF, Lee K-A, Chandran V, Cook R, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. (2016) 68:915–23. doi: 10.1002/art.39494
23. Costa-Silva M, Vide J, Lopes S, Azevedo F, Magina S. Psoriasis and comorbidities: general practitioners' awareness. Acta Dermatovenerol Alp Pannonica Adriat. (2018) 27:5–7. doi: 10.15570/actaapa.2018.2
24. Brandon A, Mufti A, Gary Sibbald R. Diagnosis and management of cutaneous psoriasis: a review. Adv Skin Wound Care. (2019) 32:58–69. doi: 10.1097/01.ASW.0000550592.08674.43
25. Savage L, Tinazzi I, Zabotti A, Laws PM, Wittmann M, McGonagle D. Defining pre-clinical psoriatic arthritis in an integrated dermato-rheumatology environment. J Clin Med. (2020) 9:3262. doi: 10.3390/jcm9103262
26. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. (2013) 2:e000062. doi: 10.1161/JAHA.113.000062
27. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases part I. Epidemiol J Am Acad Dermatol. (2017) 76:377–90. doi: 10.1016/j.jaad.2016.07.064
29. Nowell WB, Curtis D, Thai M, Wiedmeyer C, Gavigan K, Venkatachalam S, et al. Digital interventions to build a patient registry for rheumatology research. Rheum Dis Clin North Am. (2019) 45:173–86. doi: 10.1016/j.rdc.2019.01.009
30. Kakutani-Hatayama M, Kadoya M, Okazaki H, Kurajoh M, Shoji T, Koyama H, et al. Nonpharmacological management of gout and hyperuricemia: hints for better lifestyle. Am J Lifestyle Med. (2017) 11:321–9. doi: 10.1177/1559827615601973
31. Yokose C, McCormick N, Choi HK. The role of diet in hyperuricemia and gout. Curr Opin Rheumatol. (2021) 33:135–44. doi: 10.1097/BOR.0000000000000779
32. Doherty M, Jenkins W, Richardson H, Sarmanova A, Abhishek A, Ashton D, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet. (2018) 392:1403–12. doi: 10.1016/S0140-6736(18)32158-5
33. Tsuruta N, Imafuku S, Narisawa Y. Hyperuricemia is an independent risk factor for psoriatic arthritis in psoriatic patients. J Dermatol. (2017) 44:1349–52. doi: 10.1111/1346-8138.13968
34. Armstrong AW, Chambers CJ, Maverakis E, Cheng MY, Dunnick CA, Chren M-M, et al. Effectiveness of online vs in-person care for adults with psoriasis. JAMA Netw Open. (2018) 1:e183062. doi: 10.1001/jamanetworkopen.2018.3062
35. Schreier G, Hayn D, Kastner P, Koller S, Salmhofer W, Hofmann-Wellenhof R, et al. mobile-phone based teledermatology system to support self-management of patients suffering from psoriasis. Annu Int Conf IEEE Eng Med Biol Soc. (2008) 2008:5338–41. doi: 10.1109/IEMBS.2008.4650420
36. Koller S, Hofmann-Wellenhof R, Hayn D, Weger W, Kastner P, Schreier G, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. (2011) 91:680–5. doi: 10.2340/00015555-1148
37. Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis (including pre-radiographic and radiographic forms) in primary care. Ann Rheum Dis. (2005) 64:659–63. doi: 10.1136/ard.2004.028753
38. Proft F, Spiller L, Redeker I, Protopopov M, Rodriguez VR, Muche B, et al. Comparison of an online self-referral tool with a physician-based referral strategy for early recognition of patients with a high probability of axial spa. Semin Arthritis Rheum. (2020) 50:1015–21. doi: 10.1016/j.semarthrit.2020.07.018
39. Giovannini I, Zabotti A, Cicciò C, Salgarello M, Cereser L, De Vita S, et al. Axial psoriatic disease: clinical and imaging assessment of an underdiagnosed condition. J Clin Med. (2021) 10:2845. doi: 10.3390/jcm10132845
40. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. (2013) 309:1351–2. doi: 10.1001/jama.2013.393
41. Kedra J, Radstake T, Pandit A, Baraliakos X, Berenbaum F, Finckh A, et al. Current status of use of big data and artificial intelligence in RMDs: a systematic literature review informing EULAR recommendations. RMD Open. (2019) 5:e001004. doi: 10.1136/rmdopen-2019-001004
42. Yu K, Syed MN, Bernardis E, Gelfand JM. Machine learning applications in the evaluation and management of psoriasis: a systematic review. J Psoriasis Psoriatic Arthritis. (2020) 5:147–59. doi: 10.1177/2475530320950267
43. Gomolin A, Netchiporouk E, Gniadecki R, Litvinov IV. Artificial intelligence applications in dermatology: where do we stand? Front Med. (2020) 7:100. doi: 10.3389/fmed.2020.00100
44. Aggarwal SLP. Data augmentation in dermatology image recognition using machine learning. Skin Res Technol. (2019) 25:815–20. doi: 10.1111/srt.12726
45. Gossec L, Kedra J, Servy H, Pandit A, Stones S, Berenbaum F, et al. EULAR points to consider for the use of big data in rheumatic and musculoskeletal diseases. Ann Rheum Dis. (2020) 79:69–76. doi: 10.1136/annrheumdis-2019-215694
46. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. (2019) 25:30–6. doi: 10.1038/s41591-018-0307-0
47. Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. (2020) 79:700–12. doi: 10.1136/annrheumdis-2020-218456
48. Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. (2009) 27:469–74.
49. Tinazzi I, Adami S, Zanolin EM, Caimmi C, Confente S, Girolomoni G, et al. The early psoriatic arthritis screening questionnaire: a simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology. (2012) 51:2058–63. doi: 10.1093/rheumatology/kes187
50. Kampylafka E, Simon D., d'Oliveira I, Linz C, Lerchen V, Englbrecht M, et al. Disease interception with interleukin-17 inhibition in high-risk psoriasis patients with subclinical joint inflammation—data from the prospective IVEPSA study. Arthritis Res Ther. (2019) 21:178. doi: 10.1186/s13075-019-1957-0
51. Savage L, Goodfield M, Horton L, Watad A, Hensor E, Emery P, et al. Regression of peripheral subclinical enthesopathy in therapy-naive patients treated with Ustekinumab for moderate-to-severe chronic plaque psoriasis: a fifty-two-week, prospective, open-label feasibility study. Arthritis Rheumatol. (2019) 71:626–31. doi: 10.1002/art.40778
52. Solomon DH, Iversen MD, Avorn J, Gleeson T, Brookhart MA, Patrick AR, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. (2012) 172:477–83. doi: 10.1001/archinternmed.2011.1977
53. Song Y, Reifsnider E, Zhao S, Xie X, Chen H. A randomized controlled trial of the effects of a telehealth educational intervention on medication adherence and disease activity in rheumatoid arthritis patients. J Adv Nurs. (2020) 76:1172–81. doi: 10.1111/jan.14319
54. Allam A, Kostova Z, Nakamoto K, Schulz PJ. The effect of social support features and gamification on a Web-based intervention for rheumatoid arthritis patients: randomized controlled trial. J Med Internet Res. (2015) 17:e14. doi: 10.2196/jmir.3510
55. Pouls BPH, Kristensen LE, Petersson M, van den Bemt BJF, Ballerini L, Bruggraber R, et al. A pilot study examining patient preference and satisfaction for ava®, a reusable electronic injection device to administer certolizumab pegol. Expert Opin Drug Deliv. (2020) 17:705–11. doi: 10.1080/17425247.2020.1736552
56. Jørgensen TS, Skougaard M, Taylor PC, Asmussen HC, Lee A, Klokker L, et al. The parker model: applying a qualitative three-step approach to optimally utilize input from stakeholders when introducing new device technologies in the management of chronic rheumatic diseases. Patient. (2018) 11:515–26. doi: 10.1007/s40271-018-0306-8
57. Maniadakis N, Toth E, Schiff M, Wang X, Nassim M, Szegvari B, et al. A Targeted literature review examining biologic therapy compliance and persistence in chronic inflammatory diseases to identify the associated unmet needs, driving factors, and consequences. Adv Ther. (2018) 35:1333–55. doi: 10.1007/s12325-018-0759-0
58. Tatla D, Mountian I, Szegvari B, VanLunen B, Schiff M. A multicenter, open-label study to evaluate the safe and effective use of a new electromechanical auto-injection device for self-injection of certolizumab pegol. Expert Opin Drug Deliv. (2020) 17:855–62. doi: 10.1080/17425247.2020.1747430
59. Domańska B, Stumpp O, Poon S, Oray S, Mountian I, Pichon C. Using patient feedback to optimize the design of a Certolizumab pegol electromechanical self-injection device: insights from human factors studies. Adv Ther. (2018) 35:100–15. doi: 10.1007/s12325-017-0645-1
60. Knitza J, Tascilar K, Messner E-M, Meyer M, Vossen D, Pulla A, et al. German mobile apps in rheumatology: review and analysis using the Mobile Application Rating Scale (MARS). JMIR Mhealth Uhealth. (2019) 7:e14991. doi: 10.2196/14991
61. Grainger R, Townsley H, White B, Langlotz T, Taylor WJ. Apps for people with rheumatoid arthritis to monitor their disease activity: a review of apps for best practice and quality. JMIR Mhealth Uhealth. (2017) 5:e7. doi: 10.2196/mhealth.6956
62. Najm A, Gossec L, Weill C, Benoist D, Berenbaum F, Nikiphorou E. Mobile health apps for self-management of rheumatic and musculoskeletal diseases: systematic literature review. JMIR Mhealth Uhealth. (2019) 7:e14730. doi: 10.2196/14730
63. Stevenson FA, Cox K, Britten N, Dundar Y. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences for concordance. Health Expect. (2004) 7:235–45. doi: 10.1111/j.1369-7625.2004.00281.x
64. Leeb BF, Andel I, Leder S, Leeb BA, Rintelen B. The patient's perspective and rheumatoid arthritis disease activity indexes. Rheumatology. (2005) 44:360–5. doi: 10.1093/rheumatology/keh484
65. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. (2006) 4:79. doi: 10.1186/1477-7525-4-79
66. Kitchen H, Cordingley L, Young H, Griffiths CEM., Bundy C. Patient-reported outcome measures in psoriasis: the good, the bad and the missing! Br J Dermatol. (2015) 172:1210–21. doi: 10.1111/bjd.13691
67. Fortune E, Tierney M, Scanaill CN, Bourke A, Kennedy N, Nelson J. Activity level classification algorithm using SHIMMERTM wearable sensors for individuals with rheumatoid arthritis. Annu Int Conf IEEE Eng Med Biol Soc. (2011) 2011:3059–62. doi: 10.1109/IEMBS.2011.6090836
68. Connolly J, Curran K, Condell J, Gardiner P. Wearable Rehab Technology for Automatic Measurement of Patients with Arthritis. In: 2011 5th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) and Workshops. (2011). p. 508–9. doi: 10.4108/icst.pervasivehealth.2011.246010
69. Gossec L, Guyard F, Leroy D, Lafargue T, Seiler M, Jacquemin C, et al. Detection of flares by decrease in physical activity, collected using wearable activity trackers in rheumatoid arthritis or axial spondyloarthritis: an application of machine learning analyses in rheumatology. Arthritis Care Res. (2019) 71:1336–43. doi: 10.1002/acr.23768
70. Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR Mhealth Uhealth. (2019) 7:e12861. doi: 10.2196/12861
71. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. (2009) 61:1505–10. doi: 10.1002/art.24873
72. Marchesoni A, De Marco G, Merashli M, McKenna F, Tinazzi I, Marzo-Ortega H, et al. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology. (2018) 57:32–40. doi: 10.1093/rheumatology/kex079
73. Macchioni P, Salvarani C, Possemato N, Gutierrez M, Grassi W, Gasparini S, et al. Ultrasonographic and clinical assessment of peripheral enthesitis in patients with psoriatic arthritis, psoriasis, and fibromyalgia syndrome: the ULISSE study. J Rheumatol. (2019) 46:904–11. doi: 10.3899/jrheum.171411
74. de Thurah A, Stengaard-Pedersen K, Axelsen M, Fredberg U, Schougaard LMV, Hjollund NHI, et al. Tele-health followup strategy for tight control of disease activity in rheumatoid arthritis: results of a randomized controlled trial. Arthritis Care Res. (2018) 70:353–60. doi: 10.1002/acr.23280
75. Cheung PP, Ruyssen-Witrand A, Gossec L, Paternotte S, Le Bourlout C, Mazieres M, et al. Reliability of patient self-evaluation of swollen and tender joints in rheumatoid arthritis: a comparison study with ultrasonography, physician, and nurse assessments. Arthritis Care Res. (2010) 62:1112–9. doi: 10.1002/acr.20178
76. Solomon DH, Rudin RS. Digital health technologies: opportunities and challenges in rheumatology. Nat Rev Rheumatol. (2020) 16:525–35. doi: 10.1038/s41584-020-0461-x
77. Onor ML, Misan S. The clinical interview and the doctor-patient relationship in telemedicine. Telemed J E Health. (2005) 11:102–5. doi: 10.1089/tmj.2005.11.102
78. Hjelm NM. Benefits and drawbacks of telemedicine. J Telemed Telecare. (2005) 11:60–70. doi: 10.1258/1357633053499886
79. van Riel P, Alten R, Combe B, Abdulganieva D, Bousquet P, Courtenay M, et al. Improving inflammatory arthritis management through tighter monitoring of patients and the use of innovative electronic tools. RMD Open. (2016) 2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5133416/. doi: 10.1136/rmdopen-2016-000302 (cited Jan 22, 2021).
80. MacKenzie H, Thavaneswaran A, Chandran V, Gladman DD. Patient-reported outcome in psoriatic arthritis: a comparison of Web-based versus paper-completed questionnaires. J Rheumatol. (2011) 38:2619–24. doi: 10.3899/jrheum.110165
81. Sargious A, Lee SJ. Remote collection of questionnaires. Clin Exp Rheumatol. (2014) 32:S-168–172.
82. Hawkins SD, Barilla S, Feldman SR. Web app based patient education in psoriasis—a randomized controlled trial. Dermatol Online J. (2017) 23:13030/qt26d525z5. doi: 10.5070/D3234034647
83. Development and preliminary validation of smartphone sensor-based measurement tools for psoriatic arthritis. ACR Meeting Abstracts. Available from: https://acrabstracts.org/abstract/development-and-preliminary-validation-of-smartphone-sensor-based-measurement-tools-for-psoriatic-arthritis/ (cited Oct 4, 2021).
84. Norgeot B, Glicksberg BS, Trupin L, Lituiev D, Gianfrancesco M, Oskotsky B, et al. Assessment of a deep learning model based on electronic health record data to forecast clinical outcomes in patients with rheumatoid arthritis. JAMA Network Open. (2019)2:e190606. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6484652/ doi: 10.1001/jamanetworkopen.2019.0606 (cited Sep 27, 2021).
85. Lezcano-Valverde JM, Salazar F, León L, Toledano E, Jover JA, Fernandez-Gutierrez B, et al. Development and validation of a multivariate predictive model for rheumatoid arthritis mortality using a machine learning approach. Sci Rep. (2017)7:10189. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579234/ doi: 10.1038/s41598-017-10558-w (cited Sep 27, 2021).
86. Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep learning approach for evaluating knee mr images: achieving high diagnostic performance for cartilage lesion detection. Radiology. (2018) 289:160–9. Available from: https://pubmed.ncbi.nlm.nih.gov/30063195/. doi: 10.1148/radiol.2018172986 (cited Sep 27, 2021)
87. Najm A, Nikiphorou E, Kostine M, Richez C, Pauling JD, Finckh A, et al. EULAR points to consider for the development, evaluation and implementation of mobile health applications aiding self-management in people living with rheumatic and musculoskeletal diseases. RMD Open. (2019) 5:e001014. doi: 10.1136/rmdopen-2019-001014
88. Najm A, Lempp H, Gossec L, Berenbaum F, Nikiphorou E. Needs, experiences, and views of people with rheumatic and musculoskeletal diseases on self-management mobile health apps: mixed methods study. JMIR Mhealth Uhealth. (2020) 8:e14351. doi: 10.2196/14351
89. Matsumoto RA, Barton JL. Telerheumatology: before, during, and after a global pandemic. Curr Opin Rheumatol. (2021) 33:262–9. doi: 10.1097/BOR.0000000000000790
90. Schaap MJ, Broekhuis SCE. Spillekom-van Koulil S, Groenewoud HMM, de Jong EMGJ, Seyger MMB. Treatment goals and preferences of pediatric psoriasis patients, young adults, and parents. J Dermatolog Treat. (2021) 8:1–7. doi: 10.1080/09546634.2021.1985058
Keywords: Psoriatic Arthritis (PsA), psoriasis, technology, prevention, interception, early diagnosis, telerheumatology
Citation: Giovannini I, Bosch P, Dejaco C, De Marco G, McGonagle D, Quartuccio L, De Vita S, Errichetti E and Zabotti A (2021) The Digital Way to Intercept Psoriatic Arthritis. Front. Med. 8:792972. doi: 10.3389/fmed.2021.792972
Received: 11 October 2021; Accepted: 02 November 2021;
Published: 23 November 2021.
Edited by:
Philipp Sewerin, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Nicolò Girolimetto, University of Modena and Reggio Emilia, ItalyOrazio De Lucia, Istituto Ortopedico Gaetano Pini, Italy
Copyright © 2021 Giovannini, Bosch, Dejaco, De Marco, McGonagle, Quartuccio, De Vita, Errichetti and Zabotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alen Zabotti, emFib3R0aWFsZW5AZ21haWwuY29t
†These authors have contributed equally to this work
 Ivan Giovannini
Ivan Giovannini Philipp Bosch
Philipp Bosch Christian Dejaco
Christian Dejaco Gabriele De Marco4
Gabriele De Marco4 Dennis McGonagle
Dennis McGonagle Luca Quartuccio
Luca Quartuccio Enzo Errichetti
Enzo Errichetti Alen Zabotti
Alen Zabotti