- 1Department of Urology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Orthopaedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Blood Supply, Shandong Blood Center, Jinan, China
Background: Tripartite motif containing 46 was initially identified as the oncogene in several human tumors. However, the clinical value and potential functions of tripartite motif containing 46 (TRIM46) in clear cell renal cell carcinoma (ccRCC) remained largely unclear.
Methods: The expressing patterns, clinical involvement, and prognostic values of TRIM46 were analyzed using the data obtained from TCGA and GEO databases. A nomogram was constructed to examine the outcome of patients with ccRCC. We estimated the association between TRIM46 with tumor immunity in ccRCC.
Results: Tripartite motif containing 46 was highly expressed in ccRCC, and its upregulation revealed an unfavorable prognosis. A nomogram based on TRIM46 expressions and other independent prognostic factors could robustly predict the overall survival of tumor patients. TRIM46 has a strong positive correlation with NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2. Mechanically, TRIM46 displayed regulatory functions in ccRCC progression via several tumor-associated pathways. Besides, we observed that TRIM46 was distinctly related to tumor immunity in ccRCC.
Conclusions: Our findings provide a novel tumor promotive role regarding TRIM46 function in the malignant progression of ccRCC.
Introduction
Renal cell carcinoma is one of the most common urinary malignancies worldwide and its incidence is gradually increasing (1). Clear cell renal cell carcinoma (ccRCC) accounts for 70–85% of all renal tumors (2). Metastasis and recurrence are important biological characteristics, leading to a poor prognosis (3). With the development of immunotherapy and targeted therapy, a novel epoch may arrive. However, the existing treatments have several inferiorities, including drug resistance, higher systemic toxicity, and, in some cases, incidence rate and mortality (4, 5). In recent decades, the prognostic value of novel biomarkers has become increasingly attractive. The identification of novel sensitive biomarkers is eager for the optimization of the treatment plan.
Tripartite motif containing 46, a member of the family of tripartite motif (TRIM)-containing proteins, was defined as an essential modulator of oncogenesis in several malignancies (6–8). In osteosarcoma, deletion of tripartite motif containing 46 (TRIM46) suppresses the activity of tumor cells, inhibits cellular cycle, and induces cellular apoptosis, whereas upregulation of TRIM46 displays the opposite effect (9). In breast cancer, TRIM46 acts as a positive regulator in the proliferation and migration of tumor cells (10). Although TRIM46 has demonstrated its potential as a biomarker in cancers, its clinical value and specific effects on the occurrence and progression of ccRCC remained largely unclear.
In this study, we analyzed the TCGA and GEO databases to determine the expressing pattern of TRIM46 and its prognostic value in ccRCC. Then, we performed functional enrichment analyses, including Gene Ontology (GO), Kyoto Encyclopedia of Genes and genomes (KEGG0, and Gene Set Enrichment Analysis (GSEA), to delve into the possible mechanisms involved in TRIM46 function. Moreover, the association of TRIM46 and tumor immunity was equally uncovered in this study.
Materials and Methods
Data Collection
The transcriptomic profiles were downloaded from the TCGA datasets (11). After we excluded those samples with incomplete clinical information, this study included 530 ccRCC specimens and 72 non-tumor renal samples. Clinicopathological characteristics of patients with ccRCC were presented in Supplementary Table 1. Besides, the data from the GSE36895, GSE66272, and GSE53757 datasets were applied to further confirm the results of TCGA datasets.
Functional Enrichment Analysis
Metascape was applied to perform functional enrichment assays of TRIM46 and its related genes (12). Moreover, GSEA was utilized to investigate the possible mechanisms of TRIM46 in ccRCC. Gene sets with p < 0.05 were considered a significant enrichment.
Relationship of TRIM46 With Tumor-Infiltrating Immune Cells (TIICs) in CcRCC
In this study, the “Gene” module was applied to examine the association between TRIM46 expressions and six immune cell infiltration levels. CIBERSORT is a free experimental database that can measure the composition of the immune cells in tissues according to their gene expression profile (13). After the expression profile of the TCGA-KIRC, dataset with standard gene annotation was uploaded to the CIBERSORT portal, the LM22 signature, and permutation = 100 deconvolution algorithm served to calculate the immune cell infiltration. The CIBERSORT value generated was defined as the fraction of immune cell infiltration in each sample. Then, our group analyzed the differences in the content of various immune infiltrating cells between low- and high-expressing groups. Additionally, the association of TRIM46 and immunosuppressive molecules, including PDCD1, LAG3, CTLA4, CD276, and TIGIT, was analyzed in the TCGA database.
Statistical Analysis
The R 3.6.1 software (The R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. The prognostic values of TRIM46 in ccRCC were determined by the use of Kaplan-Meier survival method, followed by log-rank test. The independent prognostic factors were identified by the use of uni- and multivariate analyses. Spearman correlation analysis was used to analyze the relationship between TRIM46 and immunosuppressive molecules. The difference was considered significant when p < 0.05.
Results
The Distinct Upregulation of TRIM46 in CcRCC
Firstly, we analyzed the TIMER database, finding that TRIM46 exhibited an increased level in many types of tumors, including LIHC, KIRC, KICH, READ, PRAD, THCA, STAD, ESCA, COAD, CHOL, HNSC, BRCA, BLCA PCPG, LUSC, and LUAD (Figure 1A). However, a decreased expression of TRIM46 was observed in GBM. The data from TCGA, GSE36895, GSE66272, and GSE53757 also indicated that TRIM46 was highly expressed in ccRCC relative to normal tissue controls (both p < 0.001) (Figures 1B–E).
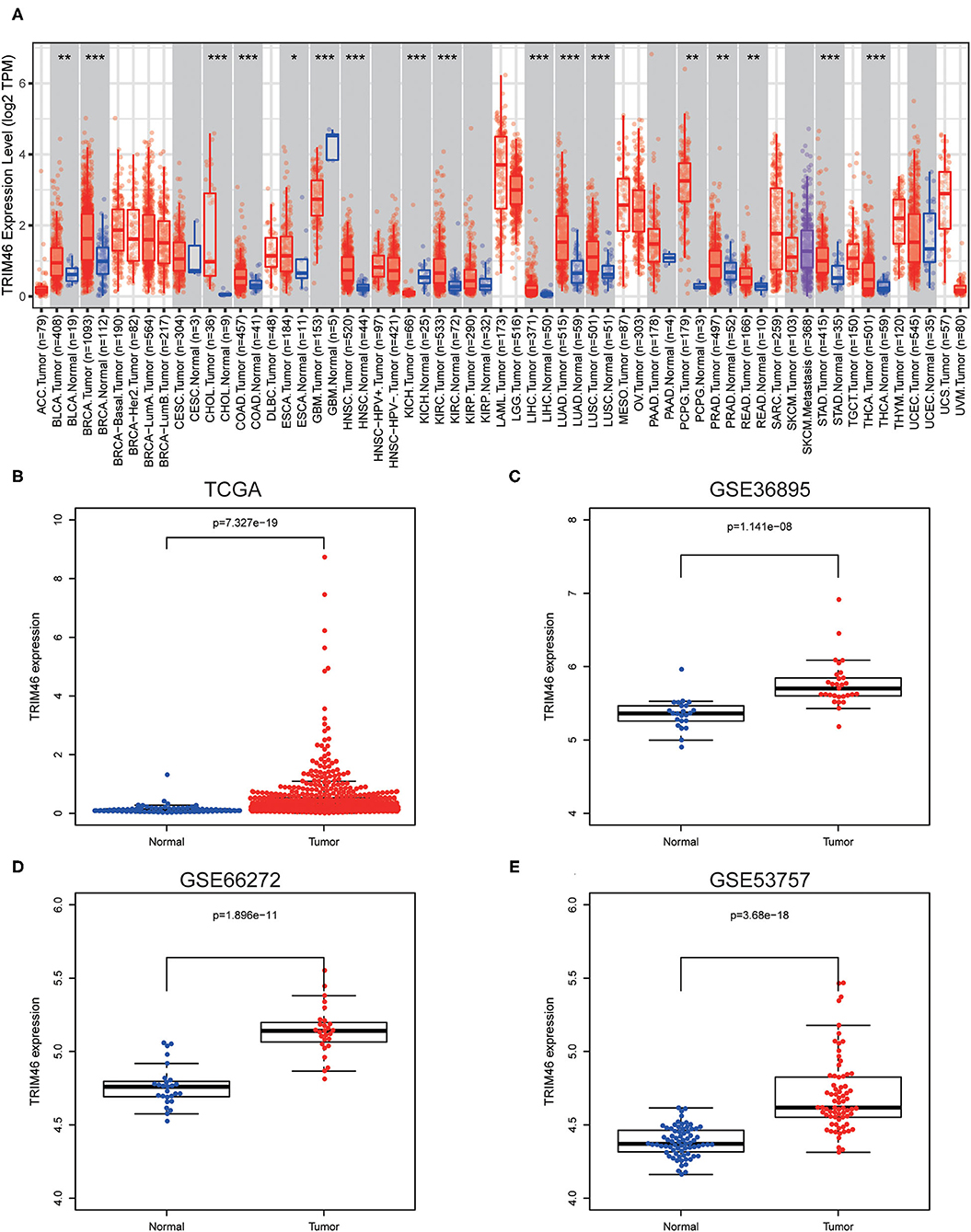
Figure 1. The distinct upregulation of tripartite motif containing 46 (TRIM46) in clear cell renal cell carcinoma (ccRCC) specimens. (A) TRIM46 expressions in several types of tumors via analyzing TCGA datasets were confirmed by the use of TIMER. (B–E) The overexpression of TRIM46 was demonstrated via analyzing data from TCGA, GSE36895, GSE66272, and GSE53757. *p < 0.05, **p < 0.01, ***p < 0.001.
The Association Between TRIM46 Expression With Clinicopathologic Variables
We next investigate the link between the expression levels of TRIM46 with clinical features of patients with ccRCC. We found that high TRIM46 expression was associated with high clinical stage (p = 0.002, Figure 2A), histologic grade (p = 0.008, Figure 2B), T classification (Figure 2C), N classification (Figure 2D), and M classification (Figure 2E).
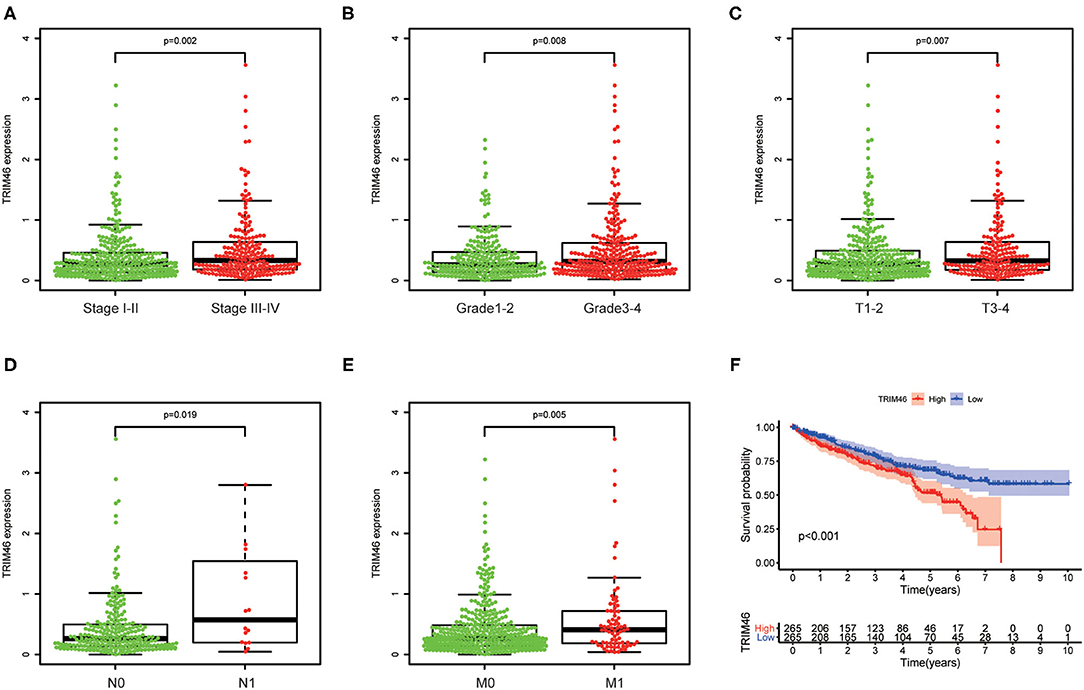
Figure 2. The relationship between TRIM46 and clinical features. (A) Stage; (B) grade; (C) T stage; (D) N stage; (E) M stage. (F) Kaplan-Meier survival analysis.
TRIM46 Overexpression Indicates a Worse Survival in CcRCC
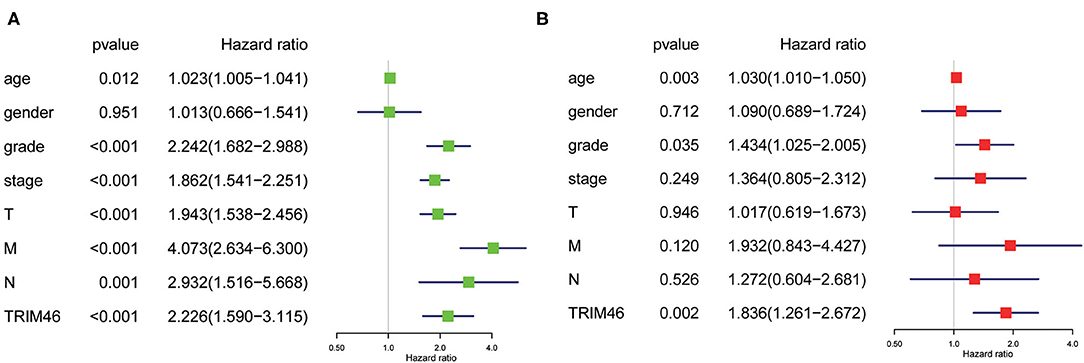
Then, we used the Kaplan-Meier survival curves to assess the prognostic values of TRIM46 in ccRCC. Kaplan-Meier assay results indicated that high TRIM46 expression subgroup had a distinctly shorter overall survival (p < 0.001) (Figure 2F). Moreover, the uni- and multivariate analyses suggested that TRIM46 expression (p = 0.002; HR = 1.836), age (p = 0.003; HR = 1.030), and histologic grade (p = 0.035; HR = 1.434) were independent biomarkers for overall survival (OS) of ccRCC (Figures 3A,B). Collectively, TRIM46 is a potential independent risk factor in ccRCC.
A Nomogram Was Developed to Predict OS of CcRCC
To better predict the long-term survivals of patients, we used the above independent of constructing nomogram to predict the long-term survivals of patients with ccRCC (Figure 4A). Importantly, the calibration curves revealed that the actual and predicted survival matched very well in terms of 1-year (Figure 4B), 3-year (Figure 4C), and 5-year OS (Figure 4D).
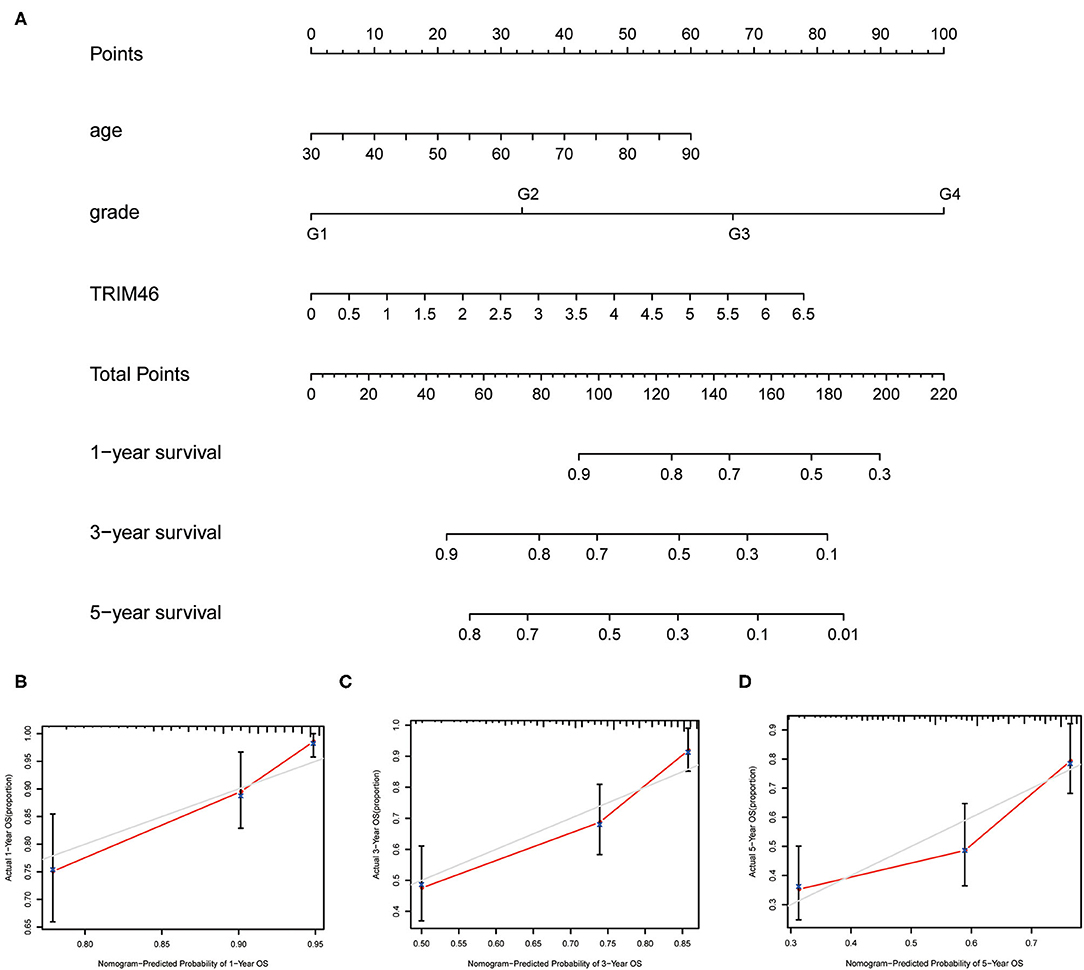
Figure 4. The prognostic value of several factors in a nomogram. (A) Construction of a predictive nomogram. (B–D) Actual and predicted survivals were shown by the use of calibration curves.
Co-expression and Functional Enrichment Analysis
In order to better examine the possible meaning of TRIM46 in ccRCC, we first performed the co-expression analysis of TRIM46; 382 genes were chosen for further analysis (Supplementary Table 2). Moreover, Figure 5A shows the heatmaps of the top 20 genes related with TRIM46. Additionally, TRIM46 has a strong positive correlation with NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2 (both p < 0.001) (Figures 5B–K). We, thus, explored their expression patterns, clinical significance, and prognostic value in ccRCC using the TCGA-KIRC data. Interestingly, higher expression levels of NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2 were found in ccRCC samples (Figure 6A), and their overexpression was all associated with advanced stage (Figure 6B) and poorer OS (Figures 6C–L), indicating TRIM46 and its 10 functional partners might be involved in ccRCC progression together. GO_BP assays revealed that TRIM46 and the related genes were mainly enriched in biological processes linked to mitotic cell cycle phase transition, DNA-dependent DNA replication, and cell division (Figures 7A–C). KEGG assays revealed that TRIM46 and its partners were distinctly enriched in valine, leucine, and isoleucine degradation; fatty acid degradation; and glycerophospholipid metabolism (Figures 7D–F), suggesting that TRIM46 and its functional partners might influence ccRCC progression by regulating cell proliferation and substance metabolism.
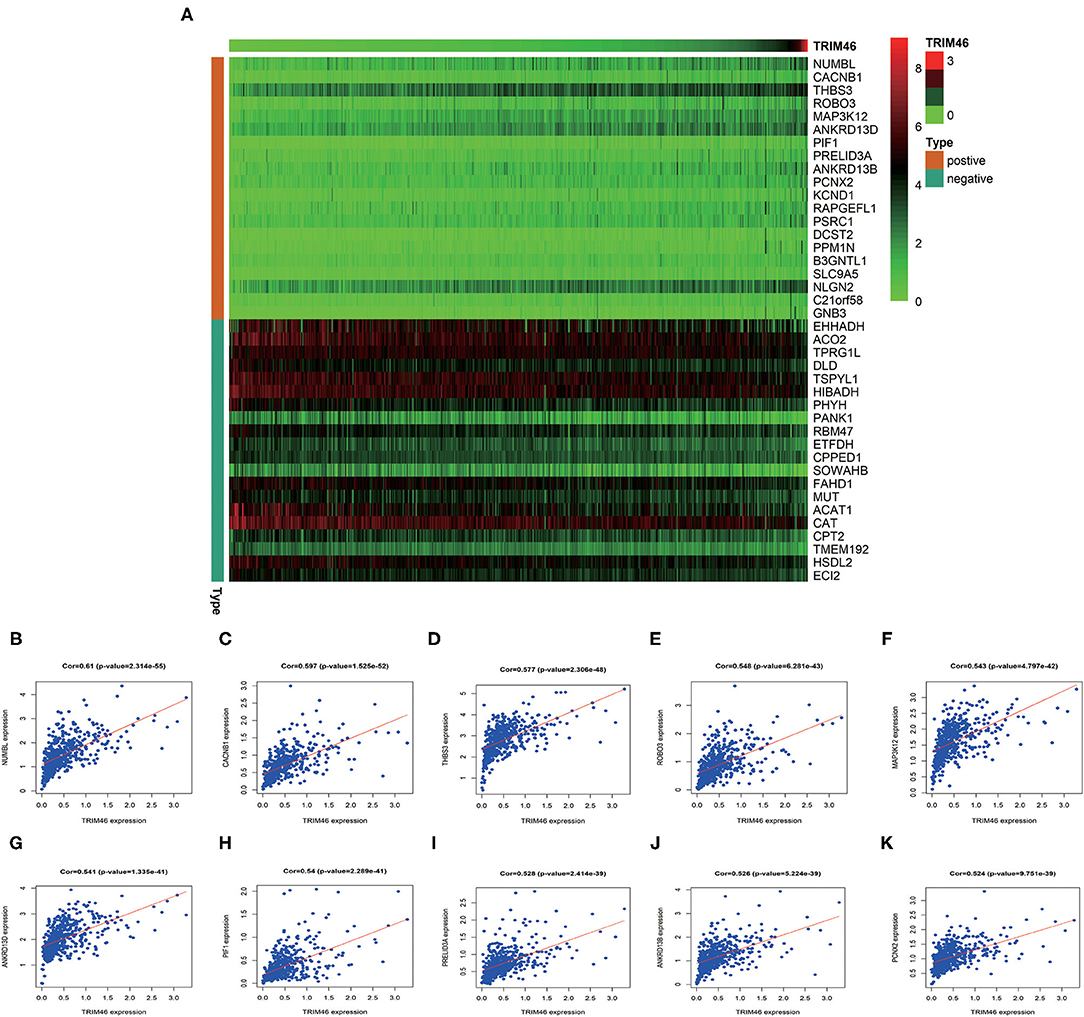
Figure 5. Co-expression assays. (A) The top 20 genes related to TRIM46. (B–K) The association of TRIM46 with NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2.
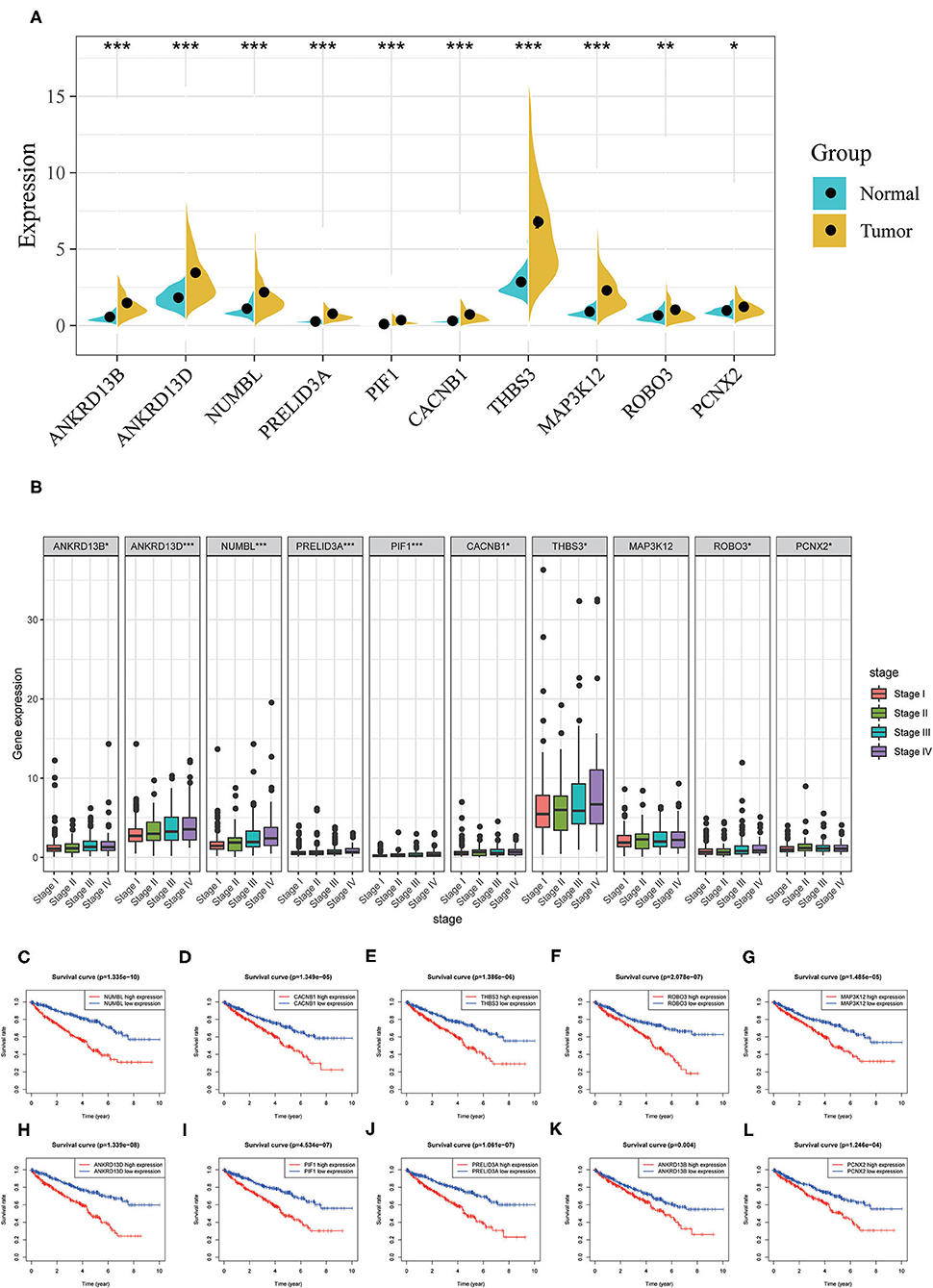
Figure 6. Analyses of co-expressed genes with TRIM46. (A) NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2 were overexpressed in ccRCC; (B) higher levels of NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2 indicated advanced clinical stage for patients with ccRCC; (C–L) higher expression level of NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2 indicated worse OS for patients with ccRCC (*p < 0.05, **p < 0.01, ***p < 0.001).
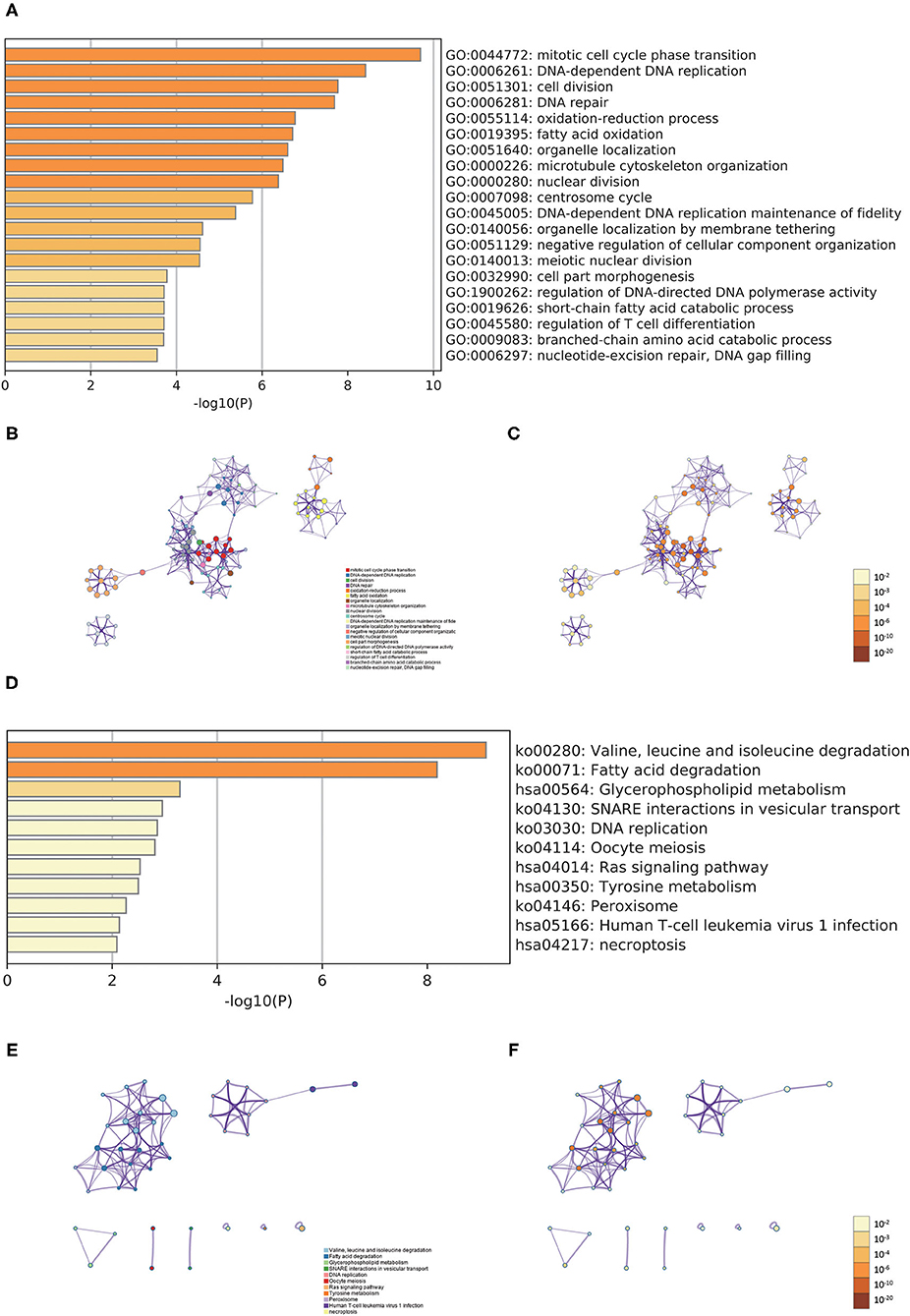
Figure 7. Functional enrichment analyses. (A) Heatmap of the biological processes enriched terms; (B) network of biological processes enriched terms; (C) network of biological processes enriched terms; (D) heatmap of the pathways enriched terms; (E) network of pathways enriched terms; (F) network of pathways enriched terms.
TRIM46-Related Signaling Pathways
We performed GSEA analysis and results showed that in the high TRIM46 expression phenotype, the four most significantly enriched signaling pathways were the cytokine cytokine–receptor interaction, chondroitin sulfate biosynthesis, taste transduction, and homologous recombination (Figures 8A–D). Whereas, in the low TRIM46 expression phenotype, the four most significantly enriched signaling pathways were citrate cycle TCA cycle, peroxisome, propanoate metabolism and valine, and leucine and isoleucine degradation (Figures 8E–H).
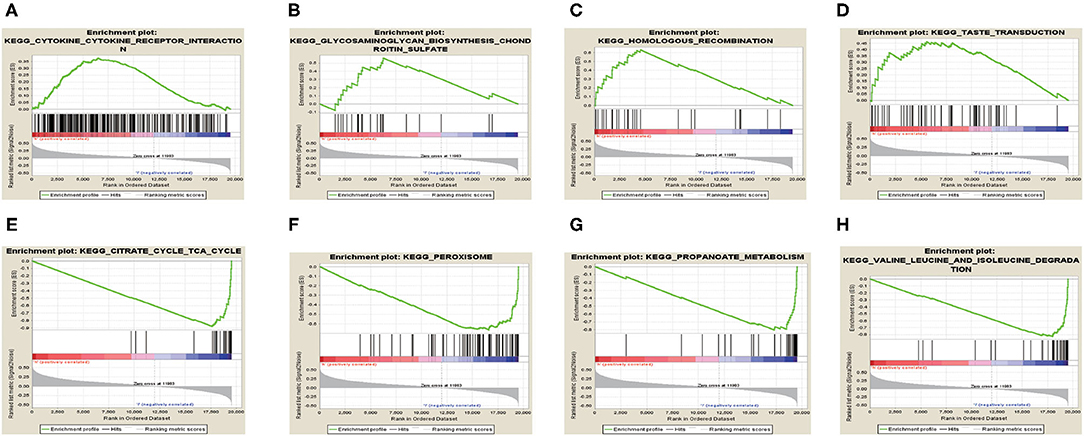
Figure 8. Gene set enrichment analysis. (A–D) The four most significantly enriched signaling pathways enriched in the high TRIM46 expression phenotype, respectively. (E–H) The four most significantly enriched signaling pathways enriched in the low TRIM46 expression phenotype, respectively.
Relationship Between TRIM46 and TIICs, Immunosuppressive Molecules
Our group observed that TRIM46 was related to the immune levels of CD 4+ T cells (cor = 0.393, p < 0.001), macrophages (cor = 0.105, p < 0.05), neutrophils (cor = 0.275, p < 0.001), and dendritic cells (cor = 0.148, p < 0.01) (Figure 9A). Moreover, CIBERSORT analysis indicated that the proportion of activated memory CD4+ T cells (p < 0.01) and M0 macrophages (p < 0.05) in the TRIM46 high-expression subgroup was significantly higher than that in the low-expression subgroup. Whereas, the proportion of naïve B cell (p < 0.05), activated NK cells (p < 0.05), eosinophils (p < 0.05), and neutrophils (p < 0.05) displayed an opposite result (Figure 9B). These altogether indicated that TRIM46 might play an important role in immune cell infiltration in ccRCC progression. Moreover, we evaluate the association between TRIM46 and several immune checkpoints (Figure 10A). The data indicated that TRIM46 was related to DCD1, LAG3, CTLA4, CD276, and TIGIT (Figures 10B–F). Taken together, these results suggested that TRIM46 might cause immunosuppression in ccRCC progression while suggesting that patients with high TRIM46 levels might be more likely to benefit from immune checkpoint inhibitors.
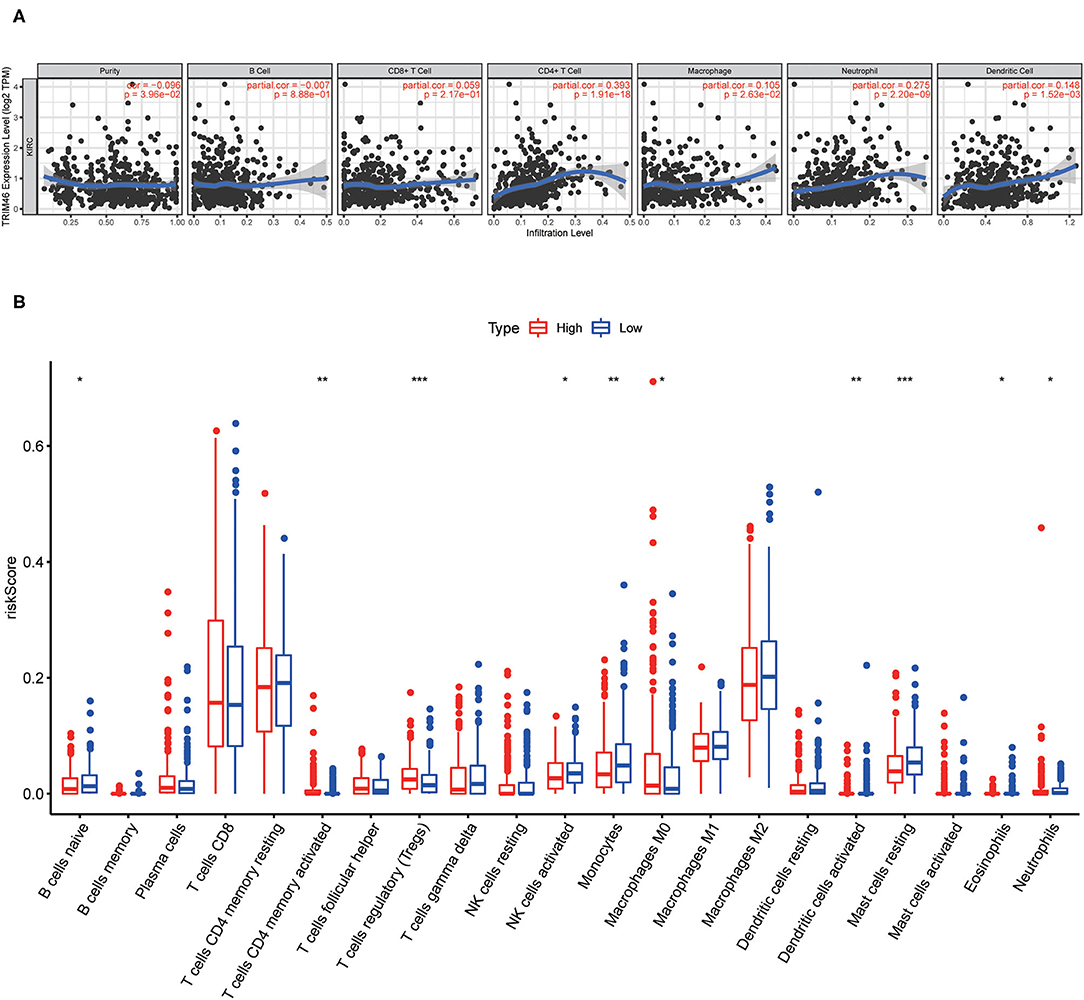
Figure 9. Associations of tumor-infiltrating immune cell (TIIC) proportion with TRIM46 expressions. (A) Six immune cell infiltration levels were involved in the expressions of TRIM46 in in ccRCC (TIMER database); (B) barplot of the proportion of 21 kinds of TIICs in ccRCC specimens (*p < 0.05, **p < 0.01, ***p < 0.001).
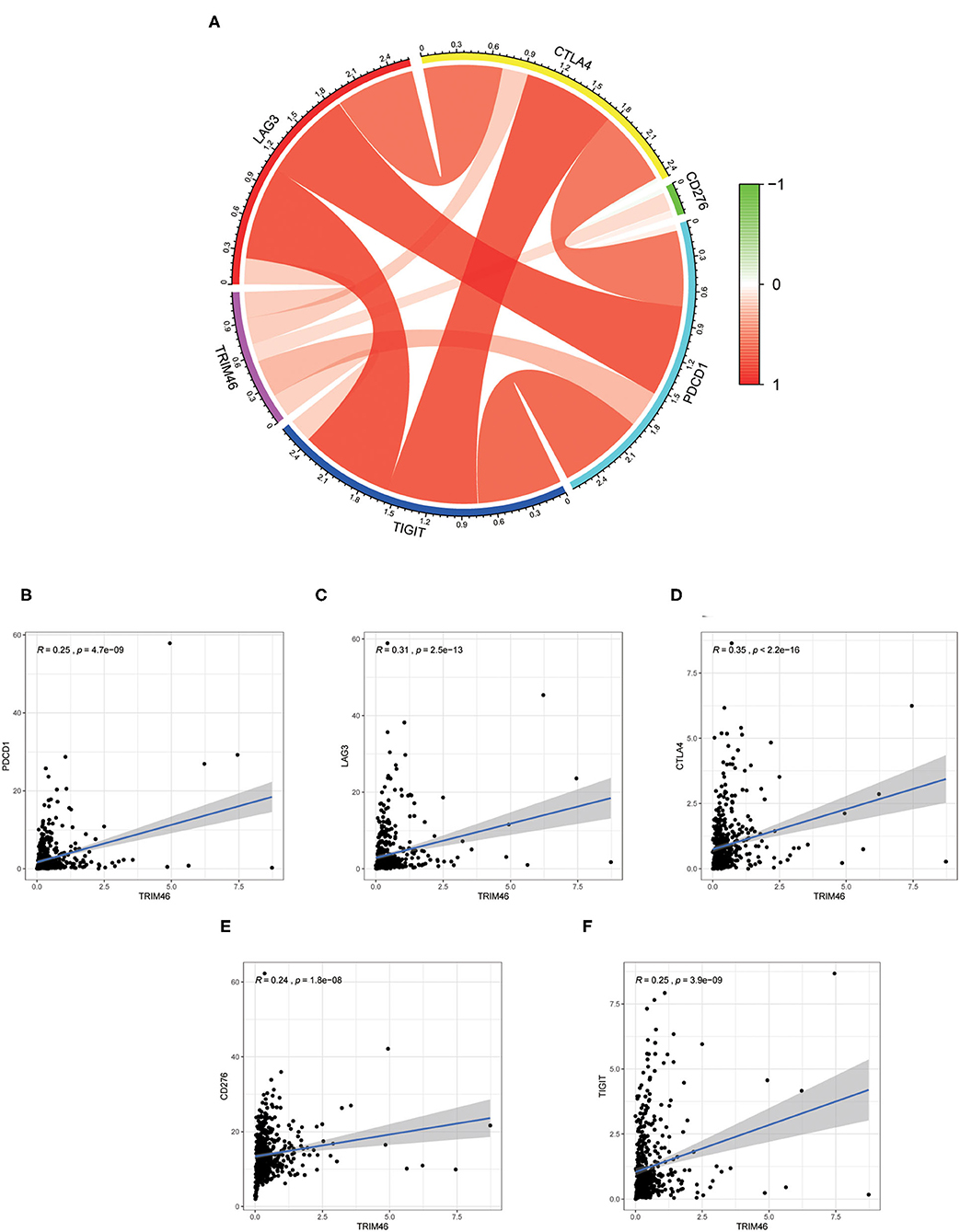
Figure 10. Correlation of the levels of immunosuppressive molecules with TRIM46 expressions. (A) Circle diagram of associations between the levels of immunosuppressive molecules and TRIM46 expressions; (B–F) TRIM46 was related to TIGIT, CD276, CTLA4, LAG3, and PD-1 according to correlation assays.
Discussion
Clear cell renal cell carcinoma is highly heterogeneous in terms of treatment options and clinical outcomes. Metastasis and recurrences are considered to be the main causes for the poor prognosis of patients (14). Moreover, the potential mechanisms involving tumor metastasis remained largely unclear. Frustratingly, unlike most malignancies, ccRCC is insensitive to radiotherapy, and despite significant progress in the study of targeted agents, many patients with the advanced or metastatic disease die due to tolerance to these agents or the drug side effects, resulting in an unsatisfactory overall prognosis (1, 4). Therefore, it is imperative to find novel therapeutic targets for ccRCC and investigate their action mechanisms.
Tripartite motif family proteins are identified as a subfamily of ring E3 ubiquitin ligases, and most of them have E3 ubiquitin ligase activities. Accumulating evidence has demonstrated that TRIM played an important role in various cellular processes, including carcinogenesis, autophagy, innate immunity, protein quality control, apoptosis, developments, and intracellular signaling. Their abnormal expression might result in many diseases such as immunological diseases, developmental disorders, and neoplasms. TRIM46, a member of the TRIM family proteins, was recognized as a crucial regulator of oncogenesis. However, the clinical value and specific effects of TRIM46 in ccRCC were rarely reported.
In the current study, we observed that TRIM46 was significantly elevated in ccRCC specimens. Additionally, we found that TRIM46 overexpression is correlated with poorer clinicopathological features and worse prognosis. Additionally, the elevated expression level of TRIM46 was an independent prognostic factor for poor OS in patients with ccRCC, indicating that TRIM46 may serve as a tumor promotor of ccRCC. Then, we established a nomogram based on the independent prognostic factors in this report, and results showed that this nomogram could efficiently predict the OS of patients with ccRCC.
Next, co-expression analysis identified 10 genes that were strongly associated with TRIM46, including NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13D, PIF1, PRELID3A, ANKRD13B, and PCNX2. Interestingly, these genes were equally overexpressed in ccRCC tissues and their increased levels were associated with advanced clinical stage and worse prognosis. Among them, some are involved in cancer progression. NUMBL was defined as an important regulator in the Notch pathway. Tao et al. (15) found that NUMBL served as a tumor suppressor in glioma and its overexpression inhibited the migration and invasion of tumor cells. It has been pointed out that CACNB1 (also known as CAB1) was overexpressed in gastric and esophageal cancer cells (16). Cristiane et al. (17) reported that high levels of THBS3 were observed in biopsies from patients with metastatic osteosarcoma. In pancreatic cancer, ROBO3 was highly expressed in tumor specimens, and its overexpression promoted cellular growth and invasion in vivo and in vitro (18). In prostate cancer, MAP3K12 exhibited a positively regulatory effect on the metastasis of tumor cells (19). In cervical cancer, loss of PIF1 suppressed the proliferation, blocked the cell cycle, and promoted apoptosis, and PIF1 deletion promoted the expressions of Caspase-3 and Bax, while suppressing the expressions of Bcl-2 (20). Despite the involvement of some of functional partners of TRIM46 in the cancer process, reports of a link between these genes and ccRCC are lacking.
Next, we found the three most significant biological processes are mitotic cell cycle phase transition, DNA-dependent DNA replication, and cell division, indicating that TRIM46 might be related to cell proliferation, depending on the cell cycle. The three most significant signaling pathways are valine, leucine, and isoleucine degradation; fatty acid degradation; and glycerophospholipid metabolism, suggesting TRIM46 might affect the metabolism of substances in tumor cells and thus be involved in ccRCC progression. Dysregulation of the cell cycle is a significant cause of cancer development and a critical factor in promoting the proliferation of ccRCC cells (21, 22). In ccRCC, changes in metabolic pathways, such as fatty acid metabolism and glycerophospholipid metabolism, modulate tumor energetics and biosynthesis (23, 24). Moreover, GESA results showed the four most significantly enriched signaling pathways enriched in the high TRIM46 expression phenotype are cytokine–cytokine receptor interaction, chondroitin sulfate biosynthesis, taste transduction, and homologous recombination. Cytokine–cytokine receptor interaction is an essential immune signaling pathway as it regulates cytokine interactions and thus the progression of cancer (25). Chondroitin sulfate is a key player in regulating cell development, cell adhesion, proliferation, and differentiation (26). Homologous recombination is a vital biological act in the acquisition of genetic information during cell proliferation (27). Taken together, the above results suggested the important association between TRIM46 and the above pathways in ccRCC progression.
TIMER database results indicated that TRIM46 was related to the immune infiltration levels of CD 4+ T cells, macrophages, neutrophils, and dendritic cells; CIBERSORT analysis indicated that the proportion of activated memory CD4+ T cells, regulatory T cells (Tregs), and M0 macrophages were significantly elevated in the TRIM46 high-expression subgroup, suggesting that TRIM46 might exhibit regulatory effects on immune infiltration. Additionally, we assessed the relationship between TRIM46 and immune checkpoints. Herein, we found that TRIM46 is significantly related to several immune checkpoints, including PDCD1, LAG3, CTLA4, CD276, and TIGIT. PDCD1 (PD-1) was reported to suppress CD8 T cell activation and resulted in depletion (28). TIGIT overexpression was demonstrated to suppress immune cell function at multiple steps (29). Our results, together with previous findings, indicated that TEIM46 promoted ccRCC progression via inhibiting the immune system.
Several limitations should be neglected. Firstly, due to the retrospective data and limited clinical information from TCGA, more clinical samples with survival data of long-term survivals were needed to demonstrate our findings. Secondly, the expression of TRIM46 at protein levels in tumor specimens was not reported, which was needed to be further demonstrated. Thirdly, the potential mechanisms involved in the effects of TRIM46 on the clinical outcome of ccRCC patients were not fully explored (concerned with cell proliferation, or metabolic reprogramming, or immune infiltrates, immunosuppression, or both?).
Conclusion
This study comprehensively explored the clinical value and potential action mechanisms of TRIM46 in ccRCC. Elevation of TRIM46 is considerably associated with ccRCC progression. TRIM46 may be linked to cell proliferation, metabolic reprogramming, immune infiltrates, and immunosuppression of ccRCC. Taken together, TRIM46 can serve as a novel biomarker for diagnosis and prognosis and potential therapeutic target in ccRCC.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This work was supported by Shandong Medical and Health Science and Technology Development Program (No. 2016WS0446) and Shandong Key Research and Development Plan (No. 2018GSF118189).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.785331/full#supplementary-material
References
1. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers. (2017) 3:17009. doi: 10.1038/nrdp.2017.9
2. Grignon DJ, Che M. Clear cell renal cell carcinoma. Clin Lab Med. (2005) 25:305–16. doi: 10.1016/j.cll.2005.01.012
3. Perazella MA, Dreicer R, Rosner MH. Renal cell carcinoma for the nephrologist. Kidney Int. (2018) 94:471–83. doi: 10.1016/j.kint.2018.01.023
4. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2019) 30:706–20. doi: 10.1093/annonc/mdz056
5. Bratu O, Mischianu D, Marcu D, Spinu D, Iorga L, Cherciu A, et al. Renal tumor biomarkers. Exp Ther Med. (2021) 22:1297. doi: 10.3892/etm.2021.10732
6. Zhang J, Qiu Q, Wang H, Chen C, Luo D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res. (2021) 407:112800. doi: 10.1016/j.yexcr.2021.112800
7. Bell M, Bachmann S, Klimek J, Langerscheidt F, Zempel H, Axonal TAU. Sorting requires the C-terminus of TAU but is independent of ANKG and TRIM46 enrichment at the AIS. Neuroscience. (2021) 461:155–71. doi: 10.1016/j.neuroscience.2021.01.041
8. Zhan W, Han T, Zhang C, Xie C, Gan M, Deng K, et al. TRIM59 promotes the proliferation and migration of non-small cell lung cancer cells by upregulating cell cycle related proteins. PLoS ONE. (2015) 10:e0142596. doi: 10.1371/journal.pone.0142596
9. Jiang W, Cai X, Xu T, Liu K, Yang D, Fan L, et al. Tripartite motif-containing 46 promotes viability and inhibits apoptosis of osteosarcoma cells by activating NF-B signaling through ubiquitination of PPAR. Oncol Res. (2020) 28:409–21. doi: 10.3727/096504020X15868639303417
10. Zhang L, Li X, Dong W, Sun C, Guo D, Zhang L. Mmu-miR-1894-3p inhibits cell proliferation and migration of breast cancer cells by targeting Trim46. Int J Mol Sci. (2016) 17:40609. doi: 10.3390/ijms17040609
11. Hutter C, Zenklusen JC. The cancer genome atlas: creating lasting value beyond its data. Cell. (2018) 173:283–5. doi: 10.1016/j.cell.2018.03.042
12. Zhou Y, Zhou B, Pache L, Chang M. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. (2019) 10:1523. doi: 10.1038/s41467-019-09234-6
13. Newman AM, Liu CL, Green MR. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. (2015) 12:453–7. doi: 10.1038/nmeth.3337
14. Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. (2014) 349:g4797. doi: 10.1136/bmj.g4797
15. Tao T, Cheng C, Ji Y, Xu G, Zhang J, Zhang L, et al. Numbl inhibits glioma cell migration and invasion by suppressing TRAF5-mediated NF-κB activation. Mol Biol Cell. (2012) 23:2635–44. doi: 10.1091/mbc.e11-09-0805
16. Kishi T, Sasaki H, Akiyama N, Ishizuka T, Sakamoto H, Aizawa S, et al. Molecular cloning of human GRB-7 co-amplified with CAB1 and c-ERBB-2 in primary gastric cancer. Biochem Biophys Res Commun. (1997) 232:5–9. doi: 10.1006/bbrc.1997.6218
17. Dalla-Torre CA, Yoshimoto M, Lee CH, Joshua AM, de Toledo SR, Petrilli AS, et al. Effects of THBS3, SPARC and SPP1 expression on biological behavior and survival in patients with osteosarcoma. BMC Cancer. (2006) 6:237. doi: 10.1186/1471-2407-6-237
18. Han S, Cao C, Tang T, Lu C, Xu J, Wang S, et al. ROBO3 promotes growth and metastasis of pancreatic carcinoma. Cancer Lett. (2015) 366:61–70. doi: 10.1016/j.canlet.2015.06.004
19. Yu J, Feng Y, Wang Y, An R. Aryl hydrocarbon receptor enhances the expression of miR-150-5p to suppress in prostate cancer progression by regulating MAP3K12. Arch Biochem Biophys. (2018) 654:47–54. doi: 10.1016/j.abb.2018.07.010
20. Wang J, Zhu X, Ying P, Zhu Y. PIF1 affects the proliferation and apoptosis of cervical cancer cells by influencing TERT. Cancer Manag Res. (2020) 12:7827–35. doi: 10.2147/CMAR.S265336
21. Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. (2004) 432:316–23. doi: 10.1038/nature03097
22. The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. (2013) 499:43–9. doi: 10.1038/nature12222
23. Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, et al. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun. (2017) 8:1769. doi: 10.1038/s41467-017-01965-8
24. Gatto F, Nookaew I, Nielsen J. Chromosome 3p loss of heterozygosity is associated with a unique metabolic network in clear cell renal carcinoma, Proc Natl Acad Sci USA. (2014) 111:E866–75. doi: 10.1073/pnas.1319196111
25. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. (2004) 4:11–22. doi: 10.1038/nrc1252
26. Bishnoi M, Jain A, Hurkat P, Jain SK. Chondroitin sulphate: a focus on osteoarthritis. Glycoconj J. (2016) 33:693–705. doi: 10.1007/s10719-016-9665-3
27. Hoppe MM, Sundar R, Tan DSP, Jeyasekharan AD. Biomarkers for homologous recombination deficiency in cancer. J Natl Cancer Inst. (2018) 110:704–13. doi: 10.1093/jnci/djy085
28. Paré L, Pascual T, Seguí E, Teixidó C, Gonzalez-Cao M, Galván P, et al. Association between PD1 mRNA and response to anti-PD1 monotherapy across multiple cancer types. Ann Oncol. (2018) 29:2121–8. doi: 10.1093/annonc/mdy335
Keywords: TRIM46, clear cell renal cell carcinoma, prognosis, biomarker, therapeutic target
Citation: Ren X-b, Zhao J, Liang X-f, Guo X-d, Jiang S-b and Xiang Y-z (2021) Identification TRIM46 as a Potential Biomarker and Therapeutic Target for Clear Cell Renal Cell Carcinoma Through Comprehensive Bioinformatics Analyses. Front. Med. 8:785331. doi: 10.3389/fmed.2021.785331
Received: 29 September 2021; Accepted: 26 October 2021;
Published: 22 November 2021.
Edited by:
Fan Feng, The 302th Hospital of PLA, ChinaReviewed by:
Li-xin Tang, Chongqing Public Health Medical Center, ChinaBin Liu, Affiliated Hospital of Hebei University, China
Copyright © 2021 Ren, Zhao, Liang, Guo, Jiang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-zhu Xiang, eXV6aHV4MTk5QDE2My5jb20=
 Xiang-bin Ren1
Xiang-bin Ren1 Yu-zhu Xiang
Yu-zhu Xiang