
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 16 December 2021
Sec. Pulmonary Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.783720
This article is part of the Research Topic Insights in Pulmonary Medicine: 2021 View all 10 articles
 Ting-Yu Lin1,2
Ting-Yu Lin1,2 Horng-Chyuan Lin1,2
Horng-Chyuan Lin1,2 Yun-Sheng Liu3
Yun-Sheng Liu3 Yu-Lun Lo1,2
Yu-Lun Lo1,2 Chun-Hua Wang1,2
Chun-Hua Wang1,2 Po-Jui Chang1,2
Po-Jui Chang1,2 Chun-Yu Lo1,2
Chun-Yu Lo1,2 Shu-Min Lin1,2*
Shu-Min Lin1,2*Background: Traffic-related pollution is associated with the onset of asthma and the development of different phenotypes of asthma. Few studies have investigated the association between traffic proximity and late-onset of asthma (LOA) and early-onset asthma (EOA). This study was conducted to investigate the associations of LOA phenotypes with a function of the distance between residence and heavy traffic roads (HTRs).
Methods: The study group consisted of 280 patients who were (LOA: 78.4%) recruited consecutively from a pay-for-performance asthma program to clarify the patient characteristics and proximity to HTRs within 1,000 m from their residences between EOA and LOA in three urban centers in Taiwan. The subsequent analysis focused on patients with LOA (n = 210) linking phenotypes and distance to HTRs.
Results: Subjects with LOA tended to be older than those with EOA and had shorter asthma duration, poorer lung function, lower atopy, and less exposure to fumes or dust at home. Patients with LOA were more likely than those with EOA to live within 900 m of two or more HTRs (14.3 vs. 3.4%, p = 0.02). Among patients with LOA, minimum distance to an HTR was negatively associated with numbers of specific IgE as well as positively associated with the age of onset and body weight significantly. A higher proportion of patients with atopy (26.3 vs. 20.6%, p = 0.001. odds ratio [OR]: 2.82) and anxiety/depression (21.0 vs. 18.1%, p = 0.047. OR: 1.81) and a trend of lower proportion of patients with obese (5.7 vs. 12.4%, p = 0.075) were found to be living within 900 m from HTRs.
Conclusions: Late-onset of asthma (LOA) tended to live in areas of higher HTR density compared to EOAs. Among patients with LOA living close to HTRs, the interaction between traffic-related pollution, allergy sensitization, and mood status were the factors associated with asthma onset early. Obesity may be the factor for later onset who live far from HTRs.
After an initial appearance in childhood, asthma may remain inactive for an extended period or reappear later in life. A number of recent studies have also described the onset of asthma during adulthood (1). Thus, early-onset asthma (EOA) and late-onset asthma (LOA) can be viewed as two distinct phenotypes, based on the categorization of disease entities according to underlying mechanisms or endotypes, such as risk factors, remission rates, co-morbidities, and gene expression profiles (2–4). The age at diagnosis determining the early vs. late-onset asthma varies from 12 or 18 years (4–6) of age to 40 (7) years of age. However, emerging evidence indicates that environmental factors also play a critical role in the development of asthma. The risk factors of EOA include single nucleotide polymorphisms on chromosome 17q21, atopic status, rhinovirus infection, and exposure to traffic-related air pollution (TRAP) (8–12). TRAP is one of the major environmental impacts of urbanization and previous research has shown the long-term effects on asthma onset in children (5, 13). Among children, proximity to traffic has been linked to an elevated risk of asthma (12, 14–16).
Despite the non-negligible incidence of adult-onset asthma, the causes have not been extensively investigated. Identifying the risk factors of LOA is crucial to understanding the underlying mechanisms and LOA was identified as the most significant independent risk factor for non-remission in patients with asthma (17). It should be noted however that the risk factors for LOA are more complex than those for EOA. Specific characteristics have been mentioned for their different influence on EOA and LOA. A population-based study in Finland showed the incidence of allergic asthma decreases with advancing age and after the age of 40, new asthma cases are almost non-atopic (18). The other study in Finland discovered the influence of the family history of asthma is higher on EOA than LOA (risk ratio (RR): 4.10 vs. 1.44) (9). A European survey discovered patients with chronic rhinosinusitis reported less EOA (RR: 0.45) but more LOA (RR: 3.09) (19). In the cluster study of asthma phenotypes, obese female is one of the major cluster of LOA (20). One large-scale genome-wide association study (GWAS) suggested that non-genetic risk factors play a more important role in LOA than in EOA. This is a clear indication that environmental factors are worthy of further consideration (21). The important role of TRAP in adult-onset asthma has also been emphasized (22). The association between traffic proximity and LOA has been identified by a certain amount of traffic volume and patients in risk (23–25). One study of our adult asthma cohort reported that higher IL-17A expression in the epithelium of patients among those living within 1,000 m from heavy traffic roads (HTRs) than among those living more than 1,000 m from HTRs (26).
In the current study, we were interested in whether the traffic proximity was different in EOA and LOA. We sought to determine whether proximity or density of HTRs is associated with the LOA in the asthma cohort of an urban medical center. We also examined patient characteristics in order to identify factors significantly associated with traffic proximity in LOA.
This was a cross-sectional study of asthma patients recruited consecutively in a pay-for-performance program at Chang Gung Memorial Hospital, Linkou branch, which has been implemented by the National Health Insurance Administration (NHIA) in Taiwan since 2001 (27). Certified physicians and case managers provide in-person training pertaining to asthma control, asthma care planning, and proper inhaler usage. The outcomes are regularly monitored by the NHIA (https://www.nhi.gov.tw/Content_List.aspx?=nEBDEAEDEC639490C&topn=5FE8C9FEAE863B46). Inclusion criteria included the diagnosis of asthma by a pulmonologist in accordance with ICD-10 code J45 at least twice within 90 days. Note that diagnoses were based on episodic respiratory symptoms (wheezing, breathlessness, chest tightness, and cough), variable or persistent obstructive pulmonary function, and response to asthma therapy. All patients provided written informed consent. The study protocol was approved by the Chang Gung Medical Foundation Institutional Review Board (No. 201900211B0).
At the initial recruitment, we recorded the characteristics of the subjects based on questionnaires or medical records, including age at the time of asthma diagnosis by a physician, asthma control test (ACT) results, family history of asthma, the use of asthma medication, co-morbidities, childhood history of dyspnea, frequency of bronchitis, exposure to fumes or dust at home or work, smoking status, and current residence (in the last 6 months). Pulmonary function and allergy-related biomarkers, including eosinophils, eosinophil cation protein (ECP), immunoglobulin E (IgE), and specific IgE (ImmunoCAP, Phadia, Sweden) were recorded. Patients with any positive specific IgE to allergens (>0.35 KU/L,) were considered atopic. The tests above were done at physicians' discretion in real-world practice. For example, according to the regulation of Taiwan healthcare insurance, the reimbursement of specific IgE would not be offered unless the total IgE > 25 KU/L. Therefore, a test of specific IgE was not mandatory and the numbers of sIgE to check were based on the physicians' discretion.
The total cohort of 283 patients was divided into two groups according to age at the time of asthma onset. Patients who were ≥ 18 years old at the time of asthma onset without a childhood history of dyspnea and frequency of bronchitis were defined as LOA. Otherwise, they were considered as EOA. It was determined that 94.7% of the total cohort were living in major urban centers: EOA (n = 58) and LOA (n = 210). This group of urban patients was subjected to further analysis to determine the proximity to HTRs within 1,000 m from their residences (26, 28) and whether distance to HTRs affected the age of asthma onset (analysis plan was illustrated in Supplementary Figure S1).
Heavy traffic roads (HTRs) were identified using open-data daily PCU (Passenger Car Unit) statistics from the Directorate General of Highways of Taiwan (https://www.thb.gov.tw/sites/en/). Based on the geographic distribution of patients, we selected the ten busiest traffic monitoring sites, each of which had a daily mean bidirectional PCU exceeding 36,329 in 2018 (Supplementary Figure S2).
Geometric data were extracted from maps obtained from the open-data service – Open Street Map (https://www.openstreetmap.org/export). Data covered the region between latitudes from 24.74 to 25.33 N and longitudes from 120.86 to 122.04 E. All map data other than roads and streets were excluded. Criteria for the selection of routing vectors were based on the Top-10 PCU routes. We sought to mitigate sphere projection bias by re-projecting the coronadite system of geometrics (e.g., routes or patient locations) from EPSG:4326 (WGS84 – World Geodetic System 1984, used in GPS) to EPSG:32651 (WGS 84 – UTM Zone 51N). This converted the spherical representation in radian units into a 2D flat surface presentation in meters, which is a convenient format for subsequent calculation. Geodata was processed using a custom script written in JavaScript under the NodeJS (version 12.9.1. OpenJS Foundation. San Francisco, California. Joyent, Inc.) runtime environment, and all exchangeable data formats were standardized according to GeoJSON format. The graphical representation of input variables and calculation results was handled using QGIS software (version 3.10.1. QGIS.org, 2021. QGIS Geographic Information System. QGIS Association. https://www.qgis.org).
Linear algebra (i.e., point to vector distance) was used to calculate the minimum distance between the domicile of each patient and the nearest HTR based on geometric data (Supplementary Figure S3). We also calculated the overall density of traffic in the areas surrounding the domicile of each patient by counting the number of HTRs within circles of various sizes, starting at 100 m and extending to a maximum distance of 1,000 m (26, 28).
All data were expressed as mean ± SD or percentage. The Student's t-test was used to compare the means of continuous variables and normally distributed data; otherwise, the Mann-Whitney test was used. Categorical variables including patients with EOA and LOA living in ≥ 1 or 2 heavy-traffic roads within indicated distance were tested using the Chi-square test or Fisher exact test. For tests done by physicians' discretion, the numbers are tested were smaller than the total cohort. The analysis was done on the patients who were checked and the number of participants who provided information and the number of participants with positive results were specified. Unadjusted odds ratio (OR) and 95% CI were calculated for selective variables in geometric analysis during the Chi-square test. The association between the minimum distance to an HTR and patient factors was tested using the Spearman rank correlation because the distance was not normally distributed. All analysis was performed using IBM SPSS Statistics version 19. Armonk, NY: IBM Corp. Statistically significant results were defined as p ≤ 0.05.
Between July 2019 and June 2020, a total of 283 asthma patients were consecutively enrolled in the pay-for-performance program. Among these asthmatics, 222 subjects (78.4%) were LOA. Table 1 shows the characteristics of patients with EOA and LOA. Supplementary Figure S4 presents the distribution of patient ages and ages of onset. The mean age of onset of EOA and LOA was 8.6 years and 52.2 years, respectively. Compared to the EOA group, participants with LOA were older, had a shorter duration of asthma onset, less association of asthma family history, less exposure to fumes or dust at home, and a higher proportion of smoking habits. There was no difference in gender, co-morbidities, weight status, exacerbation history in the last year, and ACT score between the two groups. The pre-bronchodilator forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were lower in patients with LOA than in patients with EOA. As for allergic biomarkers, IgE levels were significantly higher in the EOA group than in the LOA group. The proportion of patients with ECP levels exceeding the normal range (18 μg/L) was higher in the EOA group than in the LOA group. The two groups were comparable in terms of eosinophil count. Patients with EOA tended to be more atopic and were also more susceptible to home dust mite (HDM), cat dander, and dog dander than patients with LOA (Supplementary Table S1).
As shown in Figures 1, 2, we observed no significant differences between the LOA and EOA groups in terms of minimum distance to the nearest HTR (EOA vs. LOA: 1,124 ± 787 m vs. 1,412 ± 175 m; p = 0.07). We further analyzed the number of HTRs within regions that were measured from patient residences at set distances of up to 1,000 m. When patients resided with a distance of 900 m in the high-traffic road, more patients with LOA were living in multiple HTRs areas compared to patients with EOA (≥ 2 HTRs; 14.3 vs. 3.4%, p = 0.023. Table 2). Subgroup analysis was performed to identify patient characteristics associated with multiple HTRs within 900 m. Briefly, this involved pooling 30 patients with LOA with two patients with EOA for analysis. Compared to patients living in fewer than two HTRs within 900 m, those living in more than two HTRs had higher IgE levels (Table 3, mean ± SD, 426.9.1 ± 798.2 vs. 252.9 ± 360.8 KU/L, p = 0.05), more positive atopic status (75 vs. 49.8%, p = 0.02), and a higher sensitivity to HDM (64.3 vs. 43.2%, p = 0.04).
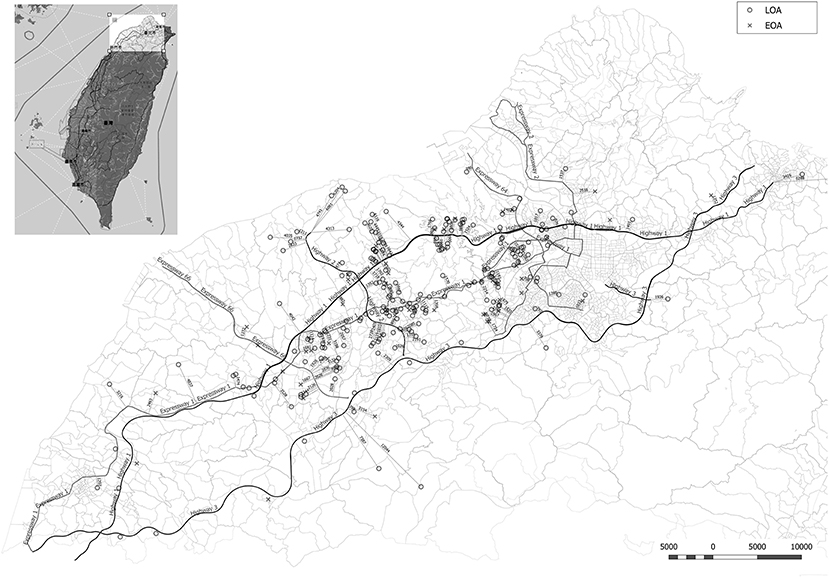
Figure 1. Minimum distance from the residence of asthmatic patients to the nearest heavy traffic road in New Taipei City, Taoyuan, and Hsinchu, Taiwan. EOA residences are indicated by X and LOA residences are indicated by circles. The heavy traffic roads in this study included three national highways (Highway 1, 2, and 3) and five expressways (Expressway 1, 2, 3, 64, and 66).
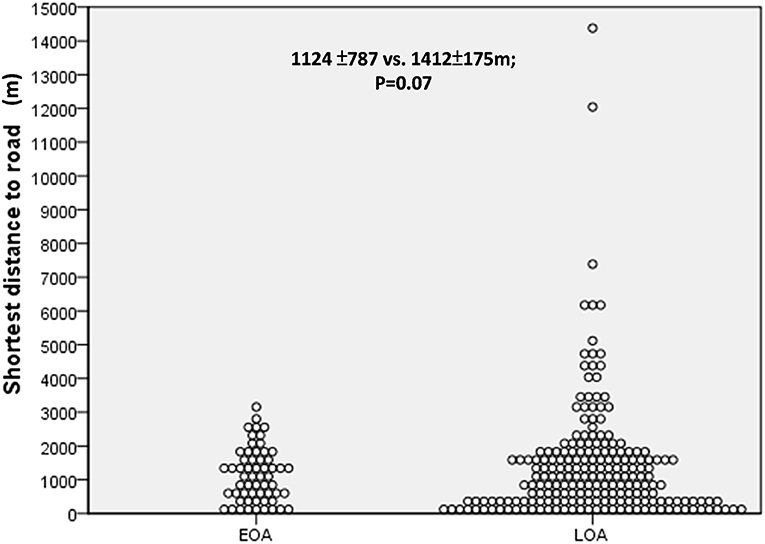
Figure 2. The minimum distance to the nearest heavy traffic road (HTR) between early-onset asthma (EOA) vs. late-onset asthma (LOA). No significant difference was observed between patients with EOA and LOA in terms of minimum distance to HTR.
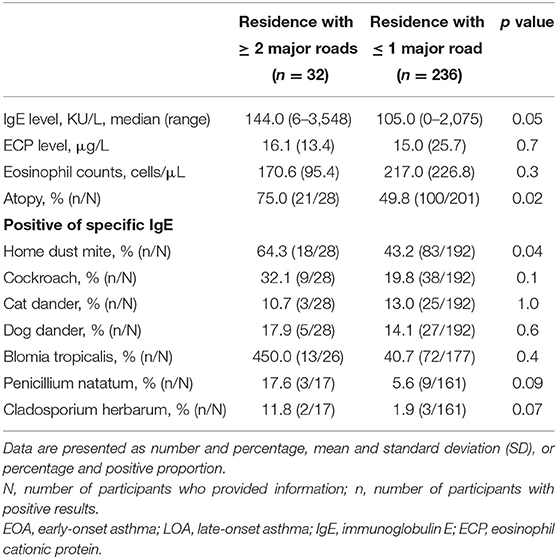
Table 3. Asthma-associated inflammatory markers in asthmatic patients living in areas with or without high-density traffic as indicated by at least two heavy traffic roads within 900 meters.
To clarify the influence of high traffic density on patients with LOA, we conducted further analysis on the correlation between the distance to HTRs and patient characteristics in the LOA group (Figure 3 and Supplementary Table S2). The minimum distance to an HTR was positively correlated with age of onset (Figure 3A, Spearman's rho 0.151, p = 0.025), BMI (Figure 3B, Spearman's rho 0.157, p = 0.023) as well as negatively correlated with the numbers of positive specific IgEs (Figure 3C, Spearman's rho −0.213, p = 0.005).
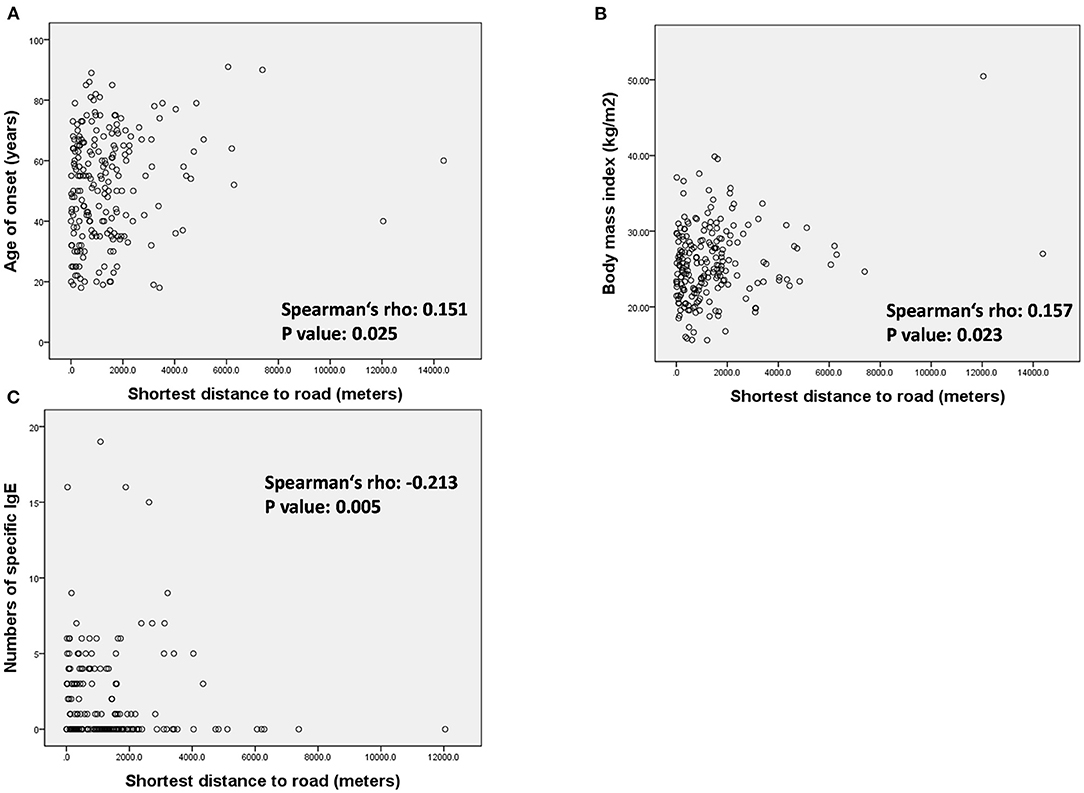
Figure 3. Association between minimum distance to heavy traffic road and (A) age of onset, (B) body mass index, and (C) number of specific IgE in the patients with LOA.
For the binary variables and confirming the results of the correlation test, we compared the atopy, mood status, and obesity (BMI ≥ 30) of patients with LOA living within or beyond 900 m of HTRs (Figure 4). A higher proportion of patients with LOA with atopic status (26.3 vs. 20.5%, p = 0.001, unadjusted OR: 2.82, 95%CI: 1.519–5.235. Figure 4A) and depression or anxiety were found to be living within 900 m from HTRs (21.0 vs. 18.1%, p = 0.047, unadjusted OR: 1.81, 95%CI: 1.031–3.165. Figure 4B). In contrast, there was a trend of a higher proportion of obese patients were living beyond 900 m from HTRs (12.4 vs. 5.7%, p = 0.075, unadjusted OR: 1.974, 95%CI: 0.936–4.165. Figure 4C). Interestingly, the proportion of obese patients living beyond 1,000 m from HTRs was statistically higher than those living within 1,000 m to HTRs (data not shown).
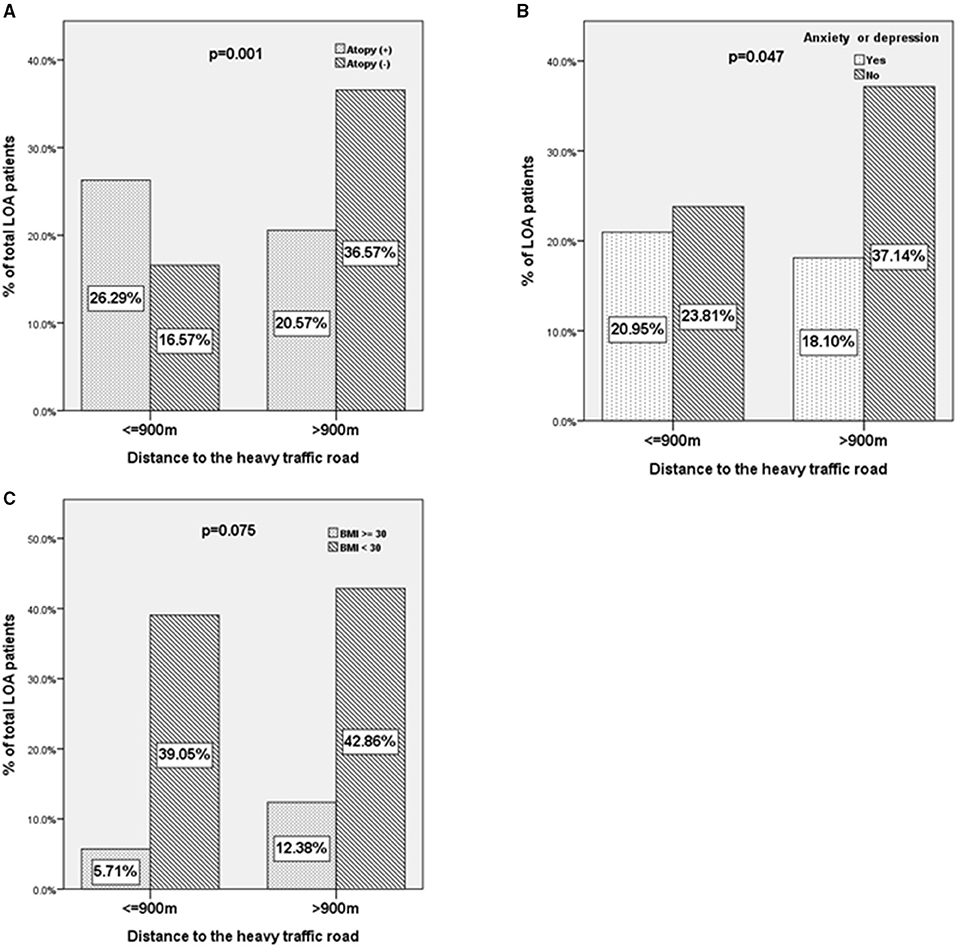
Figure 4. The characteristics of patients with LOA according to the distance away from heavy traffic roads. (A) Atopy status, (B) anxiety or depression, and (C) body mass index ≥30 of patients with LOA as a function of the distance between residence and heavy traffic roads (≤900 vs. >900 m).
Our analysis revealed that patients with LOA tended to be older than patients with EOA, to have had asthma for a shorter duration, were less likely to have a family history of asthma, were less exposed to fumes or dust in the home, were less likely to be atopic, were less sensitive to common inhaled allergens, and were more likely to have poor lung function. In urban areas, more patients with LOA had multiple HTRs (≥ 2 HTRs) within a distance of 900 m. Patients living within 900 m from multiple HTRs had a higher total IgE level, a more atopic status, and a higher sensitivity to HDM, compared to patients with ≥ 1 HTRs at a comparable distance. Among patients with LOA, the minimum distance to an HTR was positively associated with the age of onset and BMI and was negatively associated with atopy and mood status. Among patients with LOA, a higher proportion of atopic patients were living within 900 m from HTRs, a higher proportion of patients with anxiety or depression were living within 900 m from HTRs and a trend of a higher proportion of obese patients were living beyond 900 m from HTRs. To the best of our knowledge, this is the first study demonstrating the different traffic density between EOA and LOA and different phenotypes in LOA by geolocation, which showed the novel relation between asthma phenotypes and the urban environment.
Correlation between TRAP and asthma is usually assessed in terms of pollutant concentration, such as nitrogen oxide and particulate matter (PM), or the distance to HTRs. Numerous cohort studies have demonstrated a positive association between exposure to TRAP and the risk of asthma (11, 23, 29, 30); however, studies on the link between proximity to HTRs and the risk of asthma have been inconsistent. A birth cohort study in southwestern British Columbia failed to observe a significant correlation between proximity to highways and major roads (<150 m) and development of childhood asthma (15). A birth cohort study in New York City reported a significant correlation between proximity to heavy traffic (<250 m) and childhood asthma among patients without a history of moving prior to the age of 5 (16). In a cohort study of children with asthma attending elementary school in an urban area of the northeastern United States, the incidence of asthma symptoms was shown to increase inversely with the distance to major roads (12). One cohort study conducted in three cities in Sweden reported a positive association between adult-onset asthma and proximity (<50 m) to major roads (≥8,000 vehicles/day) (23). Two cross-sectional studies examined the links between subgroups of asthma and proximity to major roads. One study on adults in southern Sweden discovered that proximity (>100 m) to a major road (>10 cars/min) was associated with allergic asthma but not with non-allergic asthma (24). One study on adults in Tasmania, Australia, (i.e., an area with low air pollution levels) reported that proximity (<200 m) to a major road was associated with an elevated risk of asthma; however, this was only among carriers of glutathione S-transferase theta-1 (25). The different distances to HTR for asthma risk in the studies above may result from the different traffic volumes, the population in risks, and the local environment of individual cities. In the current study, HTRs were defined by daily mean PCU exceeding 36,329, which was much higher than that of other studies (23, 24). The range of 1,000 m to HTR was selected initially because the relevant reports included one study from our asthma patients (26, 28). By defining HTRs with the top-ten high traffic volume from the official database, we determined that living within 900 m of multiple HTRs was associated with an elevated risk of LOA compared to EOA. The current study provides new evidence of the greater impact of heavy traffic exposure for LOA. In addition to the direct distance to HTRs, exposure to the density of HTRs is also an important determinant for asthma control.
The patient profiles revealed by the subgroup analysis of patients living with multiple HTRs provided one of the possible mechanisms of asthma inception of LOA (Table 3). Patients living within 900 m from multiple HTRs presented higher total IgE levels, were more likely to be atopic and were more likely to present sensitivity to HDM, compared to patients with fewer than two HTRs at a comparable distance. We also found that asthma onset was earlier among patients with LOA living near HTRs. This suggests that an interaction between TRAP and allergic sensitization may be the force driving asthma inception at an early age among LOA living near Figures 3, 4. Similar to our results, a cross-sectional study revealed associations between exposure to high road density and the prevalence of allergic sensitization and small airway function in subjects with a family history of asthma (31). Previous animal studies on TRAP exposure and allergic sensitization support our results. In adult and neonatal mice, co-exposure to diesel exhaust particles (DEPs) and house dust mites was shown to promote the persistence of TH2/TH17 effector/memory cells in the lungs (32). In studies in an adult mouse model, DEP and HDM co-exposure has also been shown to enhance airway hyper-responsiveness and generate a mixed TH2 and TH17 response or the number of type 2 innate lymphoid cells (33, 34). Co-exposure to HDM and benzo(a)pyrene has also been shown to enhance IL-33 and TSLP production in an asthma mouse model (35). Environmental factors (e.g., ambient air polyaromatic hydrocarbons, PM, and DEP) have been linked to epigenetic changes that modify the gene expression of T regulatory cells or innate response, further promoting the Th2 response (36).
Anxiety and depression contribute to asthma symptoms and stressful life events have been shown as the risk factor of onset of asthma (37, 38). Some studies have shown psychiatric stress enhances allergic inflammation (39, 40). We found the risk of anxiety or depression increased in patients living within 900 m to HTRs (Figure 4B). This finding further demonstrated the complex interactions between the psychiatric status, traffic exposure in patients with LOA, and further studies for better analysis are required.
The incidence of obesity in patients with LOA was higher among those living >900 m from HTRs than among those living <900 m from HTRs (Figure 4C). This conflicts with a number of previous studies suggesting that air pollution plays a role in the incidence of asthma among the obese (41). By contrast, the incidence of atopic asthma in patients with LOA was lower among those living far from HTRs (Figures 3, 4). We surmise that exposure to TRAP may play a more important role in the pathogenesis of atopic asthmatics than it does in obese asthmatics. Future studies on obesity-related LOA and TRAP in urban areas are required.
The study has several limitations. First, the cohort had a smaller patient number of EOA compared to LOA. This is because we recruited adult patients consecutively without selection in the pay-for-performance program to prevent selection bias. The higher ratio of LOA to EOA in our cohort is possible due to the remission rate of EOA being much higher than LOA and LOA is suggested to be more severe than EOA (2, 42). Therefore, similar to other cohorts (43), patients with LOA were referred from local clinics to our center more frequently than patients with EOA. The present results from a single center will have to be confirmed further in subsequent longitudinal studies in a larger population. Second, self-reports pertaining to the age of the patient at the time of diagnosis by a physician were subject to recall bias. Note that we were unable to obtain documented medical records related to asthma diagnosis; however, the patient characteristics in the current study (e.g., family history of lung function) were comparable with those obtained in large-cohort studies and major review articles related to LOA (2, 3, 6, 9, 18, 44). Third, covariates of exposure to fume or dust or comorbidities were defined by questionnaires or medical records, not by real inspection of patients' environments or strict medical diagnostic criteria. The results of a negative association between exposure and comorbidities and HTR proximity in the current study are required further studies to confirm. Fourth, we were unable to obtain information related to TRAP concentrations; therefore, our findings are not necessarily generalizable to all HTRs. Our findings could have been affected by local climatic conditions, occupational exposure, and indoor air pollution in the individual urban environment.
In conclusion, the characteristics of patients with LOA were distinct from those of patients with EOA. By geolocation in urban centers, we discovered the correlation between asthma phenotypes and the urban environment in LOA. patients with LOA tended to live in areas of higher HTR density, which was associated with an elevated incidence of atopic symptoms, sensitivity to HDM, and mood disorder. Proximity to HTRs and obesity may be factors contributing to uncontrolled asthma in cases of LOA. The novel evidence of patient-environment interaction provides further explanation for asthma persistence in the modern world.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Chang Gung Medical Foundation Institutional Review Board (No. 201900211B0). The patients/participants provided their written informed consent to participate in this study.
T-YL: conceptualization, data curation, investigation, formal analysis, and writing of the original draft. Y-SL: methodology, software, formal analysis, and visualization. H-CL: data curation, resources, and formal analysis. S-ML: data curation, resources supervision, formal analysis, and writing with review and editing. Y-LL, C-HW, P-JC, and C-YL: data curation and resources. All authors contributed to the article and approved the submitted version.
Y-SL is employed by BalDr Strategic Consulting (Hong Kong) Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.783720/full#supplementary-material
1. De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G, et al. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. (2002) 110:228–35. doi: 10.1067/mai.2002.125600
2. de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. (2013) 22:44–52. doi: 10.1183/09059180.00007112
3. Amelink M, de Groot JC, de Nijs SB, Lutter R, Zwinderman AH, Sterk PJ, et al. Severe adult-onset asthma: A distinct phenotype. J Allergy Clin Immunol. (2013) 132:336–41. doi: 10.1016/j.jaci.2013.04.052
4. Hekking PP, Loza MJ, Pavlidis S, de Meulder B, Lefaudeux D, Baribaud F, et al. Pathway discovery using transcriptomic profiles in adult-onset severe asthma. J Allergy Clin Immunol. (2018) 141:1280–90. doi: 10.1016/j.jaci.2017.06.037
5. Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. (2007) 369:571–7. doi: 10.1016/S0140-6736(07)60037-3
6. Westerhof GA, Vollema EM, Weersink EJ, Reinartz SM, de Nijs SB, Bel EH. Predictors for the development of progressive severity in new-onset adult asthma. J Allergy Clin Immunol. (2014) 134:1051–6 e1052. doi: 10.1016/j.jaci.2014.05.005
7. Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther. (2017) 43:39–45. doi: 10.1016/j.pupt.2017.01.011
8. Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. (2007) 448:470–3. doi: 10.1038/nature06014
9. Paaso EM, Jaakkola MS, Rantala AK, Hugg TT, Jaakkola JJ. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: a prospective cohort study. Respir Res. (2014) 15:152. doi: 10.1186/s12931-014-0152-8
10. Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. (2008) 178:667–72. doi: 10.1164/rccm.200802-309OC
11. Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. (2015) 70:245–56. doi: 10.1111/all.12561
12. Hauptman M, Gaffin JM, Petty CR, Sheehan WJ, Lai PS, Coull B, et al. Proximity to major roadways and asthma symptoms in the School Inner-City Asthma Study. J Allergy Clin Immunol. (2020) 145:119–26 e114. doi: 10.1016/j.jaci.2019.08.038
13. McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. (2010) 118:1021–6. doi: 10.1289/ehp.0901232
14. Penard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. (2010) 36:33–40. doi: 10.1183/09031936.00116109
15. Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. (2010) 118:284–90. doi: 10.1289/ehp.0900916
16. Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, et al. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res. (2011) 111:1222–9. doi: 10.1016/j.envres.2011.08.004
17. Honkamaki J, Piirila P, Hisinger-Molkanen H, Tuomisto LE, Andersen H, Huhtala H, et al. Asthma remission by age at diagnosis and gender in a population-based study. J Allergy Clin Immunol Pract. (2020).
18. Pakkasela J, Ilmarinen P, Honkamaki J, Tuomisto LE, Andersen H, Piirila P, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. (2020) 20:9. doi: 10.1186/s12890-019-1040-2
19. Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. (2012) 67:91–8. doi: 10.1111/j.1398-9995.2011.02709.x
20. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. (2008) 178:218–24. doi: 10.1164/rccm.200711-1754OC
21. Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med. (2019) 7:509–22. doi: 10.1016/S2213-2600(19)30055-4
22. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. (2014) 383:1581–92. doi: 10.1016/S0140-6736(14)60617-6
23. Modig L, Toren K, Janson C, Jarvholm B, Forsberg B. Vehicle exhaust outside the home and onset of asthma among adults. Eur Respir J. (2009) 33:1261–7. doi: 10.1183/09031936.00101108
24. Lindgren A, Stroh E, Nihlen U, Montnemery P, Axmon A, Jakobsson K. Traffic exposure associated with allergic asthma and allergic rhinitis in adults. A cross-sectional study in southern Sweden. Int J Health Geogr. (2009) 8:25. doi: 10.1186/1476-072X-8-25
25. Bowatte G, Lodge CJ, Knibbs LD, Lowe AJ, Erbas B, Dennekamp M, et al. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol. (2017) 139:122–9 e121. doi: 10.1016/j.jaci.2016.05.008
26. Weng CM, Lee MJ, He JR, Chao MW, Wang CH, Kuo HP. Diesel exhaust particles up-regulate interleukin-17A expression via ROS/NF-kappaB in airway epithelium. Biochem Pharmacol. (2018) 151:1–8. doi: 10.1016/j.bcp.2018.02.028
27. Kao YH, Wu SC. STROBE-compliant article: Is continuity of care associated with avoidable hospitalization among older asthmatic patients? Medicine (Baltimore). (2016) 95:e4948. doi: 10.1097/MD.0000000000004948
28. Brunekreef B, Janssen NA, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. (1997) 8:298–303. doi: 10.1097/00001648-199705000-00012
29. Gehring U, Wijga AH, Koppelman GH, Vonk JM, Smit HA, Brunekreef B. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J. (2020) 56:2000147. doi: 10.1183/13993003.00147-2020
30. Kunzli N, Bridevaux PO, Liu LJ, Garcia-Esteban R, Schindler C, Gerbase MW, et al. Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. (2009) 64:664–70. doi: 10.1136/thx.2008.110031
31. Hansell AL, Rose N, Cowie CT, Belousova EG, Bakolis I, Ng K, et al. Weighted road density and allergic disease in children at high risk of developing asthma. PLoS ONE. (2014) 9:e98978. doi: 10.1371/journal.pone.0098978
32. Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol. (2015) 136:295–303 e297. doi: 10.1016/j.jaci.2014.11.043
33. Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. (2013) 132:1194–204 e1192. doi: 10.1016/j.jaci.2013.06.048
34. De Grove KC, Provoost S, Hendriks RW, McKenzie ANJ, Seys LJM, Kumar S, et al. Dysregulation of type 2 innate lymphoid cells and TH2 cells impairs pollutant-induced allergic airway responses. J Allergy Clin Immunol. (2017) 139:246–57 e244. doi: 10.1016/j.jaci.2016.03.044
35. Wang E, Liu X, Tu W, Do DC Yu H, Yang L, et al. Benzo(a)pyrene facilitates dermatophagoides group 1 (Der f 1)-induced epithelial cytokine release through aryl hydrocarbon receptor in asthma. Allergy. (2019) 74:1675–90. doi: 10.1111/all.13784
36. Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. (2010) 126:453–65. doi: 10.1016/j.jaci.2010.07.030
37. Lavoie KL, Cartier A, Labrecque M, Bacon SL, Lemiere C, Malo JL, et al. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients? Respir Med. (2005) 99:1249–57. doi: 10.1016/j.rmed.2005.03.003
38. Lietzen R, Virtanen P, Kivimaki M, Sillanmaki L, Vahtera J, Koskenvuo M. Stressful life events and the onset of asthma. Eur Respir J. (2011) 37:1360–5. doi: 10.1183/09031936.00164609
39. Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. (2002) 165:1062–7. doi: 10.1164/ajrccm.165.8.2109065
40. Theoharides TC, Enakuaa S, Sismanopoulos N, Asadi S, Papadimas EC, Angelidou A, et al. Contribution of stress to asthma worsening through mast cell activation. Ann Allergy Asthma Immunol. (2012) 109:14–9. doi: 10.1016/j.anai.2012.03.003
41. Dixon AE, Poynter ME. Mechanisms of Asthma in Obesity. Pleiotropic aspects of obesity produce distinct asthma phenotypes. Am J Respir Cell Mol Biol. (2016) 54:601–8. doi: 10.1165/rcmb.2016-0017PS
42. Westerhof GA, Coumou H, de Nijs SB, Weersink EJ, Bel EH. Clinical predictors of remission and persistence of adult-onset asthma. J Allergy Clin Immunol. (2018) 141:104–9 e103. doi: 10.1016/j.jaci.2017.03.034
43. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. (2013) 41:1308–14. doi: 10.1183/09031936.00100811
Keywords: late onset asthma, traffic proximity, urban environment, asthma phenotype, traffic density
Citation: Lin T-Y, Lin H-C, Liu Y-S, Lo Y-L, Wang C-H, Chang P-J, Lo C-Y and Lin S-M (2021) Proximity to Heavy Traffic Roads and Patient Characteristics of Late of Onset Asthma in an Urban Asthma Center. Front. Med. 8:783720. doi: 10.3389/fmed.2021.783720
Received: 26 September 2021; Accepted: 15 November 2021;
Published: 16 December 2021.
Edited by:
Hsiao-Chi Chuang, Taipei Medical University, TaiwanReviewed by:
Steven Sai Hang Ho, Desert Research Institute (DRI), United StatesCopyright © 2021 Lin, Lin, Liu, Lo, Wang, Chang, Lo and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Min Lin, c21saW4xMDBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.