- 1Department of Integrated Traditional Chinese and Western Medicine, Sichuan Provincial Pancreatitis Centre and West China-Liverpool Biomedical Research Centre, West China Hospital, Sichuan University, Chengdu, China
- 2Liverpool Pancreatitis Research Group, Liverpool University Hospitals NHS Foundation Trust and Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, United Kingdom
- 3Department of Anaesthesiology, Laboratory of Anaesthesia and Critical Care Medicine, National-Local Joint Engineering Research Centre of Translational Medicine of Anaesthesiology, West China Hospital, Sichuan University, Chengdu, China
- 4Clinical Research and Epidemiology, Institute of Liver and Biliary Sciences, New Delhi, India
- 5Division of Gastroenterology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, United States
Background: Pain management is an important priority in the treatment of acute pancreatitis (AP). Current evidence and guideline recommendations are inconsistent on the most effective analgesic protocol. This systematic review and meta-analysis of randomised controlled trials (RCTs) aimed to compare the safety and efficacy of analgesics for pain relief in AP.
Methods: A literature search was performed to identify all RCTs assessing analgesics in patients with AP. The primary outcome was the number of participants who needed rescue analgesia. Study quality was assessed using Jadad score. Pooled odds ratios (ORs) or weighted mean differences (WMDs) with 95% confidence intervals (CI) were analysed using a random-effects model.
Results: Twelve studies comprising 699 patients with AP (83% mild AP) were analysed. The tested analgesics significantly decreased the need for rescue analgesia (3 studies, OR.36, 95% CI 0.21 to 0.60) vs. placebo or conventional treatment. The analgesics also improved the pain score [Visual Analogue Scale (Δ-VAS)] at 24 h (WMD 18.46, 0.84 to 36.07) and by the 3rd to 7th days (WMD 11.57, 0.87 to 22.28). Opioids vs. non-opioids were associated with a decrease in the need for rescue analgesia (6 studies, OR 0.25, 95% CI 0.07 to 0.86, p = 0.03) but without significance in pain score. In subgroup analyses, opioids were similar to non-steroidal anti-inflammatory drugs (NSAIDs) regarding the primary outcome (4 studies, OR 0.56, 95% CI 0.24 to 1.32, p = 0.18). There were no significant differences in other clinical outcomes and rate of adverse events. Other studies, comparing epidural anaesthesia vs. patient-controlled analgesia and opioid (buprenorphine) vs. opioid (pethidine) did not show significant difference in primary outcome. Study quality issues significantly contributed to overall study heterogeneity.
Conclusions: NSAIDs and opioids are equally effective in decreasing the need for rescue analgesia in patients with mild AP. The relative paucity of trials and high-quality data in this setting is notable and the optimal analgesic strategy for patients with moderately severe and severe AP still requires to be determined.
Introduction
Pain is one of the most prevalent and costly health conditions. The estimated cost of pain in the United States was more than that of heart disease and cancer treatments, reaching $560 billion to $635 billion per year (1). Acute abdominal pain is the leading symptom and principal reason for hospital admission in patients with acute pancreatitis (AP).
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases with an increasing global incidence (2), for which there is no specific targeted therapy (3). According to the latest epidemiological investigation, aggregate annual health care cost of AP rose to $2.6 billion in the United States in 2014 (4) and £200 million in the United Kingdom (NHS). Abdominal pain not only serves as one of the diagnostic criteria (5), but also a prognostic factor (6), and is related to the length of hospital stay and other self-reported outcomes of patients with AP (7, 8).
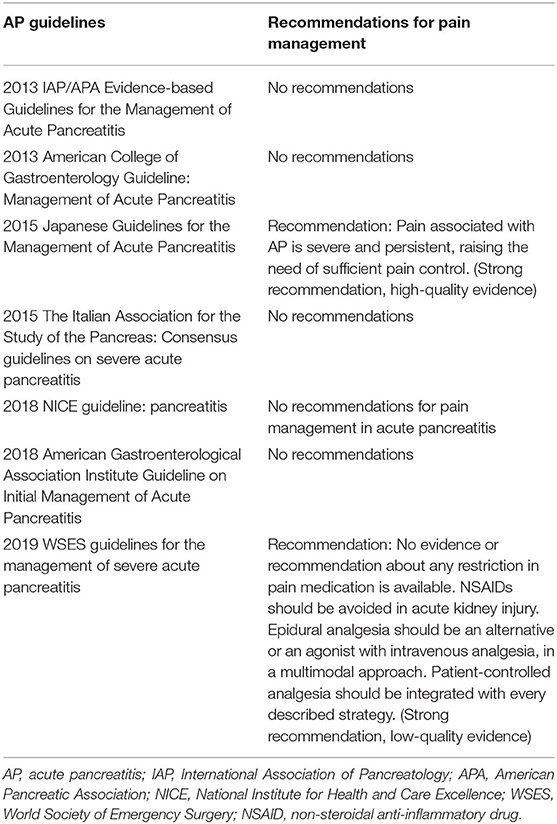
Nearly all patients with AP experienced abdominal pain which warrants prompt analgesia, and this is one of the main management priorities in the early management of AP (9). Some current practise guidelines have overlooked recommendations for pain management (10–14), while others provide a clear recommendation for pain assessment (15, 16), and best pharmacological options (15) (Table 1). No guidelines provide sufficient details regarding the type, dose, route, and frequency of analgesia administration.
There are many pharmacological options, with opioids being the most frequently prescribed analgesics for pain relief of patients with AP. Since abdominal pain in AP is secondary to pancreatic parenchymal inflammation (17, 18), non-steroidal anti-inflammatory drugs (NSAIDs) that target the enzyme cyclooxygenase (COX) are often used (19, 20). Much less frequently, local anaesthetics (i.e., procaine and bupivacaine) and paracetamol (19–21) are used.
A previous meta-analysis of only 5 studies (22) (227 participants) showed there was no significant difference between opioids vs. non-opioids (3 studies, 162 participants) regarding the need for rescue analgesia, pain intensity, clinical outcomes, and adverse events for pain relief of patients with AP. It is noted that opioid requirement during hospitalisation is strongly associated with more complications of this disease (23, 24) and there is the need for more evidence regarding the safety and efficacy of individual opioids in AP management. Further, a recent systematic review (25) demonstrated that NSAIDs reduced AP severity and mortality rate in animal models and patients. However, this review included studies that were of poor quality, the efficacy of NSAIDs for pain relief in this setting still needs to be addressed. Recently, thoracic epidural anaesthesia has been shown to significantly improve pancreatic microcirculation and splanchnic perfusion and reduce indices for multiple organ failure and mortality (26). There is the need to repeat a systematic review on this topic and it includes recent clinical studies of pain relief protocols in patients with AP to guide clinical management and trials.
The aim of this systematic review and meta-analysis was to include recent randomised clinical trials (RCTs) to evaluate the safety and efficacy of analgesics in the management of pain in patients with AP.
Methods
The study was conducted following protocols of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement (27).
Literature Search Strategy
A thorough literature search was carried out in the databases of Ovid Medline (PubMed), EMBASE, Science Citation Index Expanded, and Cochrane Library up to June 23, 2021. The detailed search strategy is shown in Appendix 1 (supplementary material). All RCTs investigating pain management in AP were collated. Reference lists of relevant reviews and other non-primary data sources regarding this context captured by the search strategy were also manually screened. Only publications in English were included. Relevant articles were manually reviewed by three investigators (W.C., F.L., and Y.W.).
Study Selection
Inclusion criteria for eligible studies were: (1) RCTs carried out in patients with AP; (2) treatment with opioids, NSAIDs, systemic, or epidural administration of local anaesthetics for abdominal pain; and (3) include at least one outcome measure for analysis. Non-RCTs, retrospective studies, reviews, abstracts, case reports/series, editorials, expert opinions, and non-English publications were excluded.
Outcomes of Interest
The primary outcome was the number of patients requiring rescue analgesia beyond the analgesic being tested during the observation time of the trial (thereafter called “need for rescue analgesia”) (28). It is provided that a patient requesting rescue analgesic for multiple times is not counted more than once. This was chosen as the primary outcome because this was widely used in the trials, and it was considered more objective than the multiple patient-reported pain scoring systems. The rescue analgesia could be the same analgesic in the trial arm or a different analgesic.
Secondary outcomes included change of pain score, all complications, mortality, length of hospital stay, and adverse event rate. Pain scores were converted to a 0–100 scale Visual Analogue Scale (VAS) from the 0–5 or 0–10 scales for pooled calculation, and their change (Δ-VAS) was assessed within 24 h, at 72 h, and/or on 3–7 d after randomisation. Any complication was defined as development of local complication (acute peripancreatic fluid collection, acute necrotic collection, walled-off necrosis, pancreatic pseudocyst, and vein thrombosis) (5), organ failure (acute respiratory failure, acute liver injury, acute kidney injury, and cardiovascular failure), and other complications (e.g., altered bowel function, ileus, ascites, and pleural effusion) that were not attributed to an adverse drug reaction.
Data Collection
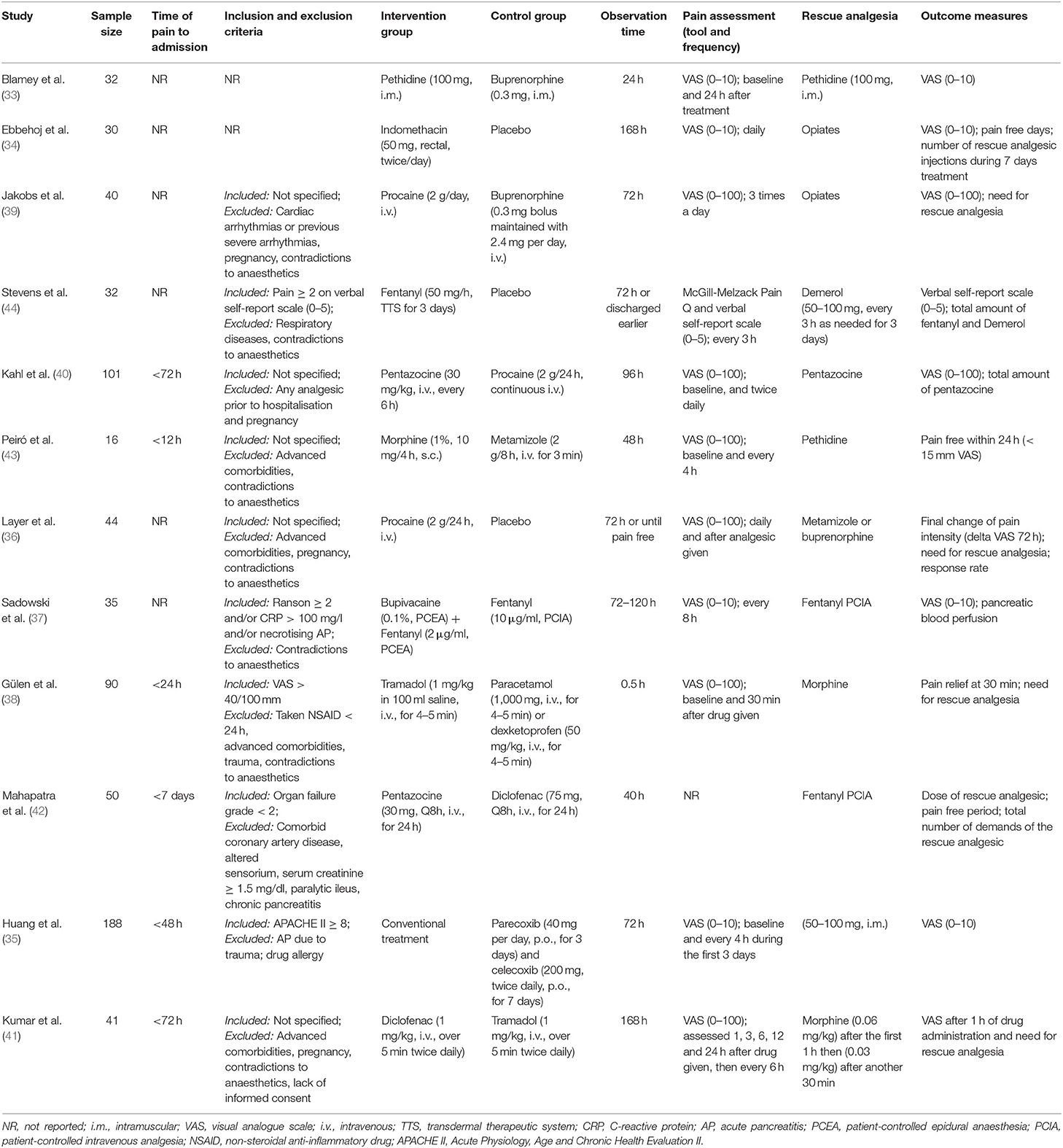
Two authors (W.C. and Y.W.) independently extracted data from included studies using a standardised pro forma designed by a senior author (W.H.). These included: authors, year of publication, country, centre(s), sample size calculation, number of patients screened (analysed), patient baseline characteristics (age, gender, weight or body mass index, aetiology, disease severity), trial criteria, and process (time of pain to admission, inclusion and exclusion criteria, treatment details of all comparison groups, observation duration, assessment tool and frequency of pain, pain scores, frequency and dose of rescue analgesic, and outcome measures).
Study Quality and Evidence Quality Assessment
Two authors (W.C. and Y.W.) scored each included study by using the Jadad system (29) that assesses randomisation (0 or 1), double blinding (0, 1, or 2), recording of dropouts and/or withdrawals (0 or 1), and allocation concealment (0 or 1), with a score ≥ 3 indicative of high quality.
The Grading of Recommendations, Assessment Development and Evaluations (GRADE) approach was used to assess the quality of the supporting evidence for selected outcomes using the GRADEpro software (https://gdt.gradepro.org). The quality of the evidence was classified as high, moderate, low, or very low based on consideration of the risk of bias, the directness of evidence, consistency, and precision of the estimates.
Statistical Analysis
Quantitative data were pooled by meta-analyses using RevMan 5.3.5 (Cochrane Collaboration; Oxford, UK). For dichotomous variables, pooled effect estimates were calculated using odds ratios (ORs) with 95% confidence intervals (c.i.). Means and SDs of continuous variables were used for meta-analysis and estimated when medians with ranges were given to generate weighted mean difference (WMD) with 95% c.i. (30). All probability (P) values were two-tailed, and a p < 0.05 was considered statistically significant. Heterogeneity was evaluated by Cochrane's Q statistic with P value. I2 values were used to quantify the degree of heterogeneity (I2 ≥ 50% or p < 0.1 indicative of high heterogeneity). A random effect model was employed to pool the overall effect estimate. Subgroup analyses of the primary outcome were conducted by separately analysing studies comparing the same type of analgesics. Sensitivity analysis of primary outcome was performed by restricting studies with high quality (Jadad ≥ 3), sample size ≥ 40, Western population, and by studies mainly including patients with mild AP.
Statistical analysis of publication bias was performed using StatsDirect 3.0 (StatsDirect Ltd; Birkenhead, UK), and p values were generated from Begg-Mazumdar (31) and Egger (32) tests as p < 0.10 was also considered significant.
Results
Design and Quality Assessment of Included Studies
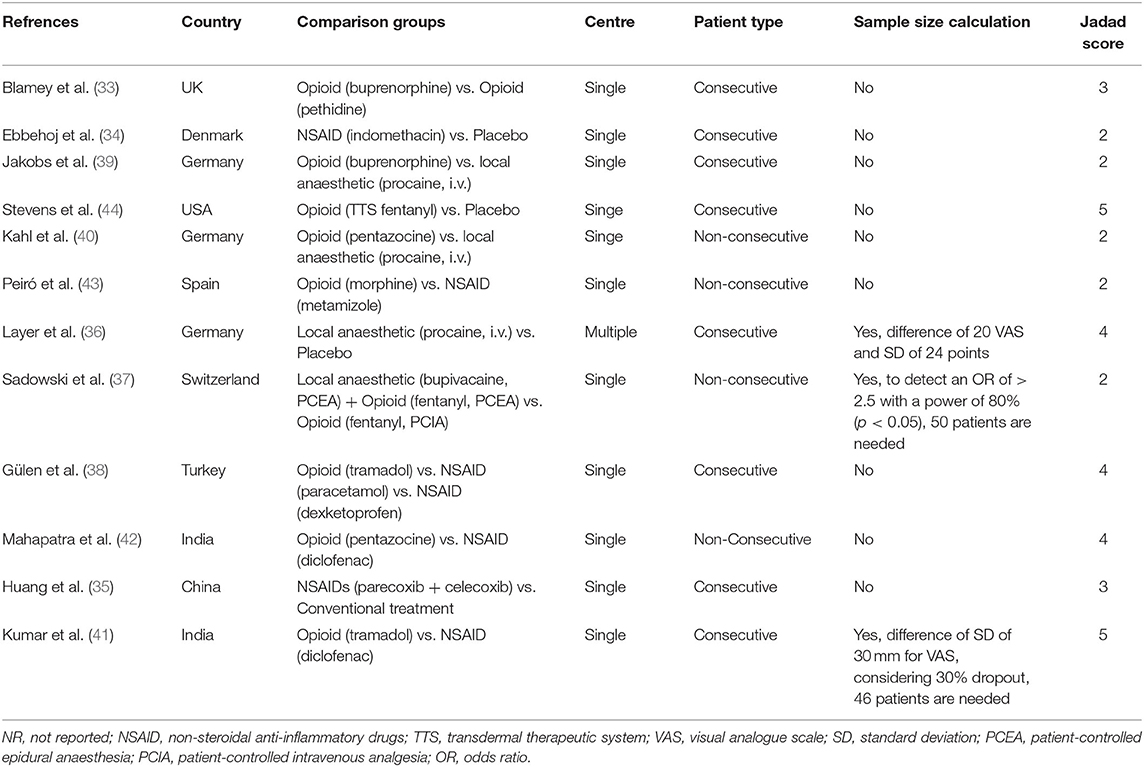
A PRISMA flow diagram for study selection is shown in Figure 1. A total of 12 studies (33–44) were included. Study design and quality assessment are displayed in Table 2 and a detailed Jadad scoring is shown in Supplementary Table 1. Only one study (36) was conducted in multiple centres. Three studies (36, 37, 41) performed sample size calculation. Seven (33, 35, 36, 38, 41, 42, 44) and 5 studies (34, 37, 39, 40, 43) had Jadad score ≥ 3 and ≤ 2, respectively.
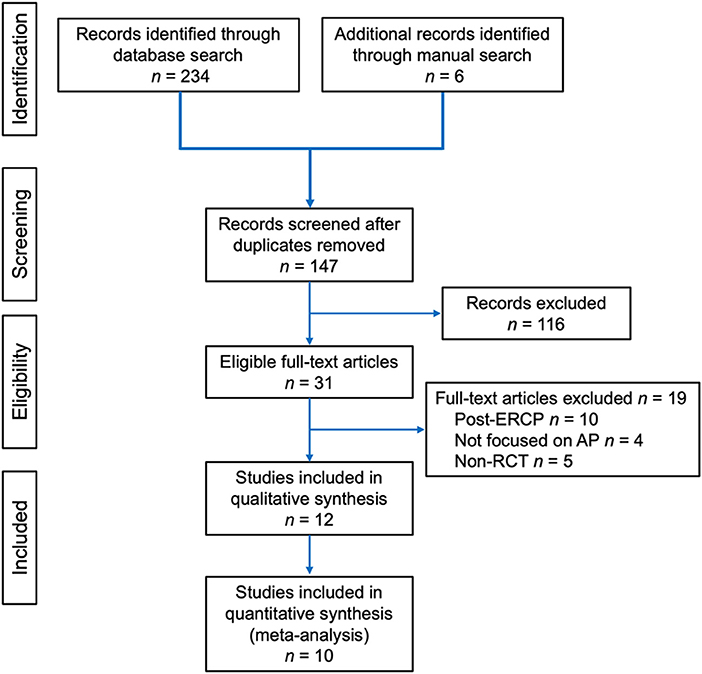
Figure 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram of article selection for the review.
Baseline Characteristics of Included Patients
These data are shown in Supplementary Table 2. A total of 800 patients with AP were screened and 699 were analysed with age ranged from 23 to 86 years old. Gender was reported in 11 studies (34–44). Two studies (39, 40) reported the weight and 3 studies (35–37) reported body mass index. Ten studies (34–43) reported aetiology of AP. Regarding AP severity classification, 2 studies (38, 44) did not report data on AP severity, 2 studies (35, 37) reported substantial proportion of patients with severe AP, and the remaining 8 studies (33, 34, 36, 39–43) mainly included patients with mild AP. Pooled prevalence of mild AP was estimated to be 83% (478/577).
Details of Trials
These are listed in Table 3. Ten studies (33–41, 43) were used the VAS to assess pain intensity, with VAS (0–100) in 6 studies (36, 38–41, 43) and VAS (0–10) in 4 studies (33–35, 37). One study (44) employed verbal self-report scale (0–5) for pain assessment and one (42) did not score pain. Study duration ranged from.5 h to 7 days.
In terms of pharmacological interventions, 4 studies compared analgesics with controls [3 studies with placebo, and one study (35) underwent the same treatments with intervention group without using tested analgesics], 6 studies compared opioids with non-opioids, one study (33) compared opioid with opioid, and one study (37) compared patient-controlled epidural analgesia (PCEA) with patient-controlled intravenous analgesia (PCIA). Rescue analgesics (the primary outcome) in all studies were opioids (4 pethidine, 2 opiates, 2 fentanyl, 2 morphine, 1 pentazocine, and 1 buprenorphine) and 1 study (36) also used metamizole as an alternative to buprenorphine.
Results for Comparative Data That Cannot Be Quantitively Synthesised
Opioid vs. Opioid
Blamey et al. (33) compared the use of opioid (buprenorphine) with another opioid (pethidine) mostly in patients with mild AP (29/32, 90.6%), reporting no significant difference (both p ≥ 0.54) in the need for rescue analgesia and adverse events between these two opioids.
PCEA vs. PICA
Sadowski et al. (37) compared the PCEA (bupivacaine and fentanyl) with PCIA (fentanyl) in patients with predicted severe AP. While the authors found a decrease in absolute VAS value on the day of PCEA implementation vs. the PCIA regimen (1.6 ± 1.8 vs. 3.5 ± 2.2, p = 0.020) and day 10 (0.2 ± 0.4 vs. 2.33 ± 2.3, p = 0.034) from respective baselines (6.6 ± 3.4 vs. 7.31 ± 3.4, p = 0.572). There were no changes in Δ-VAS from randomisation at all designated time points (all p ≥ 0.13; data not shown). The PCEA regimen significantly improved the perfusion of the pancreas compared with the PCIA regimen (13/30, 43% vs. 2/27, 7%, p = 0.0025).
Results of Meta-Analysis
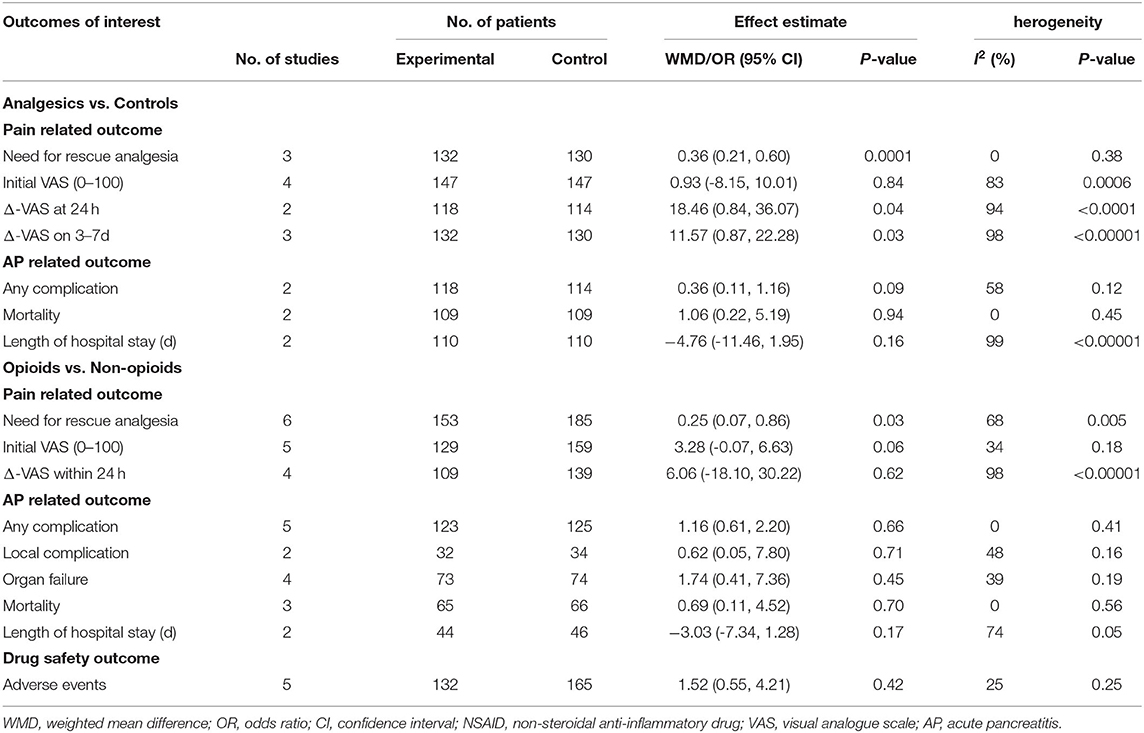
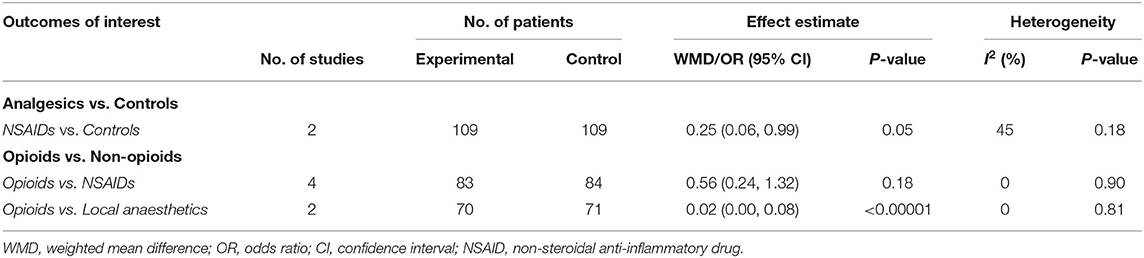
Data from 4 studies (34–36, 44) comparing analgesics with controls (placebo or conventional treatment) and 6 studies (38–43) comparing opioids with non-opioids were quantitatively synthesised for analysis. Results of the meta-analysis of primary outcome measure are presented in Figures 2, 3. All outcome measures are summarised in Table 4. Results of subgroup analysis are presented in Table 5.
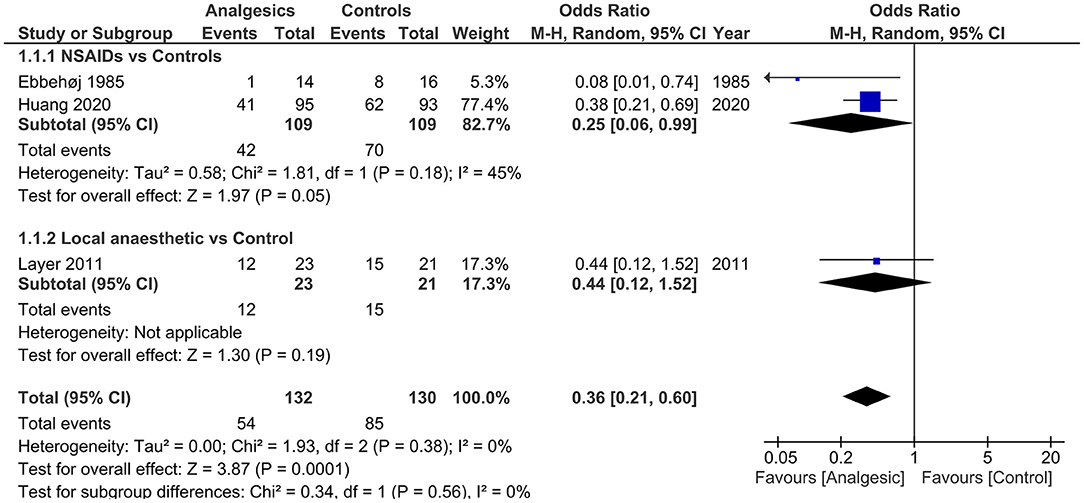
Figure 2. Forest plots of need for rescue analgesia in analgesics vs. controls. M-H, Mantel-Haenszel; CI, confidence interval; NSAIDs, non-steroidal anti-inflammatory drugs.
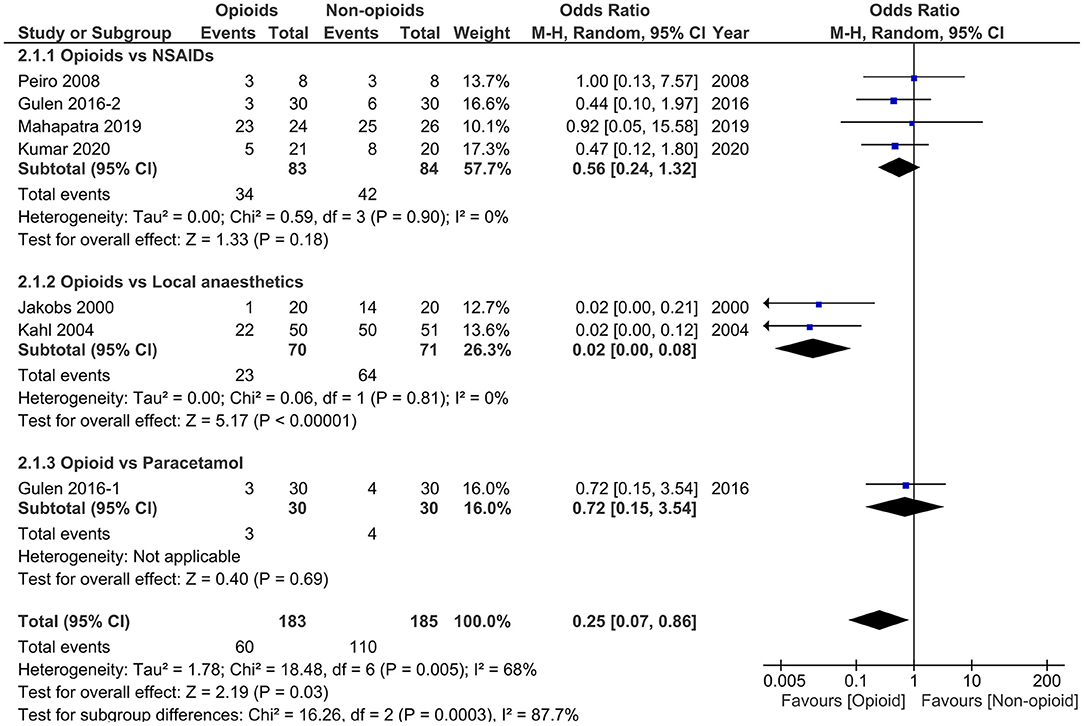
Figure 3. Forest plots of need for rescue analgesia in opioids vs. non-opioids. M-H, Mantel-Haenszel; CI, confidence interval; NSAIDs, non-steroidal anti-inflammatory drugs.
Analgesics vs. Controls
Three (34–36) (n = 262) out of the 4 included studies (34–36, 44) reported the primary outcome. The pooled results demonstrated that the use of analgesics significantly reduced the need for rescue analgesia (OR 0.36, 95% CI.0.21 to 0.60, p = 0.0001) without heterogeneity (I2 = 0) vs. controls.
With comparable initial VAS scores in both groups (p = 0.84), the analgesic group significantly improved pain intensity (Δ-VAS) within 24 h [2 studies (35, 36), n = 232; WMD 18.46, 95% CI.0.84 to 36.07, p = 0.04] and 3 to 7 days [3 studies (34–36), n = 262; WMD 11.57, 95% CI 0.87 to 22.28, p = 0.03], albeit with high heterogeneity (both I2 ≥ 94%). There were no significant differences in terms of any complication, mortality, length of hospital stay, and adverse events (all p ≥ 0.09).
Subgroup analysis of NSAIDs vs. controls still resulted in reduced need for rescue analgesia [2 studies (34, 35), n = 218; OR 0.25, 95% CI 0.06 to 0.99, p = 0.05] with low study of heterogeneity (45%).
Opioids vs. Non-opioids
All 6 studies (38–43) (n = 338) reported the primary outcome [1 (42) by personal communication]. The pooled results indicated that opioids significantly reduced the need for rescue analgesia compared with non-opioids (OR 0.25, 95% CI 0.07 to 0.86, p = 0.03) with high study heterogeneity (I2 = 68%). The initial pain score was slightly higher in opioids group than non-opioids group [5 studies (38–41, 43), n = 288; WMD 3.28, 95% CI−0.07 to 6.63, p = 0.06] and pooled data demonstrated no significant difference regarding the Δ-VAS within 24 h in these two groups [4 studies (38, 40, 41, 43), n = 248; WMD 6.06, 95% CI-18.10 to 30.22, p = 0.62] with high heterogeneity (I2 = 98%). The other clinical outcomes were not significantly different between these two groups (all p ≥ 0.17).
In subgroup analysis, when restricting studies to those comparing opioids with NSAIDs, no significant difference in the need for rescue analgesia was observed [4 studies (38, 41–43), n = 167; OR 0.56, 95% CI 0.24 to 1.32, p = 0.18]. However, the primary results remained significant when comparing opioids with systemic use of local anaesthetics [2 studies (39, 40), n = 141; OR 0.02, 95% CI 0.00 to 0.08, p < 0.00001]. There was no study heterogeneity in both subgroup analyses (both I2 = 0). These results demonstrated that the superiority of opioids in opioids vs. non-opioids analysis was driven by the results of the opioids vs. local anaesthetics.
Sensitivity Analysis
The sensitivity analysis could only be performed for opioids vs. non-opioids (Supplementary Table 3). The primary results of the need for rescue analgesia became insignificant by only analysing studies of high quality (38, 41, 42) (3 studies, n = 181; OR 0.55, 95% CI 0.24 to 1.23, p = 0.14) with improved heterogeneity (68% to 0). The results were not affected by only including studies with sample size ≥ 40 (38–42), Western population (39, 40, 43), or by studies mainly including patients with mild AP and these had similar study heterogeneity.
GRADE Assessment
The GRADE could only be applied to the studies of opioids vs. non-opioids (Table 6). Moderate quality of evidence was rated for the need for rescue analgesia. Low quality of evidence was rated for other outcomes assessed including Δ-VAS within 24 h, any complication, length of hospital stay, and adverse events.
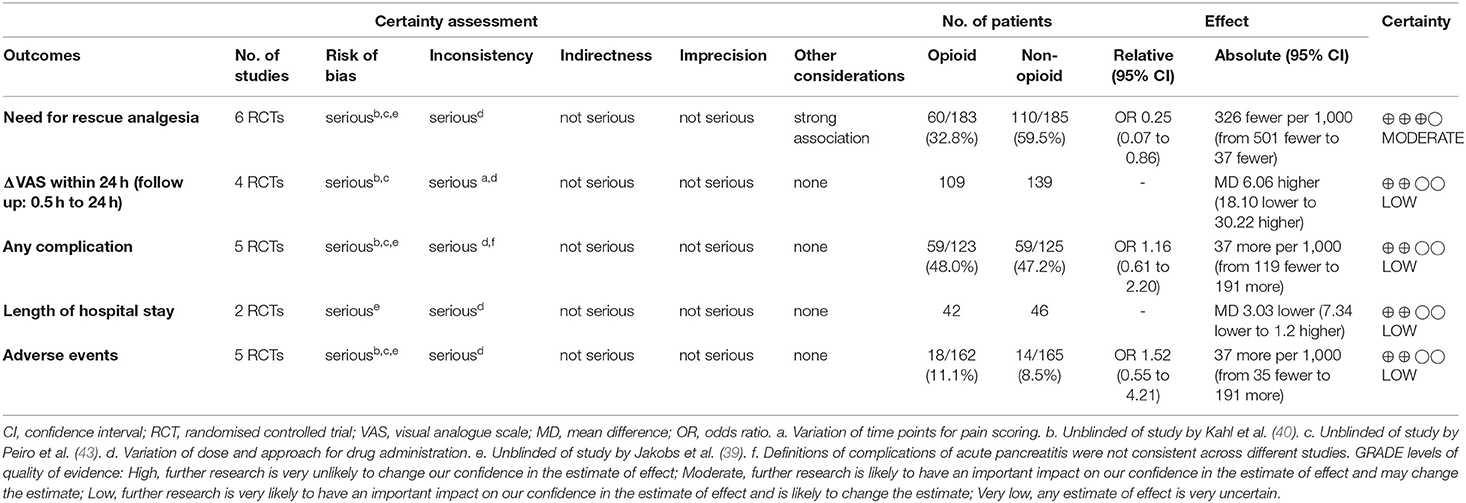
Table 6. Grading of Recommendations, Assessment Development and Evaluations (GRADE) evidence profile and summary of findings: Opioids vs. Non-opioids.
Publication Bias
There was no convincing evidence of publication bias for the primary outcome measure in opioids vs. non-opioids (Begg-Mazumdar and Egger: p > 0.1) by funnel plot analysis (Supplementary Figure 1).
Discussion
This systematic review and meta-analysis included 12 RCTs investigating analgesics for pain relief in AP and comprised a total of 699 patients, of whom 83% were mild cases. It was found that the use of analgesics was associated with reduced need for rescue analgesia and with pain score compared with controls. Unlike the previous meta-analysis (22) showing opioids were equivalent to non-opioids in decreasing the need for rescue analgesia, this meta-analysis found that opioids were superior to non-opioids in regards to the need for rescue analgesia but without significant differences in pain score, other clinical outcomes, and adverse events. It was found that NSAIDs and opioids were equally effective in decreasing the need for rescue analgesia in patients with AP, which was not the case for the systemic administration of local anaesthetics. The lack of heterogeneity in these two subgroups analyses makes the conclusion more convincing.
Of the 4 RCTs that investigated the effects of analgesics in comparison with controls, 2 reported that NSAIDs reduced the need for rescue analgesia. Ebbehoj et al. (34) showed that indomethacin was effective for pain relief. In a most recent study, Huang et al. (35) demonstrated that parecoxib plus celecoxib (selective COX-2 inhibitors) was associated with significantly less meperidine injections, reduced pain score, systemic and local complications, length of hospital stay, and cost without evidence of adverse events. Indeed, NSAIDs (i.e., celecoxib) are strongly recommended by the EARS Society as a component of a multimodal analgesic strategy and have been shown to reduce PCIA morphine consumption after major surgery in patients without contraindications (45, 46). Overall, our findings support the use of NSAIDs (in the absence of acute kidney injury) for pain relief in AP which is not a feature in previous AP guidelines.
Compared with the Cochrane database review conducted by Basurto et al. (22) in 2013 which included 5 RCTs with 227 participants, the current systematic review included 12 RCTs with 699 participants. For opioids vs. non-opioids, while Basurto et al. (22) did not show significance (3 studies with 162 patients, OR 0.41, 95% CI 0.14 to 1.19, p = 0.10), our results of an additional 3 RCTs favoured opioids over non-opioids (6 studies with 338 patients; OR 0.25, 95% CI 0.07 to 0.86, p = 0.03) in reducing the need for rescue analgesia. Subgroup analysis of 2 studies comparing opioids with procaine did not change the result of the primary outcome (OR 0.02, 95% CI 0.00 to 0.08, p < 0.00001), indicating opioids are a more effective analgesic than the systemic administration of procaine. The rationale for using procaine is because of its local analgesic effect and, possibly, its inhibitory effect on phospholipase A2 catalytic activity, which is an important enzyme involved in the early pathogenesis of AP (47). Therefore, systemic administration of local anaesthetics, mainly procaine, was already recommended as front-line analgesic in AP by consensus conferences in several European countries two decades ago (48, 49). Our results suggested that systemic use of local anaesthetics is not supported and, thus, its role in pain management in AP needs to be defined.
Subgroup analysis of 4 studies between opioids and NSAIDs revealed there was no significant difference in the need for additional analgesic (OR 0.56, 95% CI 0.24 to 1.32, p = 0.18) without heterogeneity (I2 = 0), Δ-VAS, other clinical outcomes, and adverse events. Recently, a retrospective study conducted by Kim et al. (50) showed that opioid was associated with an increased risk of developing AP compared to NSAIDs (OR 2.64, 95% CI 1.54 to 4.52). It is reported that opioids can potentially cause AP in patients with a history of cholecystectomy due to its adverse effect of sphincter of Oddi constriction (50, 51). There are other effects of opioids including ileus, dysbiosis, opioid hyperalgesia, and others (22, 52). However, opioids have been overused for pain management in AP especially in North America where 92.5% of patients with AP received opioids for impatient pain management and 64.3% have been given opioids at discharge (53). On the other hand, the over prescription or prescription of opioids without adequate supervision has led to an alarming rise of opioid overdose-associated deaths (54). From 2013 to 2019, the synthetic opioid-associated death rate increased more than 10 folds, from 1.0 to 11.4 per 100,000 age-adjusted population in the United States (55). Therefore, based on the available evidence, it appears that NSAIDs are preferred over opioids as first line analgesia in patients with AP.
Epidural analgesia is an essential component of most enhanced recovery after surgery (ERAS) pathways and is commonly used in major abdominal surgery, because it is associated with superior pain control (46). However, epidural analgesia is rarely used in patients with AP. Sasabuchi et al. (56) investigated 44,146 patients with AP in Japan between 2010 and 2013 and found that only 0.7% of patients received epidural analgesia for pain management. A large propensity score-matched retrospective observational study (57) of 1,003 patients conducted in 2018 demonstrated that the mortality of critically ill patients with AP who received epidural analgesia was significantly lower than those who did not. In our study, there was only one RCT focusing on PCEA vs. PCIA (37). It was clear that PCEA markedly improved pancreatic arterial perfusion over PCIA, and this finding is consistent with studies from experimental AP models (26) and with patients who are critically ill with AP (37). Meanwhile, a multi-centre RCT (58) is underway to elucidate the benefits of PCEA among critically ill patients with AP. PCEA is not currently recommended for patients with in mild and moderately severe AP because of potential adverse effects including catheter placement-related hypotension and epidural abscess, although at a relatively low incidence. More studies evaluating the safety and efficacy of epidural analgesia in patients with severe AP are warranted.
In our study, we did not include studies assessing the analgesic effect of acupuncture on patients with AP as we focused on the pharmacological intervention. A systematic review and meta-analysis (59) have demonstrated that acupuncture was associated with significantly reduced abdominal pain (mean difference −0.87, 95% CI −1.01 to −0.73, p < 0.00001), improved gastrointestinal function, accelerated time of resuming to diets, and shortened the length of hospital stay without noticeable adverse events. However, most of these studies are published in Chinese and more rigorously designed RCTs are needed to confirm these findings.
It is striking that there is not more level 1 evidence available to guide decision-making with optimised analgesic protocols in patients with AP. The evidence available from the 12 RCTs is limited by the quality and heterogeneity of included studies as shown by our sensitivity analysis. In order to minimise the impact of heterogeneity, the random-effects model was adopted in the quantitative analysis for all outcomes. The unblinding characteristic of some studies may influence the patient-reported outcomes including the scoring of pain. Moreover, the variation in drug administration route and dosages impacted the study outcomes and this is reflected in the low GRADE levels. For example, in opioids vs. non-opioids, Peiró et al. (43) utilised smaller doses of morphine subcutaneously while other studies administered greater doses of opioids by intravenous approach. This might also explain the differences between studies. Other aspects that impact the trial design and outcomes are differences in the timing of pain onset in relation to hospital admission or recruitment, admission pain intensity, and predicted severity. We chose the primary outcome to be “the need for rescue analgesia” as data were not available on opioid equivalent for the rescue analgesic in most studies. For future trials, there is also the need for standardised reporting of pain outcomes and inclusion the identification of objective biomarkers (electrophysiology, bioassay, omics, imaging, and behaviour) (54).
The important finding from this study is that NSAIDs are as effective as opioids and opioids are more effective than systemic local anaesthetics on reducing the need for rescue analgesia in patients with mild AP. Epidural anaesthesia shows promise but will need further trials in patients with predicted severe AP before it can be recommended or adopted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Author Contributions
WC, FL, and YW are joint first authors of this article, undertook the statistical analysis, and interpreted data. WH conceived and designed the study. WC, FL, YW, and CH undertook the systematic literature search, acquisition of data, quality cheque, and risk of bias assessment. MP contributed to the design of statistical analysis. WC, FL, YW, and WH drafted the manuscript. WH and QX obtained funding and supervised the study. VS and RS had important intellectual input. All authors have approved the final version of manuscript before submission.
Funding
This project is supported by the National Nature Science Foundation of China (No. 82104598, CH; No. 81973632, WH). NZ-China Strategic Research Alliance 2016 Award (China: 2016YFE0101800, QX and WH). WC is funded by China Scholarship Council (No. 201806240274) and West China Hospital-Liverpool Clinician Scientist Development Award.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dr. Kun Jiang (West China Hospital) for her assistance in preparation of this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.782151/full#supplementary-material
References
1. Smith TJ, Hillner BE. The cost of pain. JAMA Netw Open. (2019) 2:e191532. doi: 10.1001/jamanetworkopen.2019.1532
2. Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. (2016) 1:45–55. doi: 10.1016/S2468-1253(16)30004-8
3. Moggia E, Koti R, Belgaumkar AP, Fazio F, Pereira SP, Davidson BR, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev. (2017) 4:CD011384. doi: 10.1002/14651858.CD011384.pub2
4. Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. (2019) 156:254–72. doi: 10.1053/j.gastro.2018.08.063
5. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis−2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
6. Phillip V, Schuster T, Hagemes F, Lorenz S, Matheis U, Preinfalk S, et al. Time period from onset of pain to hospital admission and patients' awareness in acute pancreatitis. Pancreas. (2013) 42:647–54. doi: 10.1097/MPA.0b013e3182714565
7. Kapoor K, Repas K, Singh VK, Conwell DL, Mortele KJ, Wu BU, et al. Does the duration of abdominal pain prior to admission influence the severity of acute pancreatitis? Jop. (2013) 14:171–5. doi: 10.6092/1590-8577/1283
8. Wu BU, Butler RK, Chen W. Factors associated with opioid use in patients hospitalized for acute pancreatitis. JAMA Netw Open. (2019) 2:e191827. doi: 10.1001/jamanetworkopen.2019.1827
9. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. (2019) 16:175–84. doi: 10.1038/s41575-018-0087-5
10. Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN, American Gastroenterological Association Institute Clinical Guidelines C. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. (2018) 154:1096–101. doi: 10.1053/j.gastro.2018.01.032
11. Italian Italian Association for the Study of the Pancreas, Pezzilli R, Zerbi A, Campra D, Capurso G, Golfieri R, et al. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. (2015) 47:532–43. doi: 10.1016/j.dld.2015.03.022
12. Working Group IAPAPAAPG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. (2013) 13:e1–15. doi: 10.1016/j.pan.2013.07.063
13. Tenner S, Baillie J, DeWitt J, Vege SS, American American College of Group. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. (2013) 108:1400–15. doi: 10.1038/ajg.2013.218
14. Samarasekera E, Mahammed S, Carlisle S, Charnley R, Guideline C. Pancreatitis: summary of NICE guidance. BMJ. (2018) 362:k3443. doi: 10.1136/bmj.k3443
15. Leppaniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. (2019) 14:27. doi: 10.1186/s13017-019-0247-0
16. Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, et al. Japanese guidelines for the management of acute pancreatitis: Japanese guidelines 2015. J Hepatobiliary Pancreat Sci. (2015) 22:405–32. doi: 10.1002/jhbp.259
17. Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. (2004) 4:551–9. doi: 10.1159/000082180
18. Babic T, Travagli RA. Neural control of the pancreas. Pancreapedia. (2016) doi: 10.3998/panc.2016.27
19. Meng W, Yuan J, Zhang C, Bai Z, Zhou W, Yan J, et al. Parenteral analgesics for pain relief in acute pancreatitis: a systematic review. Pancreatology. (2013) 13:201–6. doi: 10.1016/j.pan.2013.02.003
20. Stigliano S, Sternby H, de Madaria E, Capurso G, Petrov MS. Early management of acute pancreatitis: a review of the best evidence. Dig Liver Dis. (2017) 49:585–94. doi: 10.1016/j.dld.2017.01.168
21. Schorn S, Ceyhan GO, Tieftrunk E, Friess H, Ekin Demir IE. Pain management in acute pancreatitis. Pancreapedia. (2015). doi: 10.3998/panc.2015.15
22. Basurto Ona X, Rigau Comas D, Urrutia G. Opioids for acute pancreatitis pain. Cochrane Database Syst Rev. (2013) CD009179. doi: 10.1002/14651858.CD009179.pub2
23. Parsa N, Faghih M, Garcia Gonzalez F, Moran RA, Kamal A, Jalaly NY, et al. Early hemoconcentration is associated with increased opioid use in hospitalized patients with acute pancreatitis. Pancreas. (2019) 48:193–8. doi: 10.1097/MPA.0000000000001240
24. Matic S, Radosavljevic I, Jankovic S, Natasa D. IL-10-1082G>a polymorphism, use of opioids and age affect the course of acute pancreatitis. Eur J Gastroenterol Hepatol. (2021) 32:178–85. doi: 10.1097/MEG.0000000000001875
25. Wu D, Bai X, Lee P, Yang Y, Windsor J, Qian J. A systematic review of NSAIDs treatment for acute pancreatitis in animal studies and clinical trials. Clin Res Hepatol Gastroenterol. (2020) 44S:100002. doi: 10.1016/j.clirex.2019.100002
26. Windisch O, Heidegger CP, Giraud R, Morel P, Buhler L. Thoracic epidural analgesia: a new approach for the treatment of acute pancreatitis? Crit Care. (2016) 20:116. doi: 10.1186/s13054-016-1292-7
27. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
28. Aubrun F, Hrazdilova O, Langeron O, Coriat P, Riou B. A high initial VAS score and sedation after iv morphine titration are associated with the need for rescue analgesia. Can J Anaesth. (2004) 51:969–74. doi: 10.1007/BF03018481
29. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
30. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
31. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
33. Blamey SL, Finlay IG, Carter DC, Imrie CW. Analgesia in acute pancreatitis: comparison of buprenorphine and pethidine. Br Med J. (1984) 288:1494–5. doi: 10.1136/bmj.288.6429.1494-a
34. Ebbehoj N, Friis J, Svendsen LB, Bulow S, Madsen P. Indomethacin treatment of acute pancreatitis. a controlled double-blind trial. Scand J Gastroenterol. (1985) 20:798–800. doi: 10.3109/00365528509088825
35. Huang Z, Ma X, Jia X, Wang R, Liu L, Zhang M, et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: a randomized controlled clinical trial. Am J Gastroenterol. (2020) 115:473–80. doi: 10.14309/ajg.0000000000000529
36. Layer P, Bronisch HJ, Henniges UM, Koop I, Kahl M, Dignass A, et al. Effects of systemic administration of a local anesthetic on pain in acute pancreatitis: a randomized clinical trial. Pancreas. (2011) 40:673–9. doi: 10.1097/MPA.0b013e318215ad38
37. Sadowski SM, Andres A, Morel P, Schiffer E, Frossard JL, Platon A, et al. Epidural anesthesia improves pancreatic perfusion and decreases the severity of acute pancreatitis. World J Gastroenterol. (2015) 21:12448–56. doi: 10.3748/wjg.v21.i43.12448
38. Gulen B, Dur A, Serinken M, Karcioglu O, Sonmez E. Pain treatment in patients with acute pancreatitis: a randomized controlled trial. Turk J Gastroenterol. (2016) 27:192–6. doi: 10.5152/tjg.2015.150398
39. Jakobs R, Adamek MU, von Bubnoff AC, Riemann JF. Buprenorphine or procaine for pain relief in acute pancreatitis. a prospective randomized study. Scand J Gastroenterol. (2000) 35:1319–23. doi: 10.1080/003655200453692
40. Kahl S, Zimmermann S, Pross M, Schulz HU, Schmidt U, Malfertheiner P. Procaine hydrochloride fails to relieve pain in patients with acute pancreatitis. Digestion. (2004) 69:5–9. doi: 10.1159/000076541
41. Kumar NS, Muktesh G, Samra T, Sarma P, Samanta J, Sinha SK, et al. Comparison of efficacy of diclofenac and tramadol in relieving pain in patients of acute pancreatitis: a randomized parallel group double blind active controlled pilot study. Eur J Pain. (2020) 24:639–48. doi: 10.1002/ejp.1515
42. Mahapatra SJ, Jain S, Bopanna S, Gupta S, Singh P, Trikha A, et al. Pentazocine, a kappa-opioid agonist, is better than diclofenac for analgesia in acute pancreatitis: a randomized controlled trial. Am J Gastroenterol. (2019) 114:813–21. doi: 10.14309/ajg.0000000000000224
43. Peiro AM, Martinez J, Martinez E, de Madaria E, Llorens P, Horga JF, et al. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. (2008) 8:25–9. doi: 10.1159/000114852
44. Stevens M, Esler R, Asher G. Transdermal fentanyl for the management of acute pancreatitis pain. Appl Nurs Res. (2002) 15:102–10. doi: 10.1053/apnr.2002.29532
45. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. J Pain. (2016) 17:131–57. doi: 10.1016/j.jpain.2015.12.008
46. Small C, Laycock H. Acute postoperative pain management. Br J Surg. (2020) 107:e70–80. doi: 10.1002/bjs.11477
47. Kunze H, Nahas N, Traynor JR, Wurl M. Effects of local anaesthetics on phospholipases. Biochim Biophys Acta. (1976) 441:93–102. doi: 10.1016/0005-2760(76)90284-8
48. Rünzi M, Layer P, Büchler MW, Beger HG, Ell C, Fölsch UR, et al. [The therapy of acute pancreatitis. general guidelines working group of the society for scientific-medical specialties]. Z Gastroenterol. (2000) 38:571–81. doi: 10.1055/s-2000-7447
49. Slavin J, Ghaneh P, Sutton R, Hartley M, Rowlands P, Garvey C, et al. Management of necrotizing pancreatitis. World J Gastroenterol. (2001) 7:476–81. doi: 10.3748/wjg.v7.i4.476
50. Kim J, Tabner AJ, Johnson GD, Brumback BA, Hartzema A. Increased risk of acute pancreatitis with codeine use in patients with a history of cholecystectomy. Dig Dis Sci. (2020) 65:292–300. doi: 10.1007/s10620-019-05803-3
51. Singh VP. Paradoxical pain from opioids: increased risk of acute pancreatitis. Dig Dis Sci. (2020) 65:13–4. doi: 10.1007/s10620-019-05909-8
52. Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. (2002) 18:S3–13. doi: 10.1097/00002508-200207001-00002
53. Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. (2020) 18:1567–75. doi: 10.1016/j.cgh.2019.11.017
54. Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol. (2020) 16:381–400. doi: 10.1038/s41582-020-0362-2
55. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. (2021) 70:202–7. doi: 10.15585/mmwr.mm7006a4
56. Sasabuchi Y, Yasunaga H, Matsui H, Lefor AK, Fushimi K, Sanui M. Epidural analgesia is infrequently used in patients with acute pancreatitis: a retrospective cohort study. Acta Gastroenterol Belg. (2017) 80:381–4.
57. Jabaudon M, Belhadj-Tahar N, Rimmelé T, Joannes-Boyau O, Bulyez S, Lefrant JY, et al. Thoracic epidural analgesia and mortality in acute pancreatitis: a multicenter propensity analysis. Crit Care Med. (2018) 46:e198–205. doi: 10.1097/CCM.0000000000002874
58. Bulyez S, Pereira B, Caumon E, Imhoff E, Roszyk L, Bernard L, et al. Epidural analgesia in critically ill patients with acute pancreatitis: the multicentre randomised controlled EPIPAN study protocol. BMJ Open. (2017) 7:e015280. doi: 10.1136/bmjopen-2016-015280
Keywords: pain management, acute pancreatitis, analgesics, randomised-controlled trial, meta-analysis
Citation: Cai W, Liu F, Wen Y, Han C, Prasad M, Xia Q, Singh VK, Sutton R and Huang W (2021) Pain Management in Acute Pancreatitis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Front. Med. 8:782151. doi: 10.3389/fmed.2021.782151
Received: 23 September 2021; Accepted: 15 November 2021;
Published: 17 December 2021.
Edited by:
Giuseppe Losurdo, University of Bari Medical School, ItalyReviewed by:
Jayanta Samanta, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaKatherine N. Theken, University of Pennsylvania, United States
Copyright © 2021 Cai, Liu, Wen, Han, Prasad, Xia, Singh, Sutton and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, d2VpLmh1YW5nLnNjdUB2aXAuMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wenhao Cai
Wenhao Cai Fei Liu
Fei Liu Yongjian Wen1†
Yongjian Wen1† Manya Prasad
Manya Prasad Robert Sutton
Robert Sutton Wei Huang
Wei Huang