- 1Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia
- 2Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 3Faculty of Medicine, Institute of Pathology, University of Ljubljana, Ljubljana, Slovenia
Introduction: Urine protein excretion is routinely measured to assess kidney allograft injury, but the diagnostic value of this measurement for kidney transplant pathology remains unclear. Here we investigated whether spot urine protein excretion in the first year following transplantation associates with allograft rejection phenotype at 1-year surveillance biopsies and de-novo occurrence of donor-specific antibodies (DSA).
Patients and Methods: This prospective, observational national-cohort study included 139 non-sensitized patients who received a deceased donor kidney transplant between December 2014 and 2018. All patients received basiliximab induction and tacrolimus-based immunosuppression. Estimated protein excretion rate (ePER) was calculated monthly from spot urine protein-to-creatinine ratios. At 1-year, all recipients underwent surveillance graft biopsy and were screened for de-novo DSA. Screening-positive sera were subjected to single antigen bead (SAB) testing. The occurrence of de-novo DSA was determined based on SAB reactivity patterns using a mean fluorescence intensity threshold >1,000.
Results: Among the 139 study patients, 27 patients (19%) had histologic evidence of T cell-mediated rejection (TCMR), and 9 patients (7%) had histologic evidence of antibody-mediated rejection (AMR) at 1-year surveillance biopsy. One year after transplant, 19 patients (14%) developed de-novo DSA. Compared with patients without rejection and no de-novo DSA, mixed-effects linear regression analysis showed a significant difference in slope of ePER during the first year in patients with AMR and de-novo DSA at 1-year (46, 95% CI 25–68 mg/day/1.73 m2 per month and 34, 95% CI 20–49 mg/day/1.73 m2 per month, respectively). Patients with vascular TCMR also showed a significant difference in ePER slope over time compared with patients with non-rejection findings (31, 95% CI 9–52 mg/day/1.73 m2 per month). The discriminatory power of ePER for intragraft rejection processes was better in patients with AMR (AUC 0.95, 95% CI 0.90–0.99; P < 0.001) than in those with TCMR (AUC 0.68, 95% CI 0.59–0.79; P = 0.002), with 89% sensitivity and 93% specificity for proteinuria >550 mg/day/1.73m2.
Conclusions: An increase in ePER in the first year following kidney transplantation associates with AMR, vascular TCMR and de-novo DSA at 1-year and may be used as a non-invasive clinical marker of intragraft endothelial cell injury.
Introduction
In recent years, antibody-mediated rejection (AMR) has been identified as the primary cause of allograft failure after kidney transplantation (1–3). This specific disease is diagnosed by means of needle biopsy. Although this invasive procedure has become safer and histologic interpretation more standardized, biopsy is usually indicated in deterioration of kidney function when allograft injury already occurs (4). Therefore, protocol biopsies have been proposed to detect changes before kidney dysfunction is apparent (5). Given that biopsy procedures are invasive, complications may occur; furthermore, sampling errors may jeopardize their diagnostic value. These shortcomings have stimulated research to identify non-invasive markers that are sufficiently diagnostic for specific transplant pathologies, and that can be used as an end point in clinical studies (6).
In patients with chronic kidney disease proteinuria is directly related to the underlying glomerular disease process and strongly associates with progression to end-stage kidney disease, with good specificity and sensitivity (7). Proteinuria is also routinely measured in kidney transplant recipients. Proteinuria, in the nephrotic range as well as lower grade, has been associated with inferior kidney transplant outcomes (8–10). Current clinical guidelines suggest that a kidney allograft biopsy should be performed when there is new onset of proteinuria or unexplained proteinuria ≥3.0 g/g creatinine or ≥3.0 g/24 h (11). However, these international guidelines are not evidence-based (evidence level 2C).
More recent data showed that proteinuria >1 g/24 h is a marker for allograft outcome with reasonable predictive accuracy, especially after the first 3 months post-transplantation (12). Although high-grade proteinuria has been related to transplant glomerulopathy and de-novo or recurrent glomerulonephritis (GN) (12–16), the association between low-grade proteinuria and the allograft pathology, in particular AMR, within the first year after transplantation has not been considered yet.
In this study, we aimed to assess the association and diagnostic performance of measuring proteinuria in spot urine samples during routine clinical follow-up in the first year following kidney transplantation with rejection phenotype at protocol-specified kidney biopsies and occurrence of de-novo donor-specific antibodies (DSA) at 1-year post-transplantation. In view of the great effect of specific diseases such as AMR on outcome after kidney transplantation, insight into the diagnostic value of proteinuria early after transplantation is particularly useful. Moreover, as many research teams are evaluating novel non-invasive biomarkers for kidney allograft injury, it is important to elucidate the diagnostic value of proteinuria measurement, a simple, inexpensive, and non-invasive marker that is already universally available.
Patients and Methods
Study Design
In this prospective, observational national-cohort study, we enrolled all consecutive adult recipients of a first deceased donor kidney transplant at the Department of Nephrology, University Medical Center Ljubljana between December 2014 and December 2018. All patients provided written informed consent. The National Medical Ethics Committee approved the study protocol.
Study Participants
Between December 2014 and December 2018, 211 adult patients received a deceased donor kidney transplant at our center. Sensitized recipients with preformed DSA and patients with prior transplants (n = 51), dual organ transplants (n = 13), and patients with early allograft loss within the first 90 days after transplantation (n = 8) were not candidates for the study. Finally, 139 patients were included in the study. All study participants were monitored regularly during the first year according to the protocol of the transplant unit of our department: twice a week in the first month, weekly in the 2nd and 3rd month, bi-weekly in the 4th and 5th month, and monthly thereafter.
The clinical data of the cohort were prospectively collected in electronic clinical patient charts, which were used for clinical patient management as well as being linked to the database used in this study.
All patients had standard immunologic risk and received basiliximab induction and tacrolimus-based immunosuppression. Patients with immediate graft function, diabetes mellitus, or previous cardiovascular events were candidates for rapid steroid withdrawal within the first week after transplantation.
Laboratory Assessment
Proteinuria was determined from second morning spot urine samples at month 1, and then monthly in the first year after transplantation in all study patients. Estimation of 24-h protein excretion rate (ePER, mg/day/1.73 m2) was obtained by multiplying protein-to-creatinine ratio (PCR) and estimated creatinine excretion rate (17, 18). Urine creatinine was measured using non-isotope-dilution mass spectrometry standardized modified Jaffe reaction (calibration traceable to IDMS). Spot urine protein was measured by pyrogallol red-molybdate complex formation using a timed endpoint method. Measurements were performed on Dimension Xpand Plus Integrated Chemistry System using manufacturer's reagents (Siemens HealthCare GmbH, Erlangen Germany). Levels of ePER in the first year were then correlated with graft rejection status, rejection phenotype, and de-novo DSA formation at 1 year after transplantation.
At all-time points, data on serum creatinine were collected on the same day as PCR measurements. Glomerular filtration rate was estimated (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (19).
Histologic Assessment of Biopsy Samples
In all patients, surveillance allograft biopsies were performed systematically at 1-year after transplantation. An indication kidney biopsy was considered if significant allograft dysfunction occurred before 1-year (e.g., associated with delayed graft function or an increase in serum creatinine of more than 20% from baseline without other obvious causes). Slides were stained with hematoxylin eosin, periodic acid–Schiff, and silver methenamine (Jones). An immunohistochemical C4d stain (monoclonal antibody, dilution 1:500; Quidel Corporation, Santa Clara, CA) was performed on frozen tissue. Two pathologists (NK and MF) independently reviewed all biopsies, blinded for the clinical data. The severity of histologic lesions was semiquantitatively scored according to the revised Banff 2013 criteria (20).
T cell-mediated rejection (TCMR) was reported as tubulo-interstitial (borderline and grade IA/B) or vascular (grade IIA/B). The phenotypes of AMR were classified as acute or chronic active. The diagnosis of acute AMR was based on morphologic evidence of acute tissue injury (i.e., peritubular capillaritis and/or glomerulitis) and positive C4d staining. The diagnosis of chronic AMR was based on the morphologic evidence of antibody-mediated chronic tissue injury, specifically glomerular double contours compatible with chronic glomerulopathy on light and/or electron microscopy.
At the time of protocol biopsies, systematic follow-up human leukocyte antigen (HLA) antibodies (ELISA HLA class I and class II Luminex Gen-Probe LifeCodes LSA screening) and their donor-specificity in case of positive screening (using Luminex Gen-Probe LifeCodes LSA Single Antigen Beads) were evaluated. The occurrence of de-novo DSA was determined based on single antigen bead reactivity patterns using a mean fluorescence intensity threshold >1,000.
Statistical Analysis
Dichotomous variables were compared with chi-squared test and continuous variables with the Student's t-test or the Wilcoxon-Mann-Whitney test as appropriate. For variance analysis of continuous variables in different groups, parametric one-way ANOVA and Kruskal-Wallis test were used.
The ePER trajectories were analyzed using linear mixed model regression, with ePER values from 1 to 12 months as dependent and time and the interaction of histologic phenotype/de-novo DSA occurrence and time as fixed effects. Furthermore, patient-specific random effect for intercept was specified. The covariance structure was specified as an autoregressive model of the first order.
Area under the receiver operator characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy of ePER and to calculate the specificity and sensitivity for discriminating between biopsy specimens showing AMR, TCMR, and other non-rejection findings.
All tests were two-sided and P < 0.05 were considered to indicate statistical significance. All analyses were performed using the SPSS statistical software (IBM SPSS statistics, version 21.0, Armonk, NY, USA).
Results
Study Population and Histologic Classification of Kidney Allograft Biopsy Specimens
The baseline patient, donor, and transplant-related characteristics of the study population according to histologic biopsy findings at 1-year after transplantation are provided in Table 1. Among the 139 patients, 36 (26%) had histologic evidence of allograft rejection at 1-year surveillance biopsies. Among them 27 patients (75%) were classified as having TCMR, and 9 patients (25%) were classified as having AMR. A total of 103 patients (74%) had no evidence of rejection and their biopsy findings are presented in Table 1. In ten patients (7%) early acute rejection occurred before 1-year. All patients underwent an indication biopsy due to allograft dysfunction in the first 3 months after transplantation. All rejection episodes were classified as TCMR (borderline or grade IA) and treated with pulse steroids (Table 1).
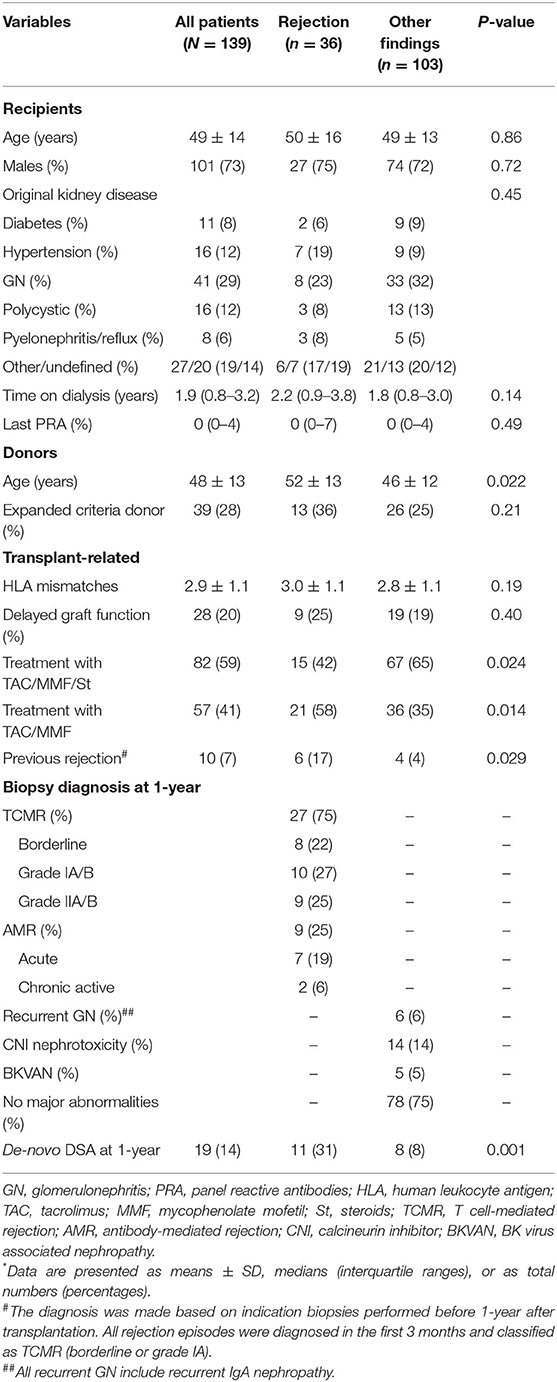
Table 1. Baseline characteristics of the study population according to histologic diagnosis in surveillance kidney allograft biopsies performed at 1 year after transplantation*.
Patients with rejection phenotypes were transplanted from older donors and more frequently undergone rapid steroid withdrawal. In addition, 6 patients with allograft rejection at 1-year surveillance biopsy experienced early TCMR (Table 1); 4 patients had TCMR (grades IA or IIA), and 2 patients had histologic evidence of chronic active AMR. At 1-year after transplant, 19 patients (14%) developed de-novo DSA (4 patients' class I, and 15 patients' class II), and incidence of de-novo DSA occurrence was significantly higher in patents with histologic evidence of allograft rejection (Table 1).
Kidney Allograft Histology and Proteinuria
At 1-year after transplantation, spot urine protein excretion was in the low range with a mean ePER of 318 ± 308 mg/day/1.73 m2. The patients were stratified into three groups according to tertiles of ePER (Table 2). eGFR did not differ significantly among patients with different levels of ePER. Greater levels of ePER were significantly associated with higher Banff histologic scores related to tubulointerstitial inflammation and microvascular injury, and patients in the highest tertile had higher incidence rates of AMR and vascular TCMR. In addition, greater levels of ePER were associated with higher c4d histologic scores, chronic glomerulopathy (cg score), and higher incidences of de-novo DSA occurrence at 1-year (Table 2).
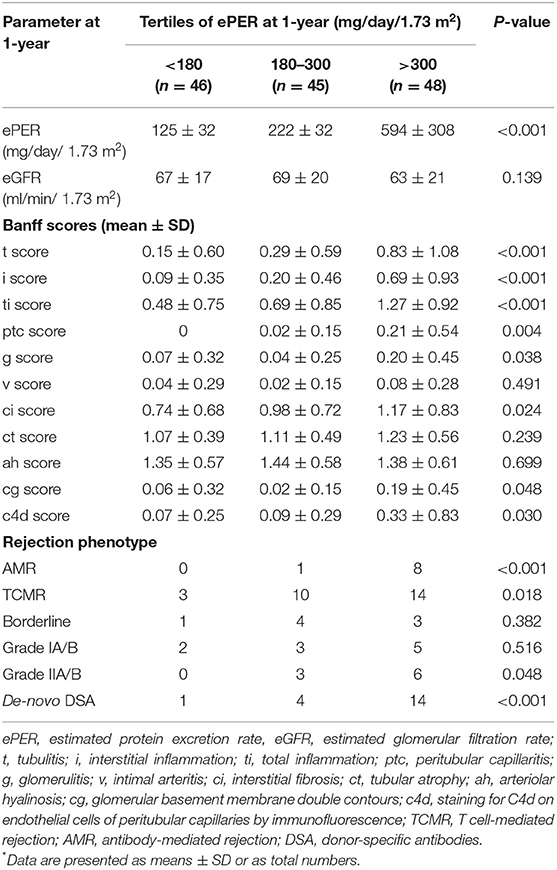
Table 2. Graft function, Banff histologic scores, and incidences of rejection phenotypes and occurrence of de-novo DSA according to levels of ePER (in tertiles) at 1-year after transplantation*.
Among patients with no evidence of rejection, levels of ePER at 1-year were slightly higher in those with recurrent GN than in those with other non-rejection findings, but the difference was not statistically significant (268 ± 109 vs. 218 ± 22 mg/day/1.73 m2; P = 0.169).
The course of ePER with respect to allograft histology and occurrence of de-novo DSA is illustrated in Figure 1. During the 12-month period, patients with AMR, TCMR, and non-rejection findings had ePER slopes of 38 (95% confidence interval [CI] 1 to 76), 5 (95% CI −11 to 22), and −6 (95% CI −11 to −1) mg/day/1.73 m2 per month, respectively (Figure 1A). The difference between patients with AMR and non-rejection findings of 46 (95% CI 25–68) mg/day/1.73 m2 per month was statistically significant (P < 0.001). The difference between patients with TCMR and no rejection of 11 (95% CI −2–25) mg/day/1.73 m2 per month was not statistically significant (P = 0.092).
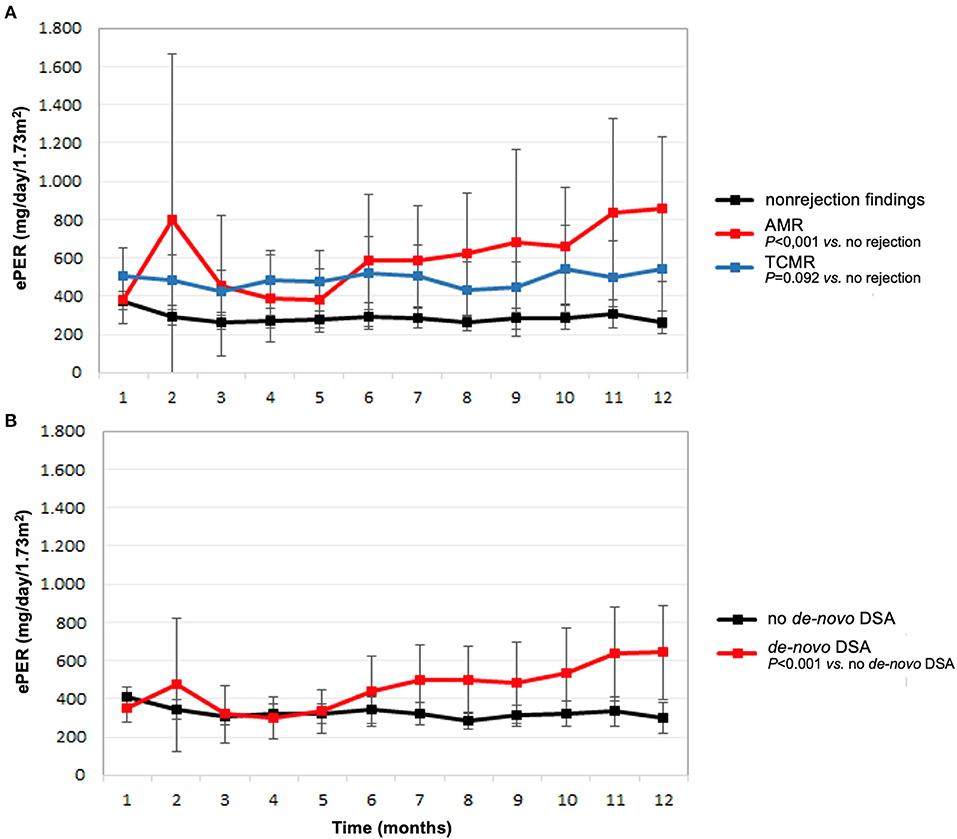
Figure 1. Estimated protein excretion rate (ePER, mean and 95% CI) in the first year following kidney transplantation according to main histologic findings at 1-year surveillance biopsies (A) and de-novo occurrence of donor-specific antibodies (DSA) (B). AMR, antibody-mediated rejection; TCMR, T cell-mediated rejection.
Patients with de-novo DSA had a significant increase in ePER during the first year compared with patients without de-novo DSA 1-year after transplantation (Figure 1B). During the 12-month period, patients with de-novo DSA and no de-novo DSA had ePER slopes of 28 (95% CI 10–46) and of −6 (95% CI −11–1) mg/day/1.73 m2 per month, respectively. The difference of 34 (95% CI 20–49) mg/day/1.73 m2 per month was statistically significant (P < 0.001).
Compared with patients without evidence of rejection, ePER slopes increased progressively in patients with higher histological grade of TCMR, and the increase was highest in patients with vascular TCMR (Figure 2). During the 12-month period, patients with borderline, tubulointerstitial, and vascular TCMR had ePER slopes of −21 (95% CI, −41 to −2), 12 (95% CI, −26–51), and 24 (95% CI, −1–50) mg/day/1.73 m2 per month, respectively. A statistically significant difference in ePER slope was noted between patients with vascular TCMR (grades II/A,B) and no rejection (31, 95% CI 9 to 52 mg/day/1.73 m2 per month; P = 0.005). The difference between patients with borderline TCMR and no rejection (15, 95% CI −7–37 mg/day/1.73 m2 per month) and between patients with TCMR grades I/A,B and no rejection (15, 95% CI −5–35 mg/day/1.73 m2 per month) was not statistically significant (P = 0.184 and 0.138, respectively).
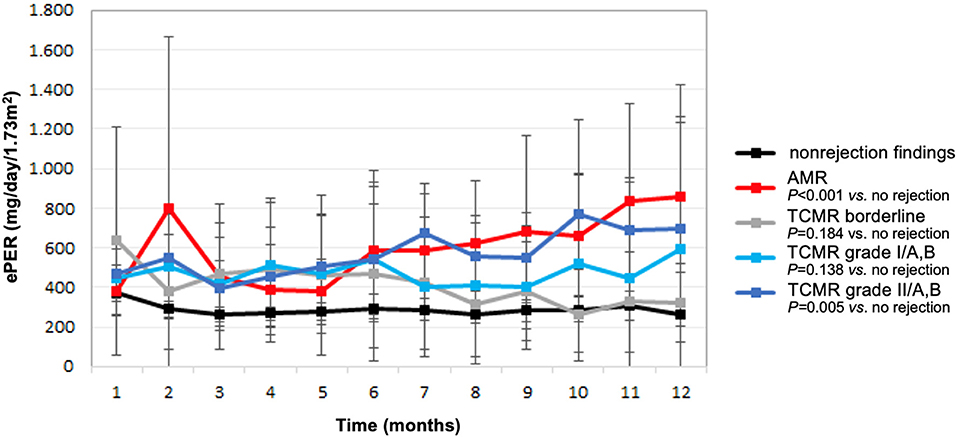
Figure 2. Estimated protein excretion rate (ePER, mean and 95% CI) in the first year following kidney transplantation according to allograft rejection phenotypes at 1-year surveillance biopsies. AMR, antibody-mediated rejection; TCMR, T cell-mediated rejection.
Proteinuria as a Biomarker for Rejection-Associated Allograft Injury
Next, we examined the diagnostic performance of ePER for rejection injury phenotypes in surveillance allograft biopsies at 1-year after transplantation. ePER at 1-year post-transplant was significantly associated with the presence of AMR. The ROC AUC was 0.95 (95% CI 0.90–0.99; P < 0.001). The threshold of ePER that gave the maximal sensitivity and specificity for AMR was 550 mg/day/1.73 m2; at this threshold, the AMR can be predicted with a sensitivity of 81%, and a specificity of 80%. The diagnostic accuracy for TCMR was lower with an AUC of 0.68 (95% CI 0.59–0.79; P = 0.002). However, the diagnostic accuracy was better for vascular TCMR with an AUC of 0.77 (95% CI 0.66–0.89; P = 0.007).
Discussion
The goal of this study was to quantify the changes in spot urine protein excretion that occur during the first year after kidney transplantation in a low-risk cohort of non-sensitized patients with stable kidney function and to investigate whether post-transplant proteinuria is associated with significant allograft pathology at 1-year. The results indicate that kidney allograft rejection and rejection phenotype at 1-year surveillance biopsies are associated with levels of ePER in the first year following transplantation. This simple diagnostic tool measured in spot urine specimens obtained longitudinally in the first-year post-transplant from patients with biopsy-confirmed tubulointerstitial TCMR and other non-rejection findings was relatively flat and distinct from the progressive increase observed in patients with AMR. In addition, de-novo DSA occurrence at 1-year was also associated with an increase in ePER in the first year. Moreover, ePER at 1-year was highly specific for endothelial response to injury associated with de-novo DSA formation, AMR and high-grade vascular TCMR. These findings are important given that spot urine protein excretion can be easily measured and followed after transplantation.
An increase in serum creatinine is often the first clinical indicator of rejection. However, it lacks sensitivity and specificity. The limitations associated with monitoring rejection by measurements of serum creatinine have been recognized previously by the observation that 30% of graft biopsies performed in patients with stable kidney function reveal histological features of rejection (21). More recently, subclinical AMR has been reported in patients with preformed anti-HLA antibodies (22). In subclinical AMR, the serum creatinine level was stable, but protocol biopsy specimens showed glomerulitis, peritubular capillary infiltration by leukocytes, and positive staining of peritubular capillaries with an anti-C4d antibody. Since AMR is associated with endothelial response-to-injury (23), one would expect an increase in urine protein excretion. Our study demonstrated that levels of proteinuria increased in the first year following transplantation among patients with histologic signs of endothelial cell injury, specifically in patients with AMR and de-novo occurrence of DSA. In addition, preceding TCMR in the first months after transplantation was associated with rejection phenotypes at 1-year, including chronic active AMR, as described previously (24). Therefore, persistent or increasing proteinuria may indicate ongoing rejection, even in the absence of allograft dysfunction and despite augmented immunosuppression.
Protein excretion from native kidneys falls rapidly after transplantation and de-novo, persistent or worsening proteinuria is usually indicative of graft pathology (25). In the largest study to date, 58% of transplanted patients with proteinuria ≥150 mg/day had transplant-specific lesions (acute rejection, transplant glomerulopathy, interstitial fibrosis/tubular atrophy) on biopsy compared with only 11% with glomerulonephritis (10). However, detailed information on the natural history of proteinuria early after transplantation and allograft injury phenotypes has not been available. In our study, we observed that in the first year following transplantation, when patients still had preserved allograft function, spot urine protein excretion was greater in patients with rejection phenotypes at 1-year surveillance biopsies. Our main observation was that proteinuria significantly increased in the patients in whom a 1-year surveillance biopsy showed AMR and in the patients who developed de-novo DSA. Additionally, the slope of proteinuria could discriminate between AMR and non-rejection findings. These findings fit well with recent observations of Fotheringham et al. who demonstrated that spot urine protein excretion is associated with DSA detection (26).
Next, ePER >550 mg/day/1.73 m2 was a specific non-invasive marker for highly relevant intragraft injury processes such as AMR and vascular TCMR in our study. The high specificity of proteinuria for these treatable diagnoses in surveillance biopsies provides the evidence of current clinical guidelines that advocate the routine measurement of proteinuria (11). In addition, clinical guidelines suggest that a kidney biopsy should be performed when there is new onset or unexplained proteinuria ≥3.0 g/g creatinine or ≥3.0 g/day. Our data illustrate that this threshold is very conservative and that early detection of proteinuria >500 mg/day could be a more sensitive threshold. However, the association between proteinuria and allograft histology was weak in the first 6 months following transplantation, likely reflecting the contribution of residual kidney function of the native kidneys in the first months (27, 28).
High specificity and sensitivity of proteinuria for AMR, vascular TCMR, and de-novo DSA formation demonstrate acceptable diagnostic performance of low-grade spot urine protein excretion for intragraft microcirculation inflammation and glomerular injury. Similarly, previous study from Naesens et al. (12) demonstrated that many patients with significant histologic injury had low-grade proteinuria <1 g/day, illustrating that surveillance biopsies could thus be warranted in the absence of significant proteinuria or allograft dysfunction for the timely detection of subclinical injury. In this light, our study confirmed that allograft rejection processes, specifically AMR, and de-novo DSA occurrence may associate with low-grade proteinuria and an increase in proteinuria already in the first year following transplantation.
The results of our study are subject to several limitations. Our strategy of including only patients with a functioning kidney beyond 90 days after transplantation may have excluded from analysis some allografts that failed early post-transplant because of rejection. This may have biased our findings toward later events. However, proteinuria in the first months after transplantation is difficult to interpret as it may originate from native kidneys or can result from injury in the grafted kidney (e.g., ischemia-reperfusion injury) (28, 29). Whether our results also apply to immunologically high-risk transplants with preformed DSA, and whether the association between the histology of AMR and proteinuria would be more pronounced in this specific high-risk patient cohort, could not be inferred from our data. Furthermore, we could not investigate the association between proteinuria in the first year and recurrent glomerulonephritis because the number of patients with recurrent disease was small and the fact that recurrence of most common glomerular diseases (e.g., IgA nephropathy) usually occurs later after transplantation. In addition, a review from Akbari et al. (30) showed that in kidney transplant population the ability of spot urine protein measurements to predict 24-h protein excretion is modest and 24-h urine collection should be considered before making further decisions. However, recent observational study showed that spot and 24-h measurements of protein excretion are similar predictors of doubling of serum creatinine, graft loss, and patient death and that spot urine samples are a suitable alternative to 24-h urine collection (31). Unfortunately, we do not have outcome data to determine whether an increase in low-grade proteinuria in the first year following transplantation associates with inferior transplant outcomes.
In conclusion, this study found that in kidney transplant recipients an increase in low-grade spot urine protein excretion in the first year following transplantation associates with AMR and de-novo DSA formation at 1-year post-transplant. The analysis of the diagnostic performance of low-grade proteinuria for treatable subclinical disease processes (specifically AMR, vascular TCMR, and de-novo DSA occurrence) in surveillance biopsies provides the scientific underpinning of the current clinical guidelines to routinely measure proteinuria early after transplantation, and to pursue a histologic diagnosis even when proteinuria >500 mg/day is detected. Further studies in an independent cohort are needed to prospectively validate these findings.
Data Availability Statement
The datasets generated during and/or analyzed during the study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by National Medical Ethics Committee of the Republic of Slovenia. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MO, GM, and MA collected data. NK and MF performed histological analysis. JB-P interpreted the data and critically revised the manuscript. MA participated in the statistical analysis. All authors participated in research design, performance of the research, preparation of the manuscript, and have approved the final version of the manuscript.
Funding
This study was funded by a research grant from the Slovenian Research Agency (ARRS ID: L3-7582) and co-funded by Astellas Pharma (Astellas ID: SI-02-RG-269). Funding sources had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data or preparation, review, or approval of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank Vanja Erčulj for her assistance in statistical analysis.
Abbreviations
AMR, antibody-mediated rejection; CI, confidence interval; DSA, donor-specific antibodies; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; MMF, mycophenolate mofetil; PCR, protein-to-creatinine ratio; ePER, estimated protein excretion rate; ROC, receiver operator characteristic; TAC, tacrolimus; TCMR, T cell-mediated rejection.
References
1. El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. (2009) 9:527–35. doi: 10.1111/j.1600-6143.2008.02519.x
2. Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. (2012) 12:388–99. doi: 10.1111/j.1600-6143.2011.03840.x
3. Naesens M, Kuypers DR, De Vusser K, Evenepoel P, Claes K, Bammens B, et al. The histology of kidney transplant failure: a long-term follow-up study. Transplantation. (2014) 98:427–35. doi: 10.1097/TP.0000000000000183
4. Williams WW, Taheri D, Tolkoff-Rubin N, Colvin RB. Clinical role of the renal transplant biopsy. Nat Rev Nephrol. (2012) 8:110–21. doi: 10.1038/nrneph.2011.213
5. Bosmans JL, Ysebaert DK, Verpooten GA. Chronic allograft nephropathy: what have we learned from protocol biopsies? Transplantation. (2008) 85:S38–41. doi: 10.1097/TP.0b013e318169c5d0
6. Anglicheau D, Naesens M, Essig M, Gwinner W, Marquet P. Establishing biomarkers in transplant medicine: a critical review of current approaches. Transplantation. (2016) 100:2024–38. doi: 10.1097/TP.0000000000001321
7. Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. (2012) 8:301–6. doi: 10.1038/nrneph.2012.42
8. Halimi JM, Matthias B, Al-Najjar A. Respective predictive role of urinary albumin excretion and nonalbumin proteinuria on graft loss and death in renal transplant recipients. Am J Transplant. (2007) 7:2775–81. doi: 10.1111/j.1600-6143.2007.02010.x
9. Halimi JM, Buchler M, Al-Najjar A, Laouad I, Chatelet V, Marlière JF, et al. Urinary albumin excretion and the risk of graft loss and death in proteinuric and non-proteinuric renal transplant recipients. Am J Transplant. (2007) 7:618–25. doi: 10.1111/j.1600-6143.2007.01665.x
10. Amer H, Fidler ME, Myslak M, Morales P, Kremers WK, Larson TS, et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant. (2007) 7:2748–56. doi: 10.1111/j.1600-6143.2007.02006.x
11. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009) 9 (Suppl. 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x
12. Naesens M, Lerut E, Emonds MP, Herelixka A, Evenepoel P, Claes K, Bammens B, et al. Proteinuria as a noninvasive marker for renal allograft histology and failure: an observational cohort study. J Am Soc Nephrol. (2016) 27:281–92. doi: 10.1681/ASN.2015010062
13. Chung J, Park SK, Park JS, Kim SC, Han DJ, Yu E. Glomerulonephritis is the major cause of proteinuria in renal transplant recipients: histopathologic findings of renal allografts with proteinuria. Clin Transplant. (2000) 14:499–504. doi: 10.1034/j.1399-0012.2000.140509.x
14. Shamseddin MK, Knoll GA. Posttransplantation proteinuria: an approach to diagnosis and management. Clin J Am Soc Nephrol. (2011) 6:1786–93. doi: 10.2215/CJN.01310211
15. Serón D, Burgos D, Alonso A. Histology and proteinuria after renal transplantation. Transplant Rev. (2012) 26:20–6. doi: 10.1016/j.trre.2011.07.009
16. Legendre C, Anglicheau D. Transplantation: proteinuria in kidney transplantation: an ongoing story. Nat Rev Nephrol. (2013) 9:251–2. doi: 10.1038/nrneph.2013.61
17. Akbari A, White CA, Shahbazi N, Booth RA, Hiremath S, Knoll GA. Spot urine protein measurements: are these accurate in kidney transplant recipients? Transplantation. (2012) 94:389–95. doi: 10.1097/TP.0b013e31825b413e
18. Mrevlje M, Oblak M, Mlinšek G, Lindič J, Jadranka-Buturović-Ponikvar, Arnol M. First and second morning spot urine protein measurements for the assessment of proteinuria: a diagnostic accuracy study in kidney transplant recipients. BMC Nephrol. (2021) 22:192. doi: 10.1186/s12882-021-02406-x
19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
20. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. (2014) 14:272–83. doi: 10.1111/ajt.12590
21. Rush DN, Henry SF, Jeffery JR, Schroeder TJ, Gough J. Histological findings in early routine biopsies of stable renal allograft recipients. Transplantation. (1994) 57:208–11. doi: 10.1097/00007890-199401001-00009
22. Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. (2009) 9:2561–70. doi: 10.1111/j.1600-6143.2009.02813.x
23. Halloran PH, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. (2016) 12:534–48. doi: 10.1038/nrneph.2016.85
24. Moreso F, Carrera M, Goma M, Hueso M, Sellares J, Martorell J, et al. Early subclinical rejection as a risk factor for late chronic humoral rejection. Transplantation. (2012) 93:41–6. doi: 10.1097/TP.0b013e31823bb647
25. Ibis A, Altunoglu A, Akgul A, Usluogullari CA, Arat Z, Ozdemir FN, et al. Early onset proteinuria after renal transplantation: a marker for allograft dysfunction. Transplant Proceed. (2007) 39:938–40. doi: 10.1016/j.transproceed.2007.02.027
26. Fotheringham J, Angel C, Goodwin J, Harmer AW, McKane WS. Natural history of proteinuria in renal transplant recipients developing de novo human leukocyte antigen antibodies. Transplantation. (2011) 91:991–6. doi: 10.1097/TP.0b013e3182126ed0
27. D'Cunha PT, Parasuraman R, Venkat KK. Rapid resolution of proteinuria of native kidney origin following live donor renal transplantation. Am J Transplant. (2005) 5:351–5. doi: 10.1111/j.1600-6143.2004.00665.x
28. Myslak M, Amer H, Morales P, Fidler ME, Gloor JM, Larson TS, et al. Interpreting post-transplant proteinuria in patients with proteinuria pre-transplant. Am J Transplant. (2006) 6:1660–5. doi: 10.1111/j.1600-6143.2006.01361.x
29. Halimi J-M, Laouad I, Buchler M, Al-Najjar A, Chatelet V, Houssaini TS, et al. Early low-grade proteinuria: causes, short-term evolution and long-term consequences in renal transplantation. Am J Transplant. (2005) 5:2281–8. doi: 10.1111/j.1600-6143.2005.01020.x
30. Akbari A, Ferguson D, Kokolo MA, Ramsay T, Beck A, Ducharme R, et al. Spot urine protein measurements in kidney transplantation: a systematic review of diagnostic accuracy. Nephrol Dial Transplant. (2014) 29:919–26. doi: 10.1093/ndt/gft520
Keywords: kidney transplantation, antibody-mediated rejection, T-cell mediated rejection, donor-specific antibodies, urine protein excretion
Citation: Oblak M, Mlinšek G, Kojc N, Frelih M, Buturović-Ponikvar J and Arnol M (2021) Spot Urine Protein Excretion in the First Year Following Kidney Transplantation Associates With Allograft Rejection Phenotype at 1-Year Surveillance Biopsies: An Observational National-Cohort Study. Front. Med. 8:781195. doi: 10.3389/fmed.2021.781195
Received: 22 September 2021; Accepted: 13 October 2021;
Published: 16 November 2021.
Edited by:
Ondrej Viklicky, Institute for Clinical and Experimental Medicine (IKEM), CzechiaReviewed by:
Takahisa Hiramitsu, Japanese Red Cross Nagoya Daini Hospital, JapanPetra Hruba, Institute for Clinical and Experimental Medicine (IKEM), Czechia
Copyright © 2021 Oblak, Mlinšek, Kojc, Frelih, Buturović-Ponikvar and Arnol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miha Arnol, bWloYS5hcm5vbEBrY2xqLnNp
 Manca Oblak1,2
Manca Oblak1,2 Gregor Mlinšek
Gregor Mlinšek Nika Kojc
Nika Kojc Jadranka Buturović-Ponikvar
Jadranka Buturović-Ponikvar Miha Arnol
Miha Arnol