- 1Department of Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Department of Cardiovascular Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Nursing, Zhongshan Hospital, Fudan University, Shanghai, China
- 4Department of Critical Care Medicine, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, China
- 5Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, China
- 6Shanghai Key Lab of Pulmonary Inflammation and Injury, Fudan University, Shanghai, China
Objective: Primary graft dysfunction (PGD) is the leading cause of early death after heart transplantation. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) can provide temporary mechanical circulatory support and time for functional recovery of the transplanted heart. The purpose of this study was to analyze the timing and prognoses of VA-ECMO in patients with severe PGD after heart transplantation.
Methods: A total of 130 patients underwent heart transplantation at the Zhongshan Hospital Affiliated with Fudan University between January 2014 and December 2020. All patients received basiliximab immunoinduction and a classic double vena cava anastomosis orthotopic heart transplantation. Among them, 29 patients (22.3%) developed severe PGD in the early postoperative period. VA-ECMO was performed in patients with difficulty weaning from cardiopulmonary bypass (CPB) or postoperative refractory cardiogenic shock. Patients were divided into two groups according to whether or not they were successfully weaned from VA-ECMO (patients who survived for 48 h after weaning and did not need VA-ECMO assistance again). The perioperative clinical data were recorded, and all patients were followed up until discharge. Early outcomes were compared between groups.
Results: A total of 29 patients with VA-ECMO support after heart transplantation were included in this study. The proportion of patients receiving VA-ECMO was 22.3% (29/130). Nineteen patients (65.5%) needed VA-ECMO due to difficulty with weaning from CPB, and 10 patients required VA-ECMO for postoperative cardiogenic shock. Nineteen patients (65.5%) were successfully weaned from VA-ECMO. Overall, in-hospital mortality of VA-ECMO support patients was 55.2%. The main causes of death were ventricular fibrillation (four cases), major bleeding (three cases), infection (four cases), and graft failure (five cases).
Conclusion: Despite advances in heart transplantation, severe PGD remains a lethal complication after heart transplantation. At present, the treatment for severe PGD after heart transplantation is a challenge. VA-ECMO provides an effective treatment for severe PGD after heart transplantation, which can promote graft function recovery.
Introduction
At present, orthotopic heart transplantation is the most effective treatment for patients with end-stage heart disease. According to the 36th Annual Adult Heart Transplantation Registry report published by the International Society of Heart and Lung Transplantation (ISHLT) registry in 2019, a total of 1,31,249 adult heart transplants were completed by June 30, 2018, although >90% of the ISHLT data came from North America and Europe (1). The perioperative success rate of heart transplantation is about 90%. Shortage of donor hearts and time of donor heart ischemia are the main limitations restricting the development of heart transplantation (2).
Early primary graft dysfunction (PGD) after heart transplantation, especially right heart failure, is an important factor leading to perioperative death, affecting 7.4–36% of heart transplant recipients (3–5). The 30-day all-cause mortality for PGD has been reported to be about 19–30% (3, 4, 6). Although advances have been achieved in the field of transplantation in the past few decades, factors leading to PGD and associated treatment remain unclear (7). ISHLT has proposed a consensus definition for PGD in 2014, which standardized and graded its diagnosis. Inotropes can be used for mild to moderate PGD to restore myocardial contractility and maintain hemodynamic stability, including catecholamines, phosphodiesterase inhibitors, and levosimendan or intraaortic balloon counterpulsation (IABP). However, for patients with severe PGD, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is needed to maintain hemodynamic stability and perfusion of vital organs (3).
Veno-arterial extracorporeal membrane oxygenation is a temporary cardiopulmonary support technology that can provide effective circulation for critically ill patients with heart failure caused by a variety of reasons (8). In heart transplantation, VA-ECMO is mainly used in patients waiting for donor hearts before the operation and in patients with cardiogenic shock or PGD after the surgery. The survival rate in patients on VA-ECMO support is notably lower, especially in the early posttransplantation period. The Zhongshan Hospital Affiliated with Fudan University started to offer ECMO support therapy in 2009 and has achieved favorable therapeutic effects. This study aimed to investigate the timing and outcomes of VA-ECMO in patients with severe PGD after heart transplantation according to the ISHLT criteria.
Materials and Methods
Patients
This retrospective single-center study reviewed the records of 130 adult patients who underwent orthotopic heart transplantation at Zhongshan Hospital, Fudan University (Shanghai, China) between January 2014 and December 2020. According to the ISHLT criteria, patients with severe PGD who received VA-ECMO support within 24 h after heart transplantation surgery were included in this study. Exclusion criteria included age of <18 years and/or pregnancy. The study was approved by the Ethics Committee of Zhongshan Hospital. Patients were divided into two groups according to whether successful weaning from VA-ECMO occurred (patients were defined as successfully weaned from VA-ECMO if they survived for longer than 48 h after VA-ECMO explantation) (9, 10). Baseline variables, including age, sex, body mass index, comorbidities, laboratory tests, and heart failure etiology, together with outcome variables, which included VA-ECMO support time, VA-ECMO weaning rate, mechanical ventilation time, length of stay in intensive care unit (ICU), length of hospital stay, complications, and in-hospital mortality, were compared between the two groups. Preoperative laboratory tests refer to the tests made on the day or the day before the heart transplantation. The changes in the Sequential Organ Failure Assessment (SOFA) score and lactate (Lac) level from baseline to day 5 were retrospectively analyzed. All data were collected from the patients' hospital records by two residents (YJ-Z and JY-H).
Surgical Procedures
After successful anesthesia, median sternotomy was performed to establish cardiopulmonary bypass (CPB). The superior vena cava (SVC), inferior vena cava (IVC), and ascending artery were blocked. The main trunk of the aorta and pulmonary artery, SVC, and IVC was cut off. The donor heart was pruned, and the posterior wall of the left atrium between the donor heart and the recipient heart was sutured continuously. The donor heart and recipient aorta were also sutured continuously, followed by aortic crossclamp removal to regain rhythmic contractions in the graft. The anastomosis with IVC, pulmonary artery, and SVC was accomplished in the beating heart to reduce the ischemia time of donor hearts.
Definition of PGD
The specific diagnostic criteria were based on the 2013 ISHLT consensus (3). The PGD diagnosis was based on the evidence of cardiac dysfunction in the first 24 h after heart transplantation, including left, right, or total heart dysfunction. The clinical manifestation included severe hemodynamic instability with cardiogenic shock, excluding acute graft failure caused by other reasons, such as pericardial tamponade and hyperacute rejection. Although the ISHLT consensus committee recommended a distinction between PGD-LV and PGD-RV, it was not feasible to include it in this study because left and right ventricular assist devices (LVADs and RVADs) were not available in our center. VA-ECMO has become the preferred treatment for patients with refractory cardiogenic shock.
Indications for VA-ECMO
The decision to use VA-ECMO was made by the cardiac surgeon in the operating room or by the intensivist in the cardiac surgery ICU. Indications for VA-ECMO therapy included difficulty weaning from CPB or postoperative refractory cardiogenic shock despite adequate volumes and high doses of inotropes, such as norepinephrine, dobutamine, epinephrine, and milrinone. A femoral venous cannula placed from the femoral vein to the right atrium was used as the VA-ECMO venous cannula. The femoral artery is most commonly used for arterial catheterization in adult patients. When femoral ECMO was initiated, an additional 8-Fr cannula was inserted distally into the femoral artery to prevent lower extremity ischemia.
General Management During VA-ECMO Support
The optimal management of VA-ECMO involves several aspects, including circulatory support, anticoagulation, infection prevention, and nutritional support (11). The VA-ECMO support management protocol has been previously described (12). Briefly, the VA-ECMO blood flow and vasoactive drug dose were adjusted to maintain the mean arterial pressure above 65 mmHg. Several indices were used for the assessment of peripheral perfusion in VA-ECMO patients, such as clinical assessment (urinary output, skin mottling, capillary refill time, and consciousness), lactate level, mixed venous oxygen saturation, central venous oxygen saturation, and regional saturation of tissue oxygen. Cardiac function and hemodynamic conditions were routinely assessed by transesophageal or transthoracic echocardiography. Heparinization therapy was titrated according to the activated clotting time (ACT) and activated partial thromboplastin time (APTT). The target ACT was maintained at 180–200 s and APTT at 50–70 s (13). Platelets were transfused when the patient's platelet count fell below 50 × 109/L (14). Major bleeding was defined if there was clinically overt bleeding recorded in the medical and/or nursing charts associated with either administration of 2 or more RBC units in 24 h or a drop in hemoglobin >2 g/L over 24 h, or if there was a hemothorax, central nervous system, or retroperitoneal bleeding, or if bleeding required surgical intervention. Midazolam and remifentanil were used for sedation.
Weaning Protocol
When the patient showed signs of partial circulatory recovery and the echocardiographic evaluation demonstrated improvement in ventricular contractility, a VA-ECMO weaning test was performed by gradually reducing the pump flow to 1.5–2 L/min (15). In this setting, the removal of VA-ECMO support was considered if the patient's hemodynamic status remained stable, the left ventricular ejection fraction (LVEF) was ≥20–25%, the left ventricular outflow tract velocity time integral was ≥10 cm, and tissue Doppler lateral mitral annulus peak systolic velocity was ≥6 cm/s under minimal VA-ECMO support (16). Epinephrine was routinely used during the weaning process. In addition, when VA-ECMO combined with continuous renal replacement therapy (CRRT) or IABP was used, VA-ECMO could be removed first, and CRRT or IABP could be removed after the condition was further improved.
Statistical Analysis
Continuous data were expressed as median (IQR). Categorical variables were expressed as percentages (%). Quantitative variables were analyzed using the Mann–Whitney U test. Categorical variables were analyzed by the chi-squared test or Fisher's exact methods as appropriate. Log-rank testing and Kaplan–Meier survival curves were used for survival analysis. Statistical significance was defined as p < 0.05. All statistical analyses were performed using the SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA).
Results
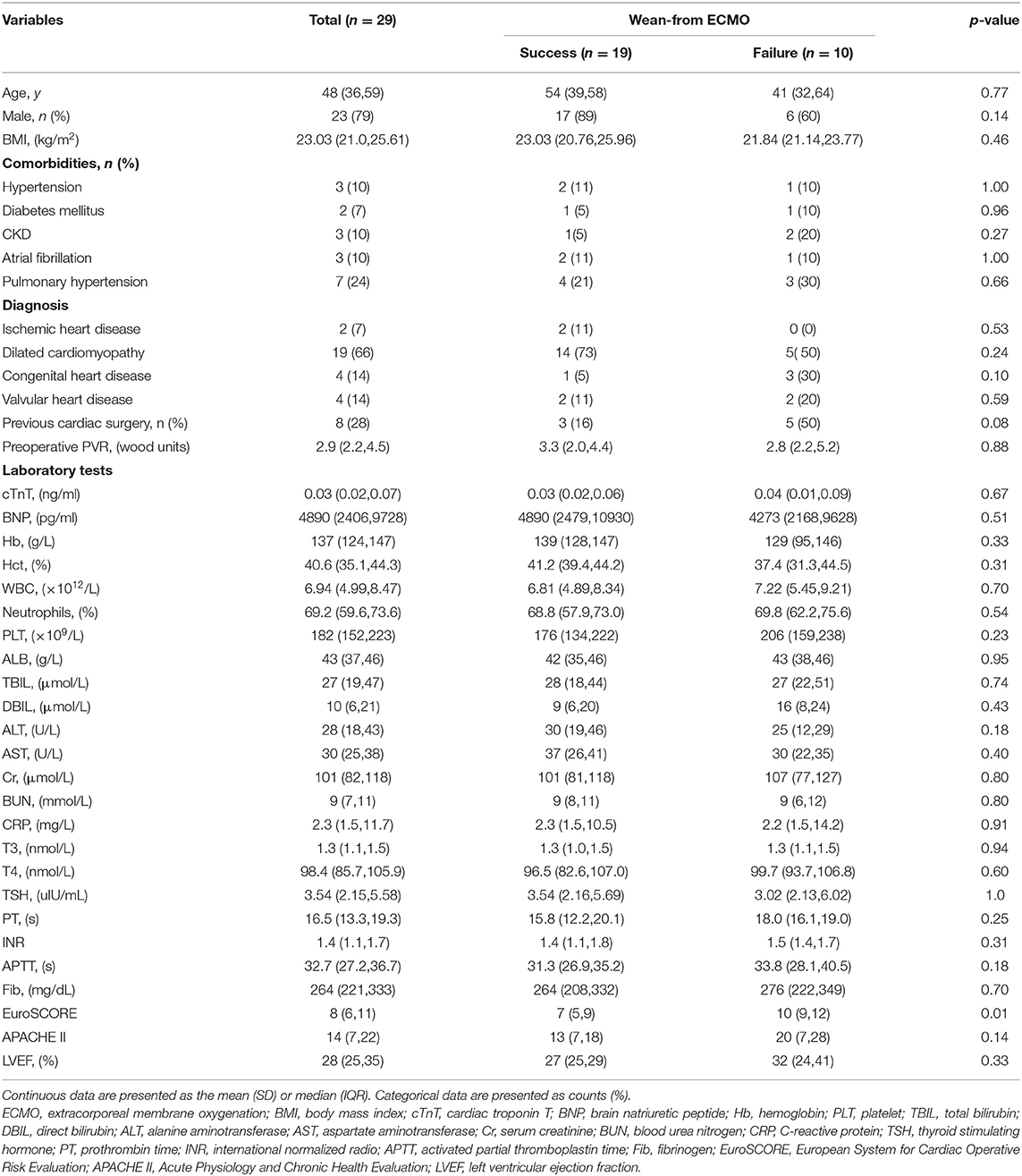
A total of 130 patients underwent an orthotopic heart transplantation procedure between January 2014 and December 2020. Twenty-nine patients who were supported with VA-ECMO following heart transplantation surgery were enrolled in the study (Figure 1). None of the patients received mechanical circulatory support preoperatively. The overall incidence of severe PGD was 22.3%. The mean recipient age was 47 ± 14 years and 23 (79%) of the recipients were men. All of the patients had a pulmonary artery catheter inserted preoperatively. Demographics, comorbidities, laboratory tests, and clinical manifestations in the successful (n = 19) and failed (n = 10) weaning groups are summarized in Table 1. Most variables were similar between the two groups. However, patients in the successful group were likely to have a lower EuroSCORE (7 ± 2 vs. 10 ± 3; p < 0.01) compared to those in the failure group.
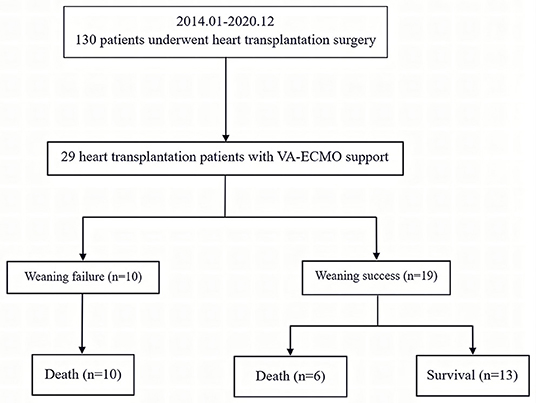
Figure 1. Enrollment, allocation, and follow-up for heart transplant patients who received VA-ECMO. VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
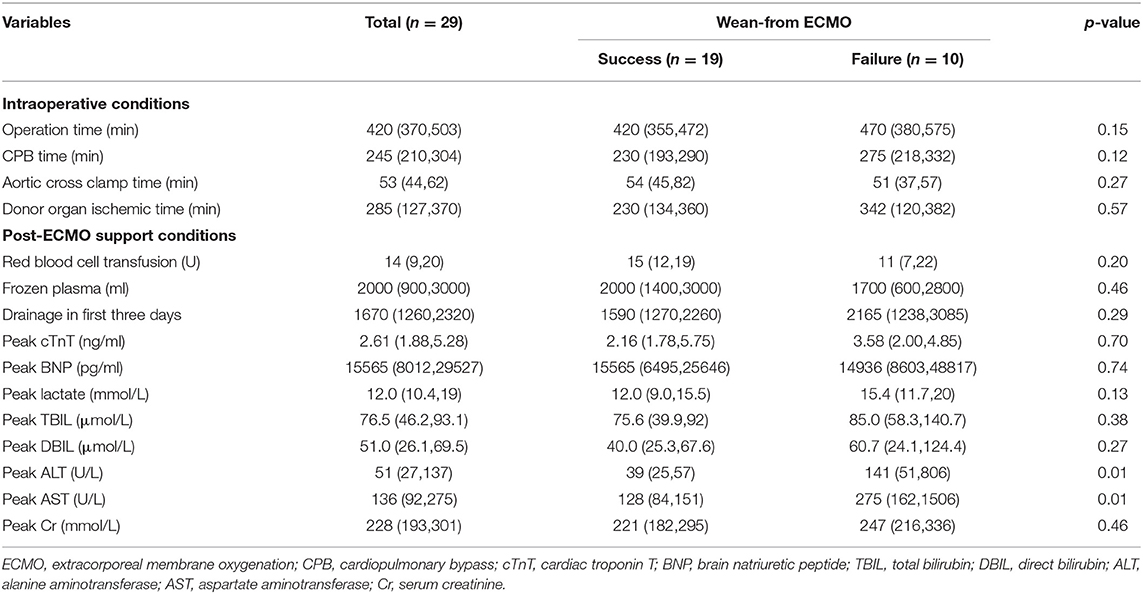
Perioperative details of the 29 patients who were supported with VA-ECMO are shown in Table 2. Although there were no significant differences in operation, CPB, or graft ischemia durations between the two groups, the durations in the failed group were longer than those in the successful group (411 ± 81 vs. 484 ± 116 min; 236 ±65 vs. 280 ± 66 min; 246 ± 118 vs. 274 ± 129 min, p > 0.05). Postoperative peak alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were both significantly higher in patients who failed to wean from VA-ECMO(39 [25–51] vs. 141 [51–806] U/L; 128 [84–151] vs. 275 [162-1,506] U/L, respectively; p < 0.01). Nineteen patients received VA-ECMO support in the operating room due to difficulty with weaning from CPB. Ten patients had VA-ECMO initiated in the cardiac surgery ICU because of refractory postoperative cardiogenic shock. The median time from the end of the operation until VA-ECMO implantation was 8.6 h in the ICU group.
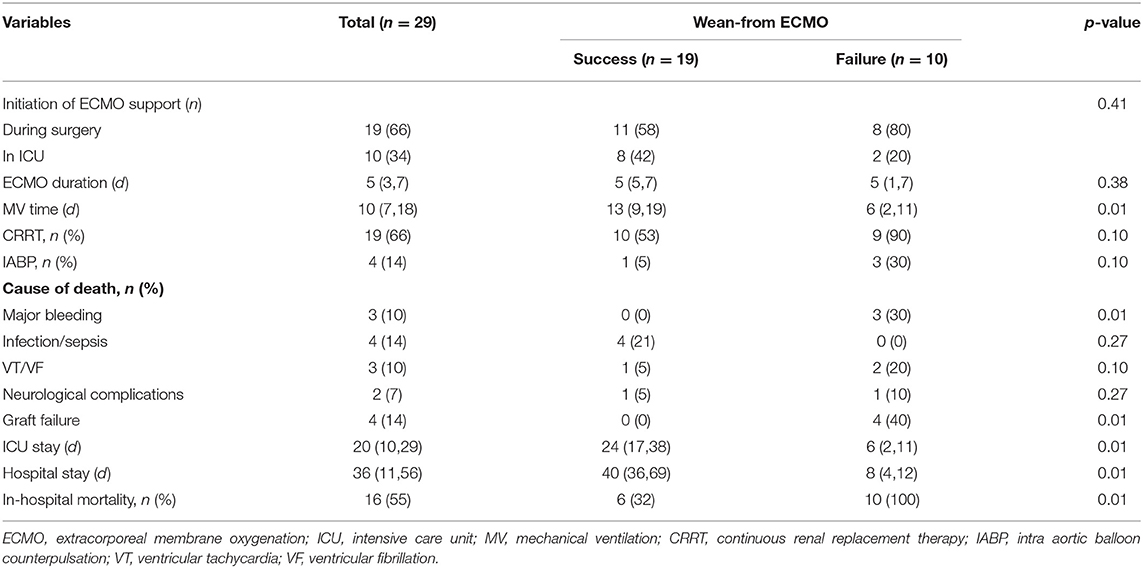
After a median support period of 5 days, 19 patients (66%) were successfully weaned from VA-ECMO support, whereas six patients died in the hospital despite successful weaning from VA-ECMO. Thirteen patients (45%) survived until hospital discharge. No patient developed leg ischemia or leg fasciotomy due to femoral arterial cannulation. The Kaplan–Meier survival curves for patients with severe PGD who required perioperative VA-ECMO support are shown in Figure 2. SOFA scores were significantly higher in the weaning failure group on days 3 and 5, whereas the difference in Lac level was significant only on day 3 (Figure 3).
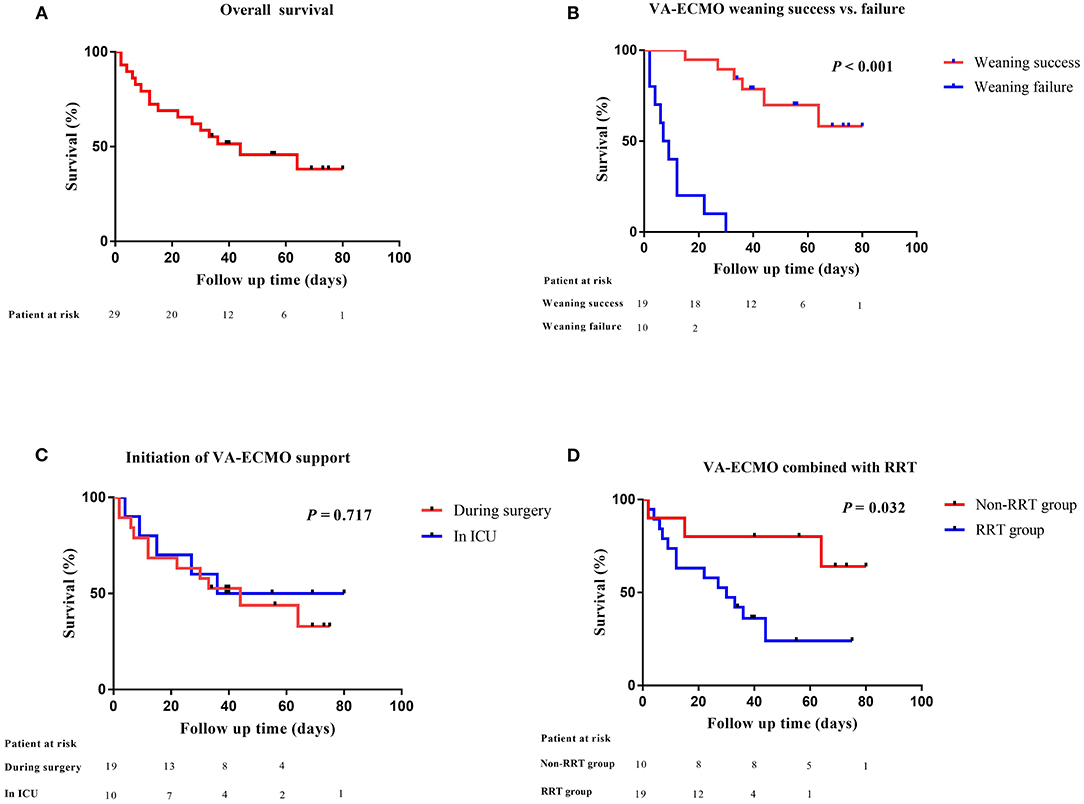
Figure 2. Comparative survival in heart transplant patients after VA-ECMO support. (A) Kaplan–Meier analysis of overall survival in heart transplant patients supported by VA-ECMO from 2014 to 2020 (n = 29); (B) VA-ECMO weaning success or failure; (C) initiation of VA-ECMO support; (D) VA-ECMO combined with RRT. VA-ECMO, veno-arterial extracorporeal membrane oxygenation; ICU, intensive care unit; RRT, renal replacement therapy.
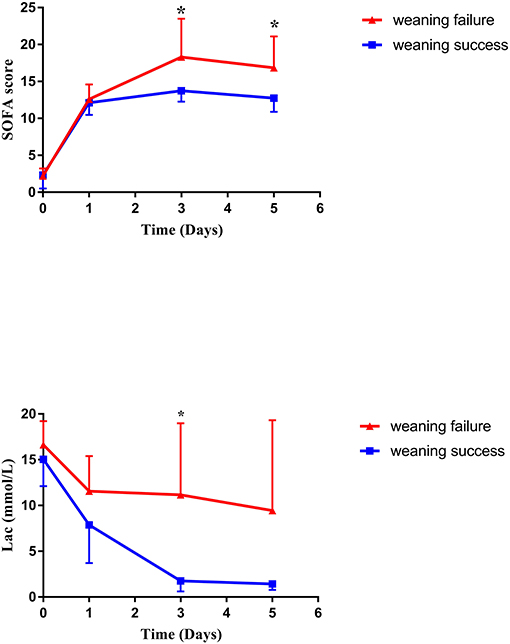
Figure 3. Changes in SOFA score and lactate level during VA-ECMO support. SOFA, sequential organ failure assessment; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Additionally, six of the patients who were successfully weaned from VA-ECMO died, including four patients died of infection or sepsis (21, 59, 8, and 30 days after weaning), one patient died of ventricular arrhythmia (31 days after weaning), and one patient died of neurological complications (19 days after weaning). No patient was lost to follow-up during this period. In addition, VA-ECMO treatment of severe PGD had no adverse effect on graft function among survivors. Details for VA-ECMO implementation and outcomes in this patient are outlined in Table 3.
Discussion
In this single-center study, 29 out of 130 heart transplant recipients received VA-ECMO and achieved relatively satisfactory results. Because LVADs and RVADs were not available in our center, VA-ECMO was the only effective treatment for patients with severe PGD after heart transplantation. To the best of our knowledge, this study is the first to apply the new ISHLT criteria to Chinese patients to investigate outcomes of severe PGD at high-volume transplantation centers.
Over the last several decades, orthotopic heart transplantation has become the standard treatment for select patients with advanced and refractory heart failure. However, severe PGD remains a major cause of perioperative death after heart transplantation. Several studies have investigated the incidence of severe PGD using the ISHLT consensus statement criteria and found that the incidence rate was 7–23% with in-hospital mortality of 19–65% (4, 6, 17–21). VA-ECMO is a viable extracorporeal life support technique for cardiac surgery patients who are difficult to wean from CPB or those with postoperative cardiogenic shock. In this study, the successful weaning rate and mortality following VA-ECMO were 66 and 55%, respectively, which are comparable to those published by Alessandro et al. (weaning: 60%, survival: 46%) (20). We believe that the main causes of this higher incidence rate and mortality may be associated with marginal donors, basic patient conditions, surgical strategies, longer cold ischemia duration, and indication for VA-ECMO despite its likelihood to be multifactorial (4, 5, 22).
The timing of VA-ECMO implantation is an important factor influencing patient outcomes. A previous study has shown that prompt implementation of VA-ECMO allows for adequate organ perfusion and promotes the recovery of graft function, while avoiding multiple organ dysfunction and complications caused by large doses of inotropic or vasoactive agents (17). This study compared outcomes in patients with different VA-ECMO initiation times and found no significant differences between the two groups in terms of weaning success rate or in-hospital mortality. It should be noted that the median time from the end of the operation to the start of VA-ECMO was 8.6 h in the ICU group. It has been shown that delayed VA-ECMO initiation for longer than 24 h in heart transplant recipients with refractory cardiogenic shock can lead to poor outcomes (6, 17, 23). Based on our previous experience, we were more aggressive in using VA-ECMO as a therapy for severe PGD in the ICU.
It has been reported that successful weaning from VA-ECMO does not mean patient survival. Several studies have assessed the predictors of death after VA-ECMO weaning in postcardiotomy shock patients. VA-ECMO implantation time, poor renal and liver function, high lactate levels, and high SOFA scores were reported to be predictors of death after weaning (24–26), which is similar to the results in this study. Many scoring systems are routinely used in cardiac surgery ICU. SOFA was developed to objectively evaluate the degree of severity in ICU patients and to simplify the prediction of patients' risk of mortality (27). Nevertheless, in patients with VA-ECMO support, the performance of the SOFA score in predicting short-term mortality is still controversial (25, 28).
Complications during VA-ECMO support may directly lead to recipient's death or forced weaning from VA-ECMO. The leading causes of death in this study were surgical bleeding, graft failure, and septic shock. The causes of major postoperative bleeding are multifactorial (29–33). Excessive loss of coagulation factors during operation, longer CPB time, thrombocytopenia, massive perioperative blood transfusion, and postoperative VA-ECMO support can lead to coagulation dysfunction and relentless bleeding. Three patients died of major bleeding in this study. Therefore, it is very important to strictly maintain hemostasis during the operation, provide infusion of fresh frozen plasma, platelets, fibrinogen, and prothrombin complex, closely observe drainage after the operation, and conduct a secondary thoracotomy if necessary. How to best maintain the balance between bleeding and clotting in VA-ECMO patients remains a challenge.
There were several limitations in this study. First, this was a single-center retrospective study. Second, although the analyses were based on the experience over the past 7 years, the sample size was too small and the follow-up time was relatively short, which meant that detailed risk factor analysis was not possible. Third, due to privacy protection, we are unable to obtain donor information. In the future, multicenter studies with large patient populations are needed to optimize management strategies and to improve outcomes in this rare but complex cardiac emergency.
Conclusion
In conclusion, the application of VA-ECMO in the perioperative period of heart transplantation provided a “bridge to recovery” in patients with severe PGD. Further research is needed to determine the optimal time for VA-ECMO use and how to prevent complications to improve patient prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongshan Hospital affiliated to Fudan University (No. B2020-169). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
J-yH, G-wT, and ZL contributed to study design. XL, Y-jZ, J-lZ, and J-fM contributed to participant enrollment. K-fG and S-gY contributed to study management and data collection. J-yH and S-gY contributed to manuscript writing. G-wT and ZL contributed to data analyses and manuscript revision. All authors have read and approved the final manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of Shanghai (20ZR1411100 to ZL, 21ZR1412900 to YS), the Science and Technology Commission of Shanghai Municipality (20DZ2261200 to ZL), the National Natural Science Foundation of China (82070085 to G-wT), the Construction Program of Key but Weak Disciplines of Shanghai Health Commission (2019ZB0105 to ZL), the Clinical Research Project of Zhongshan Hospital (2020ZSLC38 to G-wT, 2020ZSLC27 to ZL), the Smart Medical Care of Zhongshan Hospital (2020ZHZS01 to ZL), the Project for Elite Backbone of Zhongshan Hospital (2021ZSGG06 to G-wT), and the Foundation for Young Researchers of Zhongshan Hospital (2021ZSQN22, 2021ZSQN71, and 2021ZSQN72).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
References
1. Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Hsich E, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report-−2019; focus theme: Donor and recipient size match. J Heart Lung Transplant. (2019) 38:1056–66. doi: 10.1016/j.healun.2019.08.004
2. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. (2017) 36:1037–46. doi: 10.1016/j.healun.2017.07.019
3. Kobashigawa J, Zuckermann A, Macdonald P, Leprince P, Esmailian F, Luu M, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. (2014) 33:327–40. doi: 10.1016/j.healun.2014.02.027
4. Avtaar Singh SS, Banner NR, Rushton S, Simon AR, Berry C, Al-Attar N, et al. Primary graft dysfunction incidence, risk factors, and outcome: A UK national study. Transplantation. (2019) 103:336–43. doi: 10.1097/TP.0000000000002220
5. Nicoara A, Ruffin D, Cooter M, Patel CB, Thompson A, Schroder JN, et al. Primary graft dysfunction after heart transplantation: Incidence, trends, and associated risk factors. Am J Transplant. (2018) 18:1461–70. doi: 10.1111/ajt.14588
6. Takeda K, Li B, Garan AR, Topkara VK, Han J, Colombo PC, et al. Improved outcomes from extracorporeal membrane oxygenation versus ventricular assist device temporary support of primary graft dysfunction in heart transplant. J Heart Lung Transplant. (2017) 36:650–6. doi: 10.1016/j.healun.2016.12.006
7. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report-−2015; focus theme: early graft failure. J Heart Lung Transplant. (2015) 34:1244–54. doi: 10.1016/j.healun.2015.08.003
8. Rao P, Smith R, Khalpey Z. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail. (2018) 6:887. doi: 10.1016/j.jchf.2018.05.019
9. Van Herck JL, Claeys MJ, De Paep R, Van Herck PL, Vrints CJ, Jorens PG. Management of cardiogenic shock complicating acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. (2015) 4:278–97. doi: 10.1177/2048872614568294
10. Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. (2010) 139:302–11, 11 e1. doi: 10.1016/j.jtcvs.2009.10.043
11. Su Y, Liu K, Zheng JL Li X, Zhu DM, Zhang Y, et al. Hemodynamic monitoring in patients with venoarterial extracorporeal membrane oxygenation. Ann Transl Med. (2020) 8:792. doi: 10.21037/atm.2020.03.186
12. Hou JY, Wang CS, Lai H, Sun YX Li X, Zheng JL, et al. Veno-arterial extracorporeal membrane oxygenation for patients undergoing acute type A aortic dissection surgery: A six-year experience. Front Cardiovasc Med. (2021) 8:652527. doi: 10.3389/fcvm.2021.652527
13. Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. (2016) 6:97. doi: 10.1186/s13613-016-0196-7
14. Jiritano F, Serraino GF, Ten Cate H, Fina D, Matteucci M, Mastroroberto P, et al. Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med. (2020) 46:1154–69. doi: 10.1007/s00134-020-06031-4
15. Luo JC, Su Y, Dong LL, Hou JY Li X, Zhang Y, et al. Trendelenburg maneuver predicts fluid responsiveness in patients on veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care. (2021) 11:16. doi: 10.1186/s13613-021-00811-x
16. Kim D, Jang WJ, Park TK, Cho YH, Choi JO, Jeon ES, et al. Echocardiographic predictors of successful extracorporeal membrane oxygenation weaning after refractory cardiogenic shock. J Am Soc Echocardiogr. (2021) 34:414–22 e4. doi: 10.1016/j.echo.2020.12.002
17. DeRoo SC, Takayama H, Nemeth S, Garan AR, Kurlansky P, Restaino S, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after heart transplant. J Thorac Cardiovasc Surg. (2019) 158:1576–84 e3. doi: 10.1016/j.jtcvs.2019.02.065
18. Sabatino M, Vitale G, Manfredini V, Masetti M, Borgese L, Maria Raffa G, et al. Clinical relevance of the International Society for Heart and Lung Transplantation consensus classification of primary graft dysfunction after heart transplantation: Epidemiology, risk factors, and outcomes. J Heart Lung Transplant. (2017) 36:1217–25. doi: 10.1016/j.healun.2017.02.014
19. Squiers JJ, Saracino G, Chamogeorgakis T, MacHannaford JC, Rafael AE, Gonzalez-Stawinski GV, et al. Application of the International Society for Heart and Lung Transplantation (ISHLT) criteria for primary graft dysfunction after cardiac transplantation: outcomes from a high-volume centredagger. Eur J Cardiothorac Surg. (2017) 51:263–70. doi: 10.1093/ejcts/ezw271
20. D'Alessandro C, Golmard JL, Barreda E, Laali M, Makris R, Luyt CE, et al. Predictive risk factors for primary graft failure requiring temporary extra-corporeal membrane oxygenation support after cardiac transplantation in adults. Eur J Cardiothorac Surg. (2011) 40:962–9. doi: 10.1016/j.ejcts.2011.01.064
21. Bermudez CA, McMullan DM. Extracorporeal life support in preoperative and postoperative heart transplant management. Ann Transl Med. (2017) 5:398. doi: 10.21037/atm.2017.08.32
22. Mastroianni C, Nenna A, Lebreton G, D'Alessandro C, Greco SM, Lusini M, et al. Extracorporeal membrane oxygenation as treatment of graft failure after heart transplantation. Ann Cardiothorac Surg. (2019) 8:99–108. doi: 10.21037/acs.2018.12.08
23. Marasco SF, Vale M, Pellegrino V, Preovolos A, Leet A, Kras A, et al. Extracorporeal membrane oxygenation in primary graft failure after heart transplantation. Ann Thorac Surg. (2010) 90:1541–6. doi: 10.1016/j.athoracsur.2010.05.066
24. Ortuno S, Delmas C, Diehl JL, Bailleul C, Lancelot A, Naili M, et al. Weaning from veno-arterial extra-corporeal membrane oxygenation: which strategy to use? Ann Cardiothorac Surg. (2019) 8:E1–8. doi: 10.21037/acs.2018.08.05
25. Wang L, Yang F, Wang X, Xie H, Fan E, Ogino M, et al. Predicting mortality in patients undergoing VA-ECMO after coronary artery bypass grafting: the REMEMBER score. Crit Care. (2019) 23:11. doi: 10.1186/s13054-019-2307-y
26. Chang WW, Tsai FC, Tsai TY, Chang CH, Jenq CC, Chang MY, et al. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. PLoS One. (2012) 7:e42687. doi: 10.1371/journal.pone.0042687
27. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
28. Schrutka L, Rohmann F, Binder C, Haberl T, Dreyfuss B, Heinz G, et al. Discriminatory power of scoring systems for outcome prediction in patients with extracorporeal membrane oxygenation following cardiovascular surgerydagger. Eur J Cardiothorac Surg. (2019) 56:534–40. doi: 10.1093/ejcts/ezz040
29. Koerner MM, Harper MD, Gordon CK, Horstmanshof D, Long JW, Sasevich MJ, et al. Adult cardiac veno-arterial extracorporeal life support (VA-ECMO): prevention and management of acute complications. Ann Cardiothorac Surg. (2019) 8:66–75. doi: 10.21037/acs.2018.12.09
30. Koster A, Ljajikj E, Faraoni D. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann Cardiothorac Surg. (2019) 8:129–36. doi: 10.21037/acs.2018.07.03
31. Mazzeffi M, Strauss E, Meyer M, Hasan S, Judd M, Abuelkasem E, et al. Coagulation factor levels and underlying thrombin generation patterns in adult extracorporeal membrane oxygenation patients. Anesth Analg. (2019) 129:659–66. doi: 10.1213/ANE.0000000000004275
32. Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A, et al. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. (2015) 4:e002066. doi: 10.1161/JAHA.115.002066
Keywords: heart failure, heart transplantation, primary graft dysfunction, veno-arterial extracorporeal membrane oxygenation, cardiogenic shock
Citation: Hou J-y, Li X, Yang S-g, Zheng J-l, Ma J-f, Su Y, Zhang Y-j, Guo K-f, Tu G-w and Luo Z (2021) Veno-Arterial Extracorporeal Membrane Oxygenation for Patients Undergoing Heart Transplantation: A 7-Year Experience. Front. Med. 8:774644. doi: 10.3389/fmed.2021.774644
Received: 12 September 2021; Accepted: 18 November 2021;
Published: 16 December 2021.
Edited by:
Jaimin Trivedi, University of Louisville, United StatesReviewed by:
Dmitry Abramov, Loma Linda University Medical Center (LLUMC), United StatesAdam Protos, University of Mississippi Medical Center, United States
Copyright © 2021 Hou, Li, Yang, Zheng, Ma, Su, Zhang, Guo, Tu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-wei Tu, dHUuZ3Vvd2VpQHpzLWhvc3BpdGFsLnNoLmNu; Zhe Luo, ZG9jdG9ybHpAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Jun-yi Hou
Jun-yi Hou Xin Li2†
Xin Li2† Ying Su
Ying Su Guo-wei Tu
Guo-wei Tu Zhe Luo
Zhe Luo