
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 03 December 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.774345
This article is part of the Research Topic Prognostic Factors in Hepatocellular Carcinoma View all 9 articles
Objective: Apatinib is a inhibitor of vascular endothelial growth factor receptor-2. To explore the efficacy and prognostic factors of transarterial chemoembolization (TACE) combined with apatinib in the treatment of Barcelona Clinic Liver Cancer stage C (BCLC C) hepatocellular carcinoma (HCC).
Methods: Clinical data of 146 HCC patients with BCLC stage C admitted to our hospital were collected and analyzed retrospectively, of which 76 cases were treated with TACE combined with apatinib (TACE-apatinib) and 70 with TACE alone. The tumor response, survival time, and adverse events were compared between the two groups, and the factors affecting the prognosis were analyzed.
Results: The objective response rate (ORR) and disease control rate (DCR) in the TACE-apatinib group were higher than in the TACE-alone group (ORR: 42.10 vs. 25.71%, P = 0.03; DCR: 84.21 vs. 55.71%, P = 0.001). The median time to progression (TTP) and overall survival (OS) in the TACE-apatinib group were longer than in the TACE-alone group (TTP: 5.5 vs. 3.7 months, P = 0.02; OS: 10.0 vs. 6.2 months, P = 0.01). Univariate and multivariate Cox regression analysis showed that tumor size, Child-Pugh class, and the presence of the portal vein tumor thrombus affect the prognosis of patients. Subgroup analysis revealed that TACE-apatinib therapy resulted in a higher OS in patients with tumor size <10 cm, without portal vein tumor thrombus, and with Child-Pugh class A (P < 0.05). The likelihood of adverse events (hand-foot syndrome, hypertension, oral ulcer) was significantly higher in the increased in the TACE-apatinib group than in the TACE alone group (P < 0.05).
Conclusion: TACE-apatinib is an effective and safe method for the treatment of BCLC stage C HCC. Tumor size, Child-Pugh class, and portal vein tumor thrombus affect survival time in HCC patients with BCLC stage C.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world with high mortality in the digestive system (1). Due to the non-specific symptoms and the occult nature of HCC, patients are usually diagnosed at the middle or late stages of the tumor, and approximately half of them have the Barcelona Clinic Liver Cancer (BCLC) stage C. In China, local treatment based on transcatheter arterial chemoembolization (TACE) is the most important therapy for patients with advanced HCC (2, 3). TACE can control tumor growth, block the blood supply to the tumor site, and induce local ischemia and hypoxia by delivering chemotherapeutic drugs and embolic agents into the feeding artery of HCC. However, studies have shown that after TACE, vascular endothelial growth factor-α (VEGF-α) is significantly increased in the residual tumor and promotes angiogenesis. Therefore, anti-angiogenic therapy is essential in patients treated with TACE (4, 5). Sorafenib is recommended as the standard treatment for BCLC stage C HCC. Sorafenib is a molecular targeted drug, which mostly inhibits tumor cell proliferation and angiogenesis. However, for patients in the Asia-Pacific region, its therapeutic effect is limited with the median overall survival (OS) at 6.5 months and the time to progression (TTP) at 2.8 months (6).
Apatinib is a small molecular targeted drug with independent intellectual property rights in China. It selectively acts on the ATP-binding site of the VEGF receptor in tumor cells, inhibiting a variety of tyrosine kinases and block its downstream pathway. These activities inhibit the migration and proliferation of vascular endothelial cells, reduce tumor neovascularization density, and inhibit residual tumor growth (7, 8). A recent randomized controlled study showed that TACE combined with apatinib is safe and can significantly prolong the overall survival and progression-free survival of patients with advanced HCC (9). Based on these findings, the clinical data of patients with HCC stage BCLC-C treated with a combination of TACE and apatinib were retrospectively evaluated.
The clinical data of 146 patients with stage BCLC-C HCC treated in our hospital between January 2011 and July 2019 were analyzed retrospectively. All patients had been informed of the choice of taking apatinib and they made the decision based on their personal willingness. According to their decision of treatments, patients were classified into two groups. Seventy-six patients were included in the TACE combined with apatinib (TACE-apatinib) group, and 70 patients in the TACE alone group. Inclusion criteria were: (1) newly diagnosed liver cancer based on the criteria of the European Association of Liver Diseases; (2) patients with BCLC-C HCC; (3) Child-Pugh liver function class A or B; (4) Karnofsky score (KPS) > 60; and (5) ECOG score ≤ 2. Exclusion criteria were: (1) received systemic chemotherapy, targeted therapy, or radiofrequency ablation before entering the group; (2) contraindications for TACE; (3) severe coagulation disorder or active bleeding. Approval for this retrospective study was obtained from the Ethics Committee of the First Affiliated Hospital of USTC. Written informed consent was obtained from all of the patients before therapy.
In both groups, TACE was performed using the modified Seldinger method. After the guide wire was inserted into the arterial sheath, the 5.0 Fr RH catheter (Cook, Bloomington, IN, USA) was placed, and dextromethorphan and palonosetron hydrochloride were injected into the abdominal aorta. After the upper derivation tube was formed, the downward catheter was inserted into the celiac trunk at the level of the middle and lower margin of the T12 vertebra. Subsequently, DSA angiography was performed to identify the hepatic artery and its branches to understand the distribution range, size, location, and the number of blood supply arteries in tumor tissue. The 3.0 Fr micro-catheter (ProgreatTM, Terumo, Tokyo, Japan) was inserted into the tumor-related blood supply artery, and lipiodol (5–15 mL; Lipiodol Ultrafluido, Guerbet, France), pirarubicin (30–50 mg) and carboplatin (30–50 mg) were injected. The specific dose depended on the embolization condition of the patient. Finally, the proximal artery was embolized with polyvinyl alcohol particles (300–500 um, Cook, USA). Some patients with severe portal vein thrombosis, wide distribution of tumors or hepatic arteriovenous fistula were not given a sufficient dose of embolic agent because of the high risk of failure of liver function recovery after treatment.
The patients in the TACE-apatinib group were treated with apatinib on the basis of TACE. Apatinib, 500 mg/day, taken orally beginning on the 3rd day after TACE. If unbearable serious adverse reactions occurred during the treatment, the dose was reduced to 250 mg daily or suspended, and patients remained under close observation and symptomatic treatment. When the adverse reactions were alleviated or disappeared, the initial dose of apatinib was gradually restored. Apatinib was discontinued 4 days before the next course of TACE and recovered 4 days after TACE. If the drug was stopped for more than a month, the patient was excluded from the study. Patients in both groups were treated until they did not tolerate the regimen or the tumor progressed; the treatment cycle was 4 weeks.
Enhanced CT or MRI, routine blood, liver and kidney function, and AFP tests were performed 1 month and 3 months after the treatment. According to The Modified Response Evaluation Criteria in Solid Tumors (mRECIST), the local curative effect was divided into four grades: complete remission (CR) if all tumor tissue disappeared on arterial phase enhanced imaging; partial remission (PR) if tumor diameter decreased by more than 30%; on arterial phase enhanced imaging; stable disease (SD) if tumor diameter on arterial phase enhanced imaging did not decrease by at least 30% or increase by at least 20%; disease progression (PD) if tumor diameter on arterial phase enhanced imaging diameter increased by at least 20% compared with the baseline value, or new lesions emerged. Objective remission rate (ORR) was calculated as (CR+PR)/total number of cases × 100%. Disease control rate (DCR) was calculated as (CR+PR+SD)/total number of cases × 100%. Adverse events were evaluated according to The Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, the general terminology of adverse events.
The primary end-point of this study was the overall survival time (OS), defined as the time from the first interventional therapy to death or loss of follow-up. The secondary end-point was the time to progression (TPP), defined as the time from first interventional therapy to a definite disease progression or the time of death.
The SPSS 26.0 statistical software package (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Median and 95% confidence interval or mean and standard deviation were used to present continuous variables, while categorical variables are presented as a frequency and percentage. For the comparison of the percentages and frequencies between the two groups, Chi-square test was performed. The survival curve was constructed using the Kaplan-Meier method, and the logarithmic rank test was performed to detect significant differences. Univariate and multivariate Cox proportional hazard regression analysis was used to identify risk factors affecting survival. All statistical tests were two-tailed, and P < 0.05 was considered statistically significant.
There was no statistical difference in general characteristics between the TACE-apatinib group and the TACE-alone group (P > 0.05, Table 1).
After 1 month of treatment, the rates of disease remission and stable disease in the TACE-apatinib group were 42.10 and 84.21%, respectively. Corresponding values in the TACE-alone group were 25.71 and 55.71% (Table 2). The difference in local curative effect between the two groups was statistically significant (p < 0.05).
The median TTP was 5.5 months (95% CI: 4.0–6.9 months) in the TACE-apatinib group and 3.7 months (95% CI: 2.9–4.4 months) in the TACE-alone group, and this difference was statistically significant (P < 0.02, Figure 1A). The median OS was 10.0 months (95% CI: 8.2–11.7 months) in the TACE-apatinib group and 6.2 months (95% CI: 5.4–6.9 months) in the TACE-alone group and this difference was statistically significant (P < 0.01, Figure 1B).
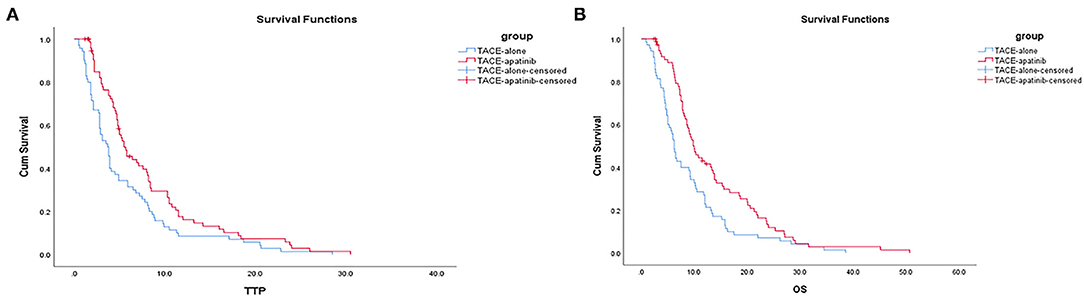
Figure 1. (A) Time to progression curves in the two groups of patients. (B) Overall survival curves in the two groups of patients.
Univariate analysis showed that ECOG score, AFP, number of tumors, distant metastasis, and history of hepatitis were not related to the median OS and TTP, but portal vein tumor thrombus, tumor size, Child-Pugh grade, and treatment modality were all related to the prognosis of the patients. Cox regression multivariate analysis showed that tumor thrombus, tumor size, Child-Pugh grade, and treatment modality were independent risk factors for prognosis (P < 0.05, Table 3).
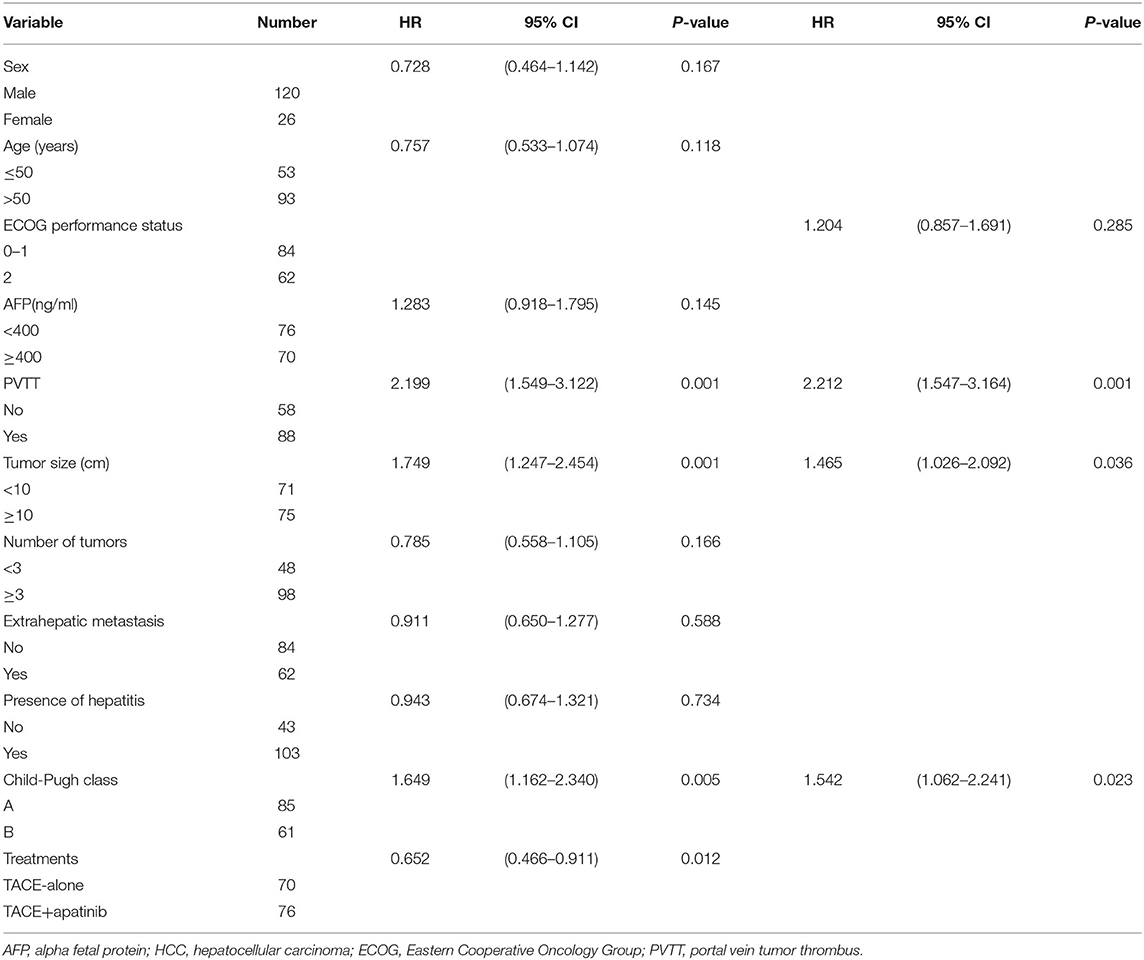
Table 3. Univariate analysis and multivariate analysis of prognosis in patients with stage BCLC-C HCC.
In patients with stage BCLC-C, the median OS after TACE-apatinib was higher than after TACE alone in patients with Child-Pugh class A, any tumor size, and with or without portal vein tumor thrombus (P < 0.05). There was no significant difference between TACE-apatinib and TACE-alone treatment in patients with Child-Pugh class B (P = 0.08) (Figures 2A–F).
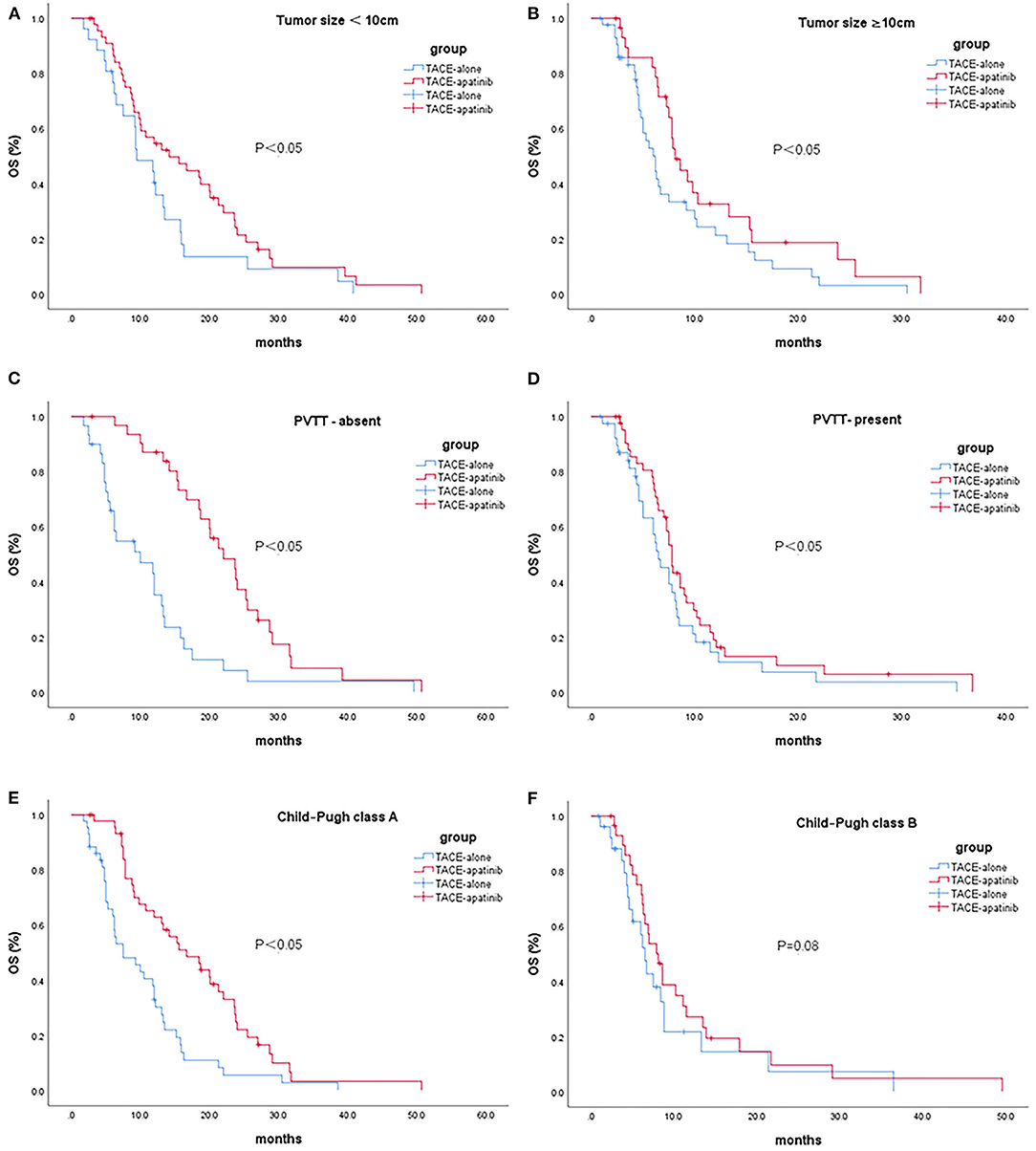
Figure 2. Kaplan-Meier curves showed overall survival in the TACE-apatinib and TACE-alone groups in patients with BCLC stage C HCC [(A) tumor size <10 cm, (B) tumor size ≥10 cm, (C) PVTT-absent, (D) PVTT-present, (E) Child-Pugh class A, and (F) Child-Pugh class B].
The most frequent adverse events in the TACE-apatinib group were nausea, fever, and hand-foot syndrome, and in the TACE-alone group, nausea, fever, and fatigue. The incidence of hand and foot syndrome, hypertension, and oral ulcer in the combined treatment group was higher than in the TACE-alone group, and the difference was statistically significant (P < 0.05). All these adverse events disappeared after symptomatic treatment.
HCC patients with BCLC stage C have a vascular invasion or extrahepatic metastasis, and most of them cannot be treated surgically. Their liver function is impaired, and the prognosis is poor (10). At present, the internationally recognized treatment standard for these patients is systemic sorafenib therapy. However, due to the high price of sorafenib and possible drug resistance, it is not widely used clinically (11). In China, local interventional therapy based on TACE remains the most important treatment for HCC patients with BCLC stage C. Apatinib is an oral small-molecule targeted drug produced independently in China, which has been widely used in the treatment of gastric cancer, small cell lung cancer, esophageal cancer, and other tumors (12–14). At present, the treatment of BCLC stage C HCC with TACE-apatinib is still at the research stage. In principle, TACE can embolize the vessels supplying the tumor, promote tumor cell apoptosis, and inhibit tumor cell proliferation. TACE can rapidly reduce the tumor load of patients and improve clinical symptoms and quality of life. Apatinib can inhibit angiogenesis and vessel remodeling after TACE treatment, suppress the recurrence of the tumor and metastasis of liver cancer cells, and improve the local efficacy and long-term survival rate of patients with liver cancer (15). Therefore, these two treatments play a complementary role in the treatment of liver cancer.
In our study, the objective remission rate (ORR) and disease control rate (DCR) in the TACE-apatinib group were 42.10 and 84.21%, respectively. These values were higher than those of TACE combined with sorafenib or sorafenib alone. This difference reflects the fact that apatinib, a receptor tyrosine kinase inhibitor selectively targeting VEGFR-2, has a binding affinity 10 times higher than sorafenib (16). Apatinib is generally well-tolerated and is associated with controllable adverse reactions; the most common drug-related adverse reactions are hand and foot syndrome, hypertension, and albuminuria, which is similar to the previously reported study of apatinib as the only treatment and TACE combined with apatinib (17, 18). In the present study, the incidence of adverse reactions, particularly hypertension, hand and foot syndrome, and oral mucositis, was higher in the TACE-apatinib group than in the TACE-alone group. There was no significant difference in post-embolization syndrome between the two groups.
Recent clinical studies have shown that TACE combined with apatinib can prolong the survival of patients with advanced liver cancer (19, 20). The current analysis showed that for HCC patients with BCLC stage C, the median survival time in the TACE-apatinib group was 3.8 months longer than in the TACE-alone group, and the difference was statistically significant (P < 0.05). The median TTP in the TACE-apatinib group was 5.5 months, and in the TACE-alone group, 3.7 months, indicating that the combined therapy could delay tumor progression and reduce tumor load. Some studies have confirmed that the median survival time of BCLC stage C HCC patients treated with TACE alone is 7.1 months and in patients treated with supportive therapy is 5.1 months, indicating that TACE can significantly prolong the survival time of patients (21). Our work documented that the median survival time in the TACE-apatinib group was 10.0 months, implying that the combined therapy further improved the survival time of patients with BCLC stage C HCC. From the point of view of clinical practice, TACE combined with apatinib can significantly prolong the survival time of patients with liver cancer.
The conducted univariate and multivariate analysis demonstrated that tumor size, Child-Pugh class, and portal vein tumor thrombus were independent prognostic factors for the survival of patients with BCLC stage C HCC. Earlier studies documented that the 1- and 3-year survival rate of patients with small liver cancer treated with TACE is significantly longer than that of patients with large liver cancer. Tumor size also significantly affects the prognosis of primary liver cancer treated with the combination of TACE and apatinib; with the increase in tumor size, the median survival time of patients was shortened (22). Current studies have confirmed that the larger the tumor size and the higher the activity, the more difficult it is to completely inhibit tumor growth, and the easier it is to relapse and metastasize, negatively affecting the prognosis of patients (23, 24). Liver function grade was also shown to be related to prognosis. The main reason underlying this relationship is that the higher grade of liver function is associated with a worse compensatory ability of liver function, lower liver tolerance, and poor conditions for TACE re-treatment, which can not only fail to provide a good effect but may even accelerate liver failure in some patients (25, 26). Although portal vein tumor thrombus is present in a considerable number of HCC patients with BCLC stage C, this study shows that cancer thrombus is still an independent risk factor for the prognosis of patients. The portal vein is mainly derived from the tumor, so portal vein tumor thrombus is more common. Tumor thrombus in the portal vein system can not only cause intrahepatic or extrahepatic metastasis of the tumor but can also block the portal vein system, resulting in an increased portal vein pressure. Additionally, it can cause dilatation and tortuosity of the portal vein system, especially in the lower segment of the esophagus and gastric fundus vein, increasing the probability of upper gastrointestinal bleeding (27). As the normal liver tissue is primarily supplied by the portal vein, portal hypertension will reduce the effective blood supply to the liver tissue and lead to further impairment of liver function.
This study has some limitations. The number of cases is relatively small, and the follow-up time is relatively short. Moreover, this is an observation and retrospective study, and thus may be affected by subjective selection bias. More large-scale multicenter prospective studies are needed to verify the efficacy of the combination of TACE and apatinib.
In conclusion, TACE combined with apatinib is a safe and effective treatment of advanced liver cancer and may represent a novel and feasible treatment modality for BCLC stage C HCC.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of USTC (Anhui Provincial Hospital) Medical Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
W-FL conceived the study and wrote the manuscript. DL, X-HZ, BJ, Y-LT, and G-XW collected the data. SL and K-CL performed statistical analyses. SL and W-FL provided support and helped with manuscript revision. All authors were involved in analyzing the results. All authors contributed to the article and approved the submitted version.
This work was supported by the Fundamental Research Funds for the Central Universities (No. WK9110000061) and by the Anhui Natural Science Foundation (No. 1808085MH254).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
2. Xie L, Yin J, Xia R, Zhuang G. Cost-effectiveness of antiviral treatment after resection in hepatitis B virus-related hepatocellular carcinoma patients with compensated cirrhosis. Hepatology. (2018) 68:1476–86. doi: 10.1002/hep.29922
3. Xie D, Ren Z, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. (2020) 9:452–63. doi: 10.21037/hbsn-20-480
4. Nahm J, Rhee H, Kim H, Yoo J, San Lee J, Jeon Y, et al. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: A biopsy and resection matched study. Oncotarget. (2017) 8:99359–71. doi: 10.18632/oncotarget.22078
5. Schicho A, Hellerbrand C, Krüger K, Beyer L, Wohlgemuth W, Niessen C, et al. Impact of different embolic agents for transarterial chemoembolization (TACE) procedures on systemic vascular endothelial growth factor (VEGF) levels. J Clin Transl Hepatol. (2016) 4:288–92. doi: 10.14218/jcth.2016.00058
6. Cheng A, Kang Y, Chen Z, Tsao C, Qin S, Kim J, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. (2009) 10:25–34. doi: 10.1016/s1470-2045(08)70285-7
7. Ding J, Chen X, Gao Z, Dai X, Li L, Xie C, et al. Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humans. Drug Metab Dispos. (2013) 41:1195–210. doi: 10.1124/dmd.112.050310
8. Zhou C, Yao Q, Zhang H, Guo X, Liu J, Shi Q, et al. Combining transcatheter arterial embolization with iodized oil containing Apatinib inhibits HCC growth and metastasis. Sci Rep. (2020) 10:2964. doi: 10.1038/s41598-020-59746-1
9. Lu W, Jin X, Yang C, Du P, Jiang F, Ma J, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol Ther. (2017) 18:433–8. doi: 10.1080/15384047.2017.1323589
10. Kaneko S, Tsuchiya K, Yasui Y, Inada K, Kirino S, Yamashita K, et al. Strategy for advanced hepatocellular carcinoma based on liver function and portal vein tumor thrombosis. Hepatol Res. (2020) 50:1375–85. doi: 10.1111/hepr.13567
11. Liu K, Hao Y, Lv W, Jia W, Ji C, Zhou C, et al. Transarterial chemoembolization combined with sorafenib in patients with BCLC stage C hepatocellular carcinoma. Drug Des Devel Ther. (2020) 14:3461–8. doi: 10.2147/dddt.S248850
12. Wang E, Xia D, Bai W, Yuan J, Li X, Niu J, et al. Tumor hypervascularity and hand-foot-skin reaction predict better outcomes in combination treatment of TACE and Sorafenib for intermediate hepatocellular carcinoma. BMC Cancer. (2019) 19:409. doi: 10.1186/s12885-019-5570-z
13. Liang J, Gu W, Jin J, Zhang H, Chen Z, Tang Y, et al. Efficacy and safety of apatinib as third- or further-line therapy for patients with advanced NSCLC: a retrospective study. Ther Adv Med Oncol. (2020) 12:1758835920968472. doi: 10.1177/1758835920968472
14. Park S, Nam C, Kim S, Mun J, Rha S, Chung H. Comparative efficacy and tolerability of third-line treatments for advanced gastric cancer: A systematic review with Bayesian network meta-analysis. Euro J Cancer. (2020) 144:49–60. doi: 10.1016/j.ejca.2020.10.030
15. Liu X, Deng L, Guo R, Liang Q, Liu S, Hu P. [Evaluation of total liver perfusion imaging of CT for efficacy of transcatheter arterial chemoembolization combined with apatinib on rabbit VX2 liver tumors]. Zhong nan da xue xue bao Yi xue ban. (2019) 44:477–84. doi: 10.11817/j.issn.1672-7347.2019.05.002
16. Zhang Y, Huang G, Miao H, Song Z, Zhang X, Fan W, et al. Apatinib treatment may improve survival outcomes of patients with hepatitis B virus-related sorafenib-resistant hepatocellular carcinoma. Ther Adv Med Oncol. (2020) 12:1758835920937422. doi: 10.1177/1758835920937422
17. Chen S, Yu W, Zhang K, Liu W. Comparison of the efficacy and safety of Transarterial chemoembolization with and without Apatinib for the treatment of BCLC stage C hepatocellular carcinoma. BMC Cancer. (2018) 18:1131. doi: 10.1186/s12885-018-5081-3
18. Yang Z, Chen G, Cui Y, Xiao G, Su T, Yu J, et al. The safety and efficacy of TACE combined with apatinib on patients with advanced hepatocellular carcinoma: a retrospective study. Cancer Biol Ther. (2019) 20:321–7. doi: 10.1080/15384047.2018.1529099
19. Wei Y, Liu J, Yan M, Zhao S, Long Y, Zhang W. Effectiveness and safety of combination therapy of transarterial chemoembolization and apatinib for unresectable hepatocellular carcinoma in the Chinese population: a meta-analysis. Chemotherapy. (2019) 64:94–104. doi: 10.1159/000502510
20. Zhao S, Zhang T, Dou W, Wang E, Wang M, Wang C, et al. A comparison of transcatheter arterial chemoembolization used with and without apatinib for intermediate- to advanced-stage hepatocellular carcinoma: a systematic review and meta-analysis. Ann Trans Med. (2020) 8:542. doi: 10.21037/atm.2020.02.125
21. Lv W, Liu K, Lu D, Zhou C, Cheng D, Xiao J, et al. Transarterial chemoembolization for hepatocellular carcinoma combined with portal vein tumor thrombosis. Cancer Manag Res. (2018) 10:4719–26. doi: 10.2147/cmar.S166527
22. Ni J, Sun H, Chen Y, Luo J, Chen D, Jiang X, et al. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. (2014) 20:17483–90. doi: 10.3748/wjg.v20.i46.17483
23. Jun C, Yoon J, Cho E, Shin S, Cho S, Kim H, et al. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: A novel approach to subclassification and treatment. Medicine. (2017) 96:e6745. doi: 10.1097/md.0000000000006745
24. Zhang W, Wang X, Jiang R, Hou J, Mu X, Li G, et al. Effect of tumor size on cancer-specific survival in small hepatocellular carcinoma. Mayo Clin Proc. (2015) 90:1187–95. doi: 10.1016/j.mayocp.2015.06.018
25. Qiu Z, Shen L, Chen S, Qi H, Cao F, Xie L, et al. Efficacy of apatinib in transcatheter arterial chemoembolization (TACE) refractory intermediate and advanced-stage hepatocellular carcinoma:a propensity score matching analysis. Cancer Manag Res. (2019) 11:9321–30. doi: 10.2147/cmar.S223271
26. Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. (2013) 24:2565–70. doi: 10.1093/annonc/mdt247
Keywords: hepatocellular carcinoma, TACE, apatinib, efficacy, prognostic factors
Citation: Liu S, Liu K-C, Lv W-F, Lu D, Zhu X-H, Jiang B, Tan Y-L and Wang G-X (2021) The Efficacy and Prognostic Factors of the Combination of TACE and Apatinib for the Treatment of BCLC Stage C Hepatocellular Carcinoma. Front. Med. 8:774345. doi: 10.3389/fmed.2021.774345
Received: 11 September 2021; Accepted: 08 November 2021;
Published: 03 December 2021.
Edited by:
Marcello Dallio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Mario Romeo, University of Campania Luigi Vanvitelli, ItalyCopyright © 2021 Liu, Liu, Lv, Lu, Zhu, Jiang, Tan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Fu Lv, d2VpZnVsdkB1c3RjLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.