
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 08 November 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.771206
This article is part of the Research TopicMolecular Mechanism of Metastasis in Gastrointestinal CancerView all 8 articles
 Xianghui Li1†
Xianghui Li1† Zhiyan Li1†
Zhiyan Li1† Ping Liu2†
Ping Liu2† Shichao Ai1
Shichao Ai1 Feng Sun1
Feng Sun1 Qiongyuan Hu1
Qiongyuan Hu1 Yuxiang Dong3*
Yuxiang Dong3* Xuefeng Xia1
Xuefeng Xia1 Wenxian Guan1*
Wenxian Guan1* Song Liu1*
Song Liu1*Gastric cancer (GC) is one of the most prevalent malignancies with an unfavorable survival rate. Immunotherapy may contribute to a better prognosis. However, several phase III trials failed. Circular RNA (circRNA) is a novel type of non-coding RNA, plays a vital role in the progression of tumors. The expression and function of circRNA in the GC immune microenvironment remain obscure. In this study, we utilized a bioinformatic analysis to construct a circRNA/microRNA (miRNA)/messenger RNA (mRNA) network involved in the progression and prognosis of GC. CircRNA DYRK1A_017, circRNA FLNA_118, miR-6512-3p, miR-6270-5p, and VCAN were identified as the key molecules in the hub regulatory axis. Dysregulation of this axis contributed to the cancer-associated signaling pathways (epithelial-mesenchymal transition [EMT], Nuclear factor kappa β-Tumor necrosis factor-α (NFκβ-TNFα) signaling, and angiogenesis) and aberrant immune microenvironment (infiltration by tumor associated macrophage, regulatory T cell, and mast cell). More importantly, the immunosuppressive tumor microenvironment may reveal the mechanism of novel circRNAs in tumors and serve as the target of immunotherapy.
As one of the most prevalent malignant tumors in both genders, gastric cancer (GC) is a major leading cause of cancer-related death worldwide in recent years. In 2018, there were an estimated more than 1 million GC cases globally (1, 2). Especially in East Asia, the incidence and mortality of GC remain high (3, 4). Unfortunately, since patients with early GC lack obvious and specific symptoms, the early diagnosed rate is low. Therefore, the 5-year survival rate with or without lymph node metastasis is <20 and 10%, respectively (5, 6). The WHO classification and tumor node metastasis (TNM) stage are generally utilized for hierarchical treatment. However, the prognosis among the patients with the same pathological stage varies, suggesting the important roles of gene heterogeneity and tumor microenvironment (TME) (7).
The tumor immune microenvironment is critical for the survival of patients (8, 9). Enormous effort is made to discover the immune targets and explore the optimal therapeutic interventions (10). In a cancer biological process, the immunosuppressive microenvironment is established to avoid an antitumor immune response. The mechanism that involves selective immune cell infiltration and immunosuppressive molecules is employed to escape from the immunological surveillance (11). The essential issues are proposed for the clinicians to uncover the status of the immune response, identify key immune elements, and integrate into the field of immunopathology (12). Compared with the routine strategies, immunotherapy (e.g., immune checkpoint inhibitors) shed light on patient management in various tumors. Several phase III trials were conducted to estimate the efficacy in advanced GC. However, in the randomized trial JAVELIN 300, Avelumab did not improve overall survival (OS) or post-progression survival (PFS) compared with chemotherapy (13). Similarly, as first-line or second-line therapy for advanced gastric cancer, Pembrolizumab was noninferior to chemotherapy in OS (14, 15). Despite some other trials that are currently ongoing (e.g., ATTRACTION-04, CheckMate-649, and KEYNOTE-859), these outcomes suggested an aberrant immune microenvironment in a GC tissue.
With the rapid development of molecular biology and high-throughput sequencing technology, non-coding RNA (ncRNA) is recognized in the process of tumor initiation and progression (16–18). Among them, circular RNA (circRNA) attracted attention for its regulatory function in various cellular, biological, and pathological processes (19, 20). Different from the linear RNA molecules, a circRNA holds a covalently closed continuous loop. This unique structure endows circRNA with resistance to RNase and being more stable than their linear counterparts. Most of the circRNAs are unable to translate into proteins; By sponging microRNA (miRNA), a circRNA regulates the transcriptional and translational behavior. Various circRNAs are identified as aberrantly expressed in the tumor tissues (18). For example, a circRNA CSNK1G3 interacts with miR-181 and promotes prostate cancer growth (21). A circRNA CCDC66 promotes colon cancer growth and metastasis (22). In terms of GC, only several studies have demonstrated the essential functions of circRNAs. Ding et al. (23) found that circ-DONSON facilitates GC growth and invasion via activation of transcription factor SOX4. CircNHSL1 was reported by Zhu et al. (24) that promotes the GC progression via miR-1306-3p/SIX1/vimentin axis. In addition, a circular RNA circ_HN1 was found as a modulator of the miR-302b/ROCK2 axis and facilitating GC progression (25). As an impact factor in GC, circRNA may pave the way for novel approaches for the management of patients (26–28). However, the association between a circRNA and TME of GC remains to be elucidated (29, 30).
In this study, we investigated the association between circRNAs and GC immune microenvironment. We constructed a ceRNA network and identified the hub circRNAs/miRNA/messenger RNA (mRNA) axis. Its underlying mechanism in the progression of GC and regulatory function in a tumor immune microenvironment were explored and discussed. Our findings demonstrated the regulatory mechanisms of circRNAs and contribute to the development of personalized immunotherapy for patients with GC.
In this study, we obtained a collection of expression data related to GC from The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga), including miRNA-seq, RNA-seq, and the corresponding clinical information (Supplementary Tables S1–S3). Based on the edger R software package (R Foundation for Statistical Computing, Vienna, Austria), the expression data with the corresponding clinical information and deduplication were analyzed to acquire the differentially expressed mRNAs and miRNAs (DEmRNAs and DEmiRNAs, respectively). Next, we used the |Log 2-fold change| ≥ 1.0 and P < 0.05 as cut-off conditions to screen mRNAs and miRNAs for further analysis. The relevant data obtained from TCGA are publicly available and do not require the approval of any local ethics committee. Besides, we searched gastric tumor circRNA expression microarray information from the public database Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) (Supplementary Table S4). GSE141977 (data from the three primary GC tissues and three matched gastric normal tissues) was selected for further analysis. Limma package (version: 3.40.2) of R software was used to study the differential expression of circRNAs (DEcircRNAs). |Log 2-fold change| ≥ 0.9 and P < 0.05 were defined as the thresholds for the screening of differential expression of circRNAs. The probe sets without corresponding circBase ID (http://www.circbase.org) were discarded.
The miRNA binding sites of the circRNAs were predicted using the online website Cancer-Specific CircRNA (CSCD) (http://gb.whu.edu.cn/CSCD/). The candidate miRNAs were selected using the following criteria: upregulated circRNA paired with downregulated miRNA. On the contrary, the downregulated circRNAs were paired with upregulated miRNA. Further analyses were restricted to the intersection of the predicted miRNAs and DEmiRNAs from TCGA. Except for hsa_circ_0081069, 15 miRNAs without repetition were predicted by a surplus of five circRNAs in total. Using the miRPath V3.0, DEmiRNAs were enriched. Meanwhile, the structure diagram of potential circRNA was carried out through the web tool Cancer-Specific CircRNA (CSCD).
We used miRDB, TargetScan, and miRTarbase to predicate the interactions between the selected miRNAs and messenger RNAs (mRNAs). Likewise, matching paired miRNA-mRNA combinations were selected. In total, 151 overlappings predicted mRNAs were selected for further analysis.
To realize the major functions of the above-mentioned 151 mRNAs, we conducted the gene function enrichment analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) and gene ontology (GO) analysis were applied. To identify enriched KEGG and GO terms, Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) was employed.
The network consisted of potential target genes to the miRNAs, potential target miRNAs to the differentially expressed circRNAs (DECs), and corresponding DECs. Cytoscape software (version 3.7.2, ISB, WA, USA) was utilized to visualize the circRNA-miRNA-mRNA network.
To study the prognostic value of the DEmRNAs of the network, we used mRNA expression data and the clinical information downloaded from TCGA for survival analysis. The DEmRNAs with a log-rank P < 0.05 were considered as potential DEmRNAs and draw their survival curves through the R package “Survminer.” Ultimately, seven mRNAs revealed significant differences in the expression profile and survival outcomes synergistically and were selected as hub genes. Of which, STX1A and VCAN with a p < 0.01 were enrolled in further procedures. In addition, the clinical characteristics of the two mRNAs, such as age, gender, grade, stage, and TNM were analyzed. To compare the performance of STX1A and VCAN, the Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/) was processed.
As a computational method, gene set enrichment analysis (GSEA) is considered for the interpretation of gene expression data. We obtained the pathways that were significantly related to the central gene with GSEA analysis, and the cut-off value was set to p < 0.05. The immunohistochemical staining results were obtained from the Human Protein Atlas. An immune infiltration analysis was performed using TIMER (http://cistrome.dfci.harvard.edu/TIMER/) and TISIDB (http://cis.hku.hk/TISIDB/).
All the analyses were performed using R (version3.6.1; https://www.r-project.org/). All the statistical comparisons between the two groups were performed with the Student's t-test, and p < 0.05 was considered significant.
The microarray GSE141977 from the GEO dataset was contained in the study. After normalization of batch effect, six circRNAs were defined as DECs between tumor and normal tissue: hsa_circ_0005280, hsa_circ_0049192, hsa_circ_0050301, hsa_circ_0061695, hsa_circ_0081069, and hsa_circ_0091994 (Supplementary Table S5). Among which, hsa_circ_0005280, hsa_circ_0049192, and hsa_circ_0050301 were downregulated, while hsa_circ_0061695, hsa_circ_0081069, and hsa_circ_0091994 were upregulated in tumor. The circRNAs were displayed in the heatmap and volcano map (Figures 1A,B).
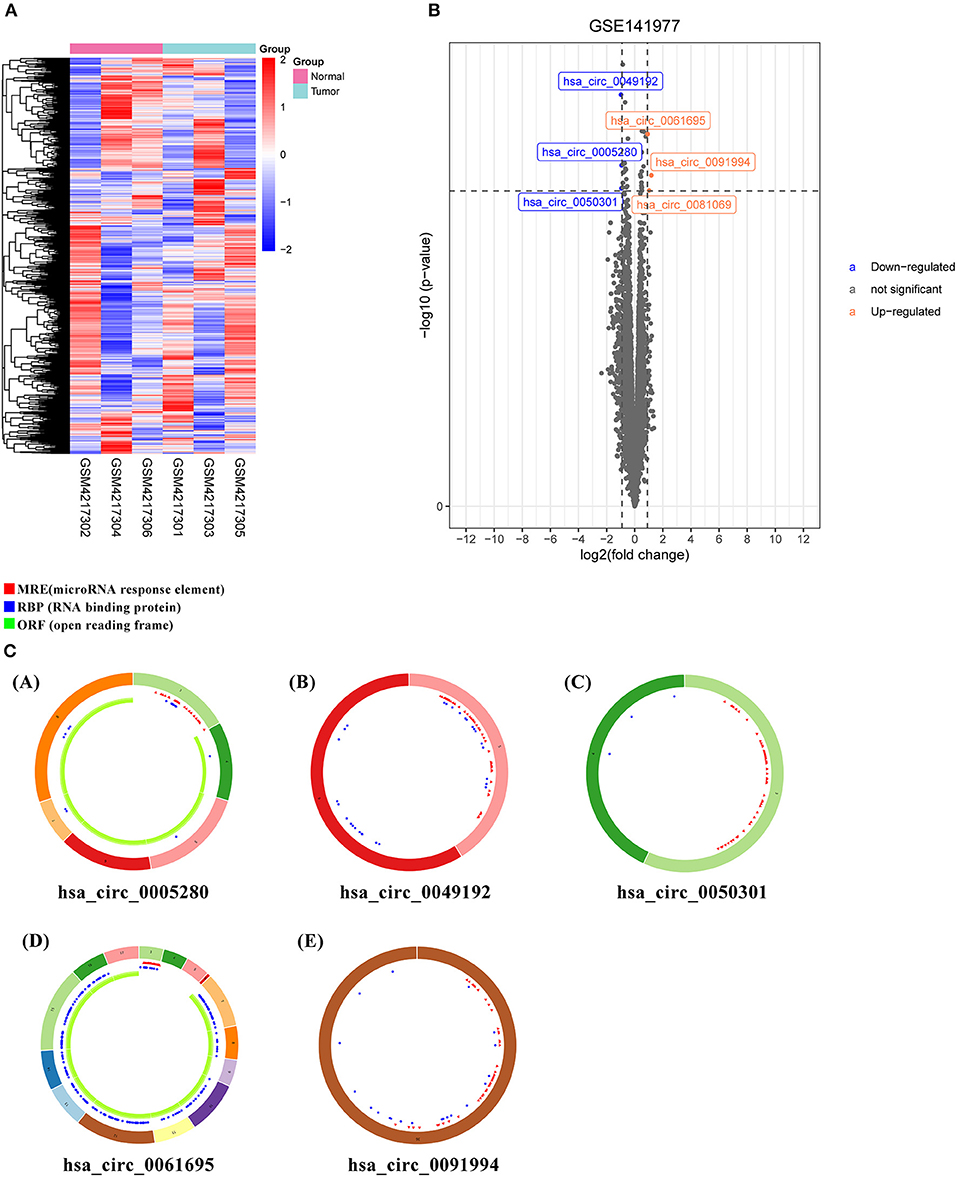
Figure 1. Differently expressed circular RNAs (DECs) and predicted CircRNA structure. (A) Heatmap of top six DECs. (B) Volcano plot of DECs. (C) Predicted CircRNA structure of the top six DECs. Each arc with a numeric ID depicts one exon. The red spots represent microRNA (miRNA) response elements (MRE), the blue spots represent RNA binding protein (RBP), and the green curves represent open reading frame (ORF).
To identify miRNA response element (MRE) and target miRNA, the above-mentioned DECs were predicted by the Cancer-Specific CircRNA Database (CSCD), among which the five DECs can be identified. The pattern map of five DECs, namely MRE, RNA binding protein, and open reading frame information, was obtained from the CSCD (Figure 1C). From the TCGA database, we obtained the quantitative data of miRNA and mRNA expression in 270 GC samples via sequencing (miRNA-seq and mRNA-seq). R software was used to screen the DEmiRNAs and DEmRNAs extracted from TCGA. After the intersection of the above-predicted miRNAs and DEmiRNAs, 15 miRNAs were identified as target miRNAs (Figures 2A,B; Supplementary Table S5). Subsequently, the MiRDB database, the TargetScan database, and the DIANA database were utilized to predict the target mRNA of these 15 miRNAs, respectively. In total, 151 eligible genes (mRNAs) were selected as the final target genes by the intersection with DEmRNAs (Figures 2C,D; Supplementary Table S6).
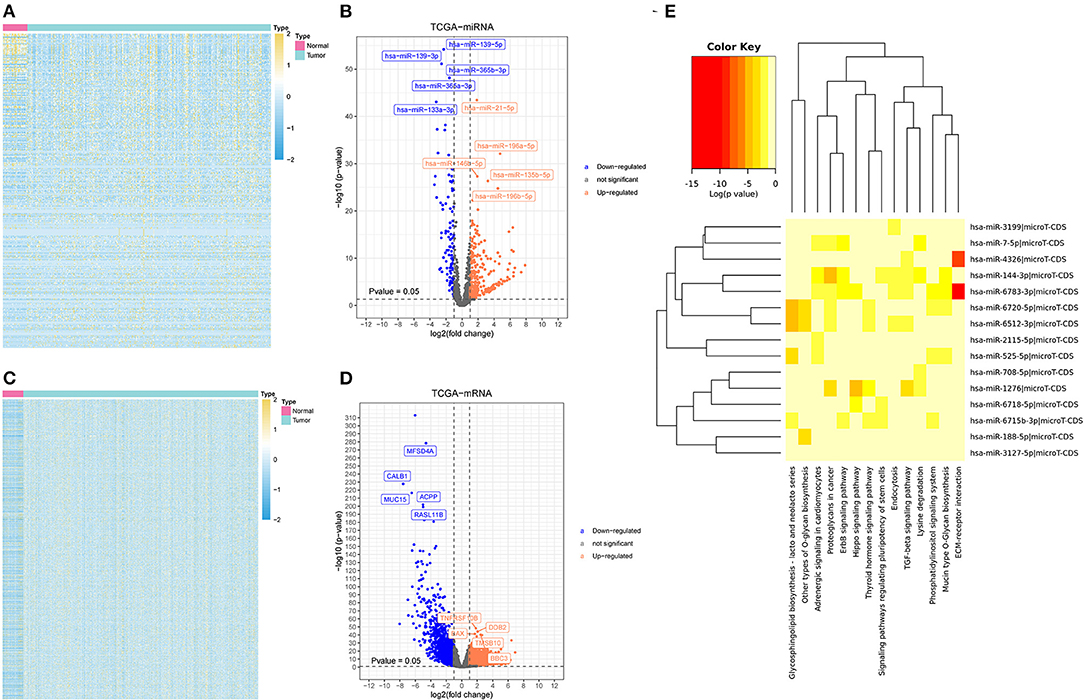
Figure 2. Differently expressed miRNAs and messenger RNAs (mRNAs) (DEmiRNAs and DEmRNAs, respectively) and the possible associated signal pathways (A) a heatmap of DEmiRNAs. (B) A volcano plot of DEmiRNAs. (C) A heatmap of DEmRNAs. (D) A volcano plot of DEmRNAs. (E) The possible signal pathways in which 15 miRNAs may be involved.
A software named as DIANA-MiRPath V3.0 was utilized to investigate the possible signal pathways in which 15 miRNAs may be involved. The results indicated that several miRNAs were closely related to the tumor-associated signal pathways (Figure 2E). To explore the understanding of the biological relevance of these 151 target genes, KEGG and GO analyses were conducted. According to the results of DAVID, the top enriched biological process, cellular components, and molecular function terms were: regulation of transcription from RNA polymerase II promoter, plasma membrane, and transcription factor activity, respectively (Supplementary Figure S1).
After multiple filters, five circRNAs, 15miRNAs, and 151 target mRNAs were screened out for subsequent investigation. The ceRNA (circRNA/miRNA/mRNA) network was established by a comprehensive consideration of the circRNA/miRNA interaction and miRNA/mRNA interaction, and initially displayed the overview of the ceRNA regulation network (Figure 3). The detailed matching information is shown in Supplementary Table S6.
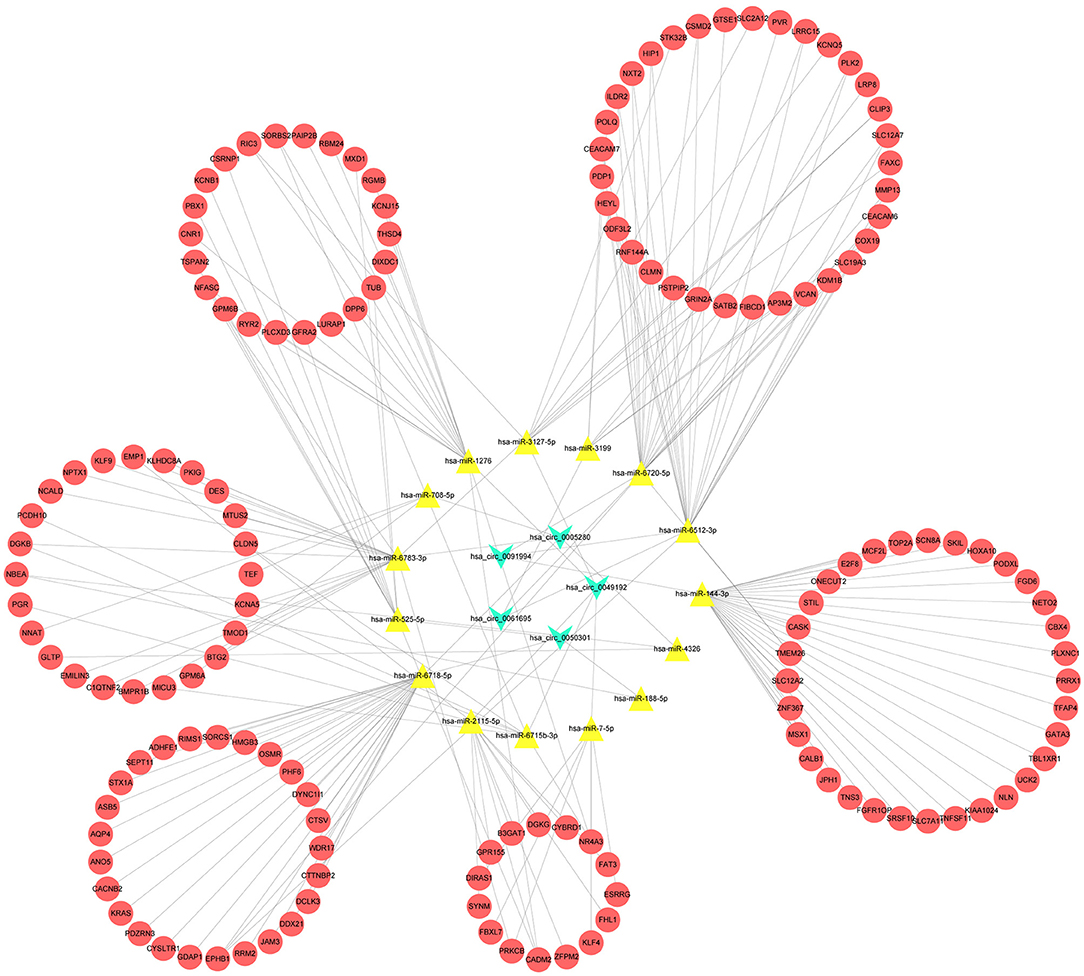
Figure 3. A competing endogenous RNA (CeRNA) regulation network constructed by the circRNAs, 15 miRNAs, and 151 target mRNAs.
A survival analysis of the 151 target genes was conducted, and the target genes significantly associated with the OS were shown in Supplementary Table S7. Seven genes were holding the same tendency between the differentially expressed analysis and survival analysis. Among them, the two target genes (VCAN and STX1A) with a P < 0.01 were selected for further analysis (Figure 4; Supplementary Figure S2). Particularly, VCAN was significantly associated with disease-free survival (P = 0.016) while STX1A was not (P = 0.076). Based on Wilcox's of R software, the association between each subset of age, gender, grade, stage, and TNM with the two candidate genes was analyzed. We found that VCAN was significantly associated with grade (P = 0.0013), stage (P = 0.028), and T stage (P = 8.4 × 10−5; Figure 5), while STX1A was significantly associated with age (P = 0.035) and grade (P = 0.00086; Supplementary Figure S3).
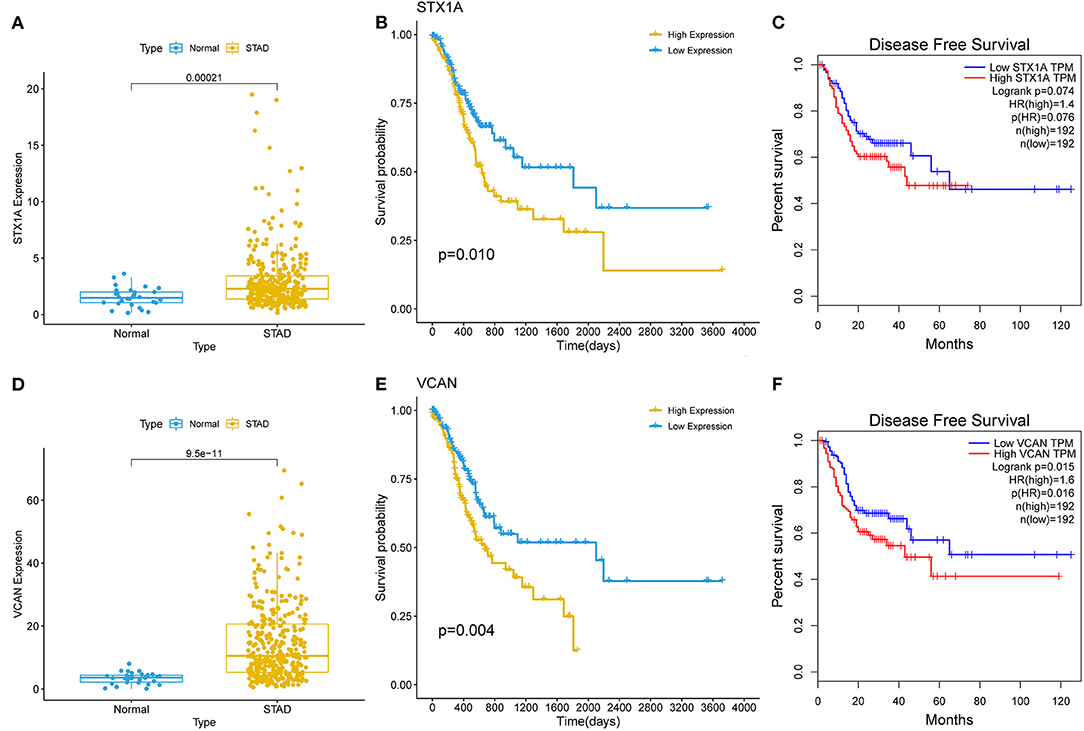
Figure 4. The survival analyses of two target genes (VCAN and STX1A). (A) Kalpan–Meier (K–M) plot of STX1A. (B) Expression of STX1A between gastric cancer (GC) and normal tissues. (C) Disease-free survival of STX1A. (E) K–M plot of VCAN. (D) Expression of VCAN between the GC and normal tissues. (F) Disease-free survival of VCAN. The relationship between a gene and survival was analyzed through the K–M curve which was evaluated by log-rank test.
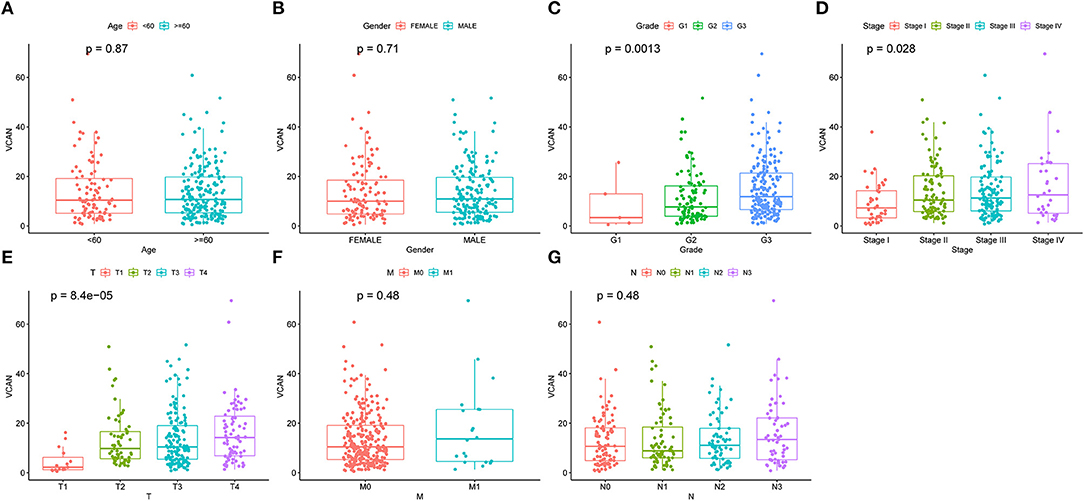
Figure 5. An Upregulated VCAN is associated with the GC progression. The clinical characteristics, including (A) age. (B) Gender. (C) Grade. (D) Stage. (E) T stage. (F) M stage, and (G) N stage of VCAN. Of which, upregulated VCAN indicates higher pathological grade (p = 0.0013), stage (p = 0.028), and T stage (p = 8.4e-5). The relationship between the VCAN and clinical characteristics was analyzed by Wilcox's of R software.
Based on the previous analysis, we identified VCAN as the star hub gene in the regulatory ceRNA network and the hub regulatory axis was defined as hsa_circ_0061695&hsa_circ_0091994/hsa-miR-6512-3p&hsa-miR-6720-5p/VCAN (Figure 6A). These two novel circRNAs were first identified as GC promoters. Furthermore, we employed the Human Protein Atlas for subsequent verification. The immunohistochemical staining results indicated that a higher expression level of VCAN was confirmed in the tumor tissues (Figures 6B–D).
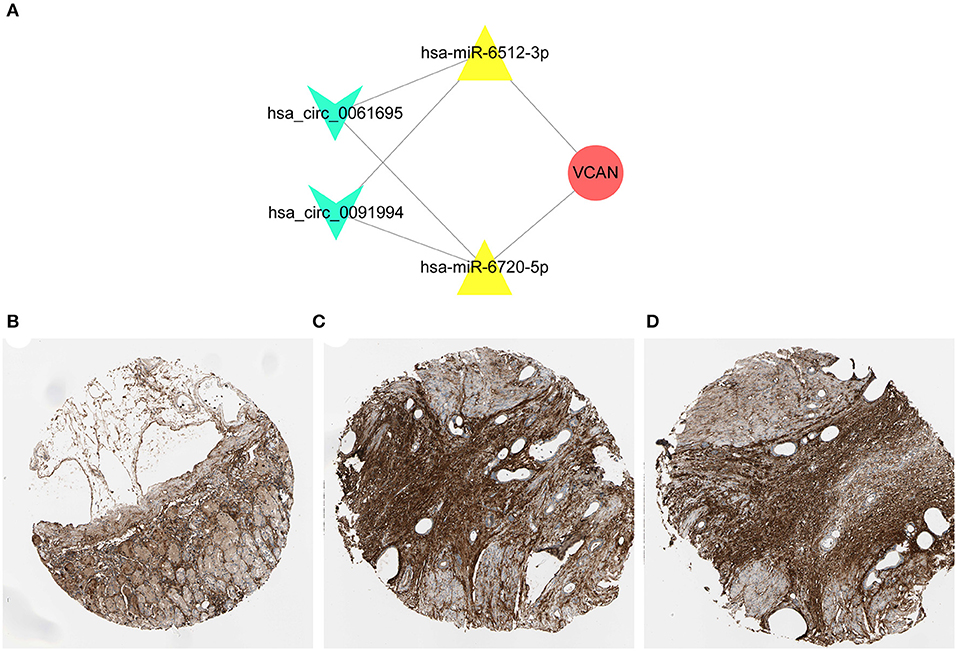
Figure 6. Construction of regulatory axis. (A) The key axis in the circRNA-miRNA-mRNA network of GC was constructed by hsa_circ_0061695, hsa_circ_0091994, hsa-miR-6512-3p, hsa-miR-6720-5p, and VCAN. (B–D) The immunohistochemical staining results indicated that a higher expression level of VCAN was confirmed in the tumor tissues. The samples of normal tissue (B) and two tumor tissues (C,D) were obtained from one patient. Concrete information at https://www.proteinatlas.org/.
To investigate the biological relevance between the hub regulatory axis and GC initiation and progression, a GSEA was conducted. The result indicated that most signal pathways enriched were mainly associated with the regulation of GC tumorigenesis and progression process, such as TGF β signaling, epithelial-mesenchymal transition (EMT), KRAS signaling, hypoxia, TNFα-NFKβ signaling, inflammatory response, IL6-JAC-STAT signaling, angiogenesis, and NOTCH signaling (Figure 7).
The importance of the immune system in the tumor process is now recognized. The correlation between the regulatory axis and immune infiltration was evaluated. The results indicated that the expression levels, including CD1C, THBD, CD14, FCGR3A, CD80, CD86, HLA-G, CCR8, IL2RA, and FOXP3, increased stepwise with VCAN expression level rising. The increased expression level of CCR8, interleukin 2 receptor alpha-chain (IL2RA), and Foxp3 indicate regulatory T cell (Treg) infiltration and contribute to the immunosuppression of T cells against cancer (31). The upregulated CD80 and CD86 enhance the suppressive function of the induced Treg cells (32). Moreover, the tumor-associated macrophagocytes, mast cells, regulatory T cells, dendritic cells were positively associated with the upregulated axis (Figure 8, all P < 0.05). In addition, we analyzed the impacts of VCAN copy number variation on several infiltration levels of the pivotal immune cell subtypes. The arm-level deletion is the most obvious variation type and significantly reduce B cell (p < 0.05), CD8+T cell (p < 0.001), CD4+T cell (p < 0.001), macrophage (p < 0.001), neutrophil (p < 0.001), and dendritic cell (p < 0.001). Deep deletion of VCAN also exhibited the suppressor effect on immune system, with a low infiltration level of CD8+T cell (p < 0.05), macrophage (p < 0.05), neutrophil (p < 0.01), and dendritic cell (p < 0.01; Supplementary Figure S4A). Spearman's correlations between the expression of VCAN and tumor-infiltrating lymphocytes (Y-axis) across human cancers (X-axis) were also demonstrated (Supplementary Figure S4B). These results indicated that the regulatory axis mediated immunosuppressive microenvironment contributed to the GC progression. The molecular mechanisms are worthy of further discussion.
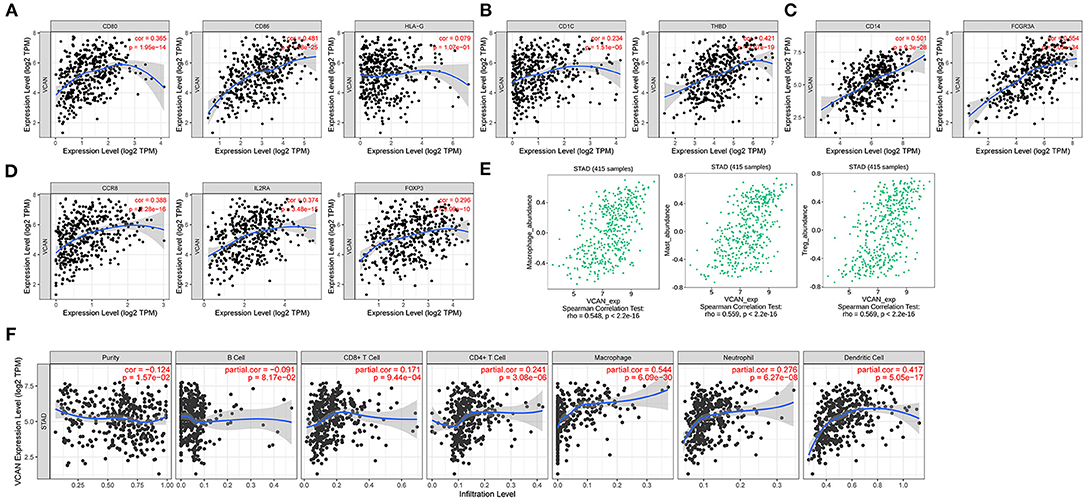
Figure 8. Immune infiltration of VCAN. (A–C,E) Biomarkers of (A) dendritic cell, (B) monocyte, (C) tumor-associated macrophage (TAM), and (E) regulatory T cell (Treg). (D) VCAN expression is associated with the macrophage, mast cell, and Treg infiltration. (F) Major immune cells infiltration in the GC tissues is correlated with the VCAN expression.
GC is a digestive malignancy with high incidence and mortality around the world. With a diagnostic rate of early GC of <10%, most of the patients were diagnosed at an advanced stage and succumb to a worse prognosis and quality of life. About 50–70% of the patients with advanced GC suffered from recurrence and resulted in an unsatisfied 5-year survival rate after routine treatment (33). Because of gene heterogeneity, not everyone was benefitted from surgery or chemotherapy. Immunotherapy may be a promising option. In this scenario, understanding the immune microenvironment and exploring the regulatory factors of GC are urgently expected. Herein, we determined the key circRNA-miRNA-mRNA axis of GC. We found that hsa_circ_0061695, hsa_circ_0091994, hsa-miR-6512-3p, hsa-miR-6720-5p, and VCAN are the key nodes in the network. Among these, hsa_circ_0061695 and hsa_circ_0091994 were the first found associated with tumor progression. To better understand the mechanism, we performed the GO and KEGG analysis and GESA. We eventually extracted the infiltrated immune cell subsets that contribute to an immunosuppressive microenvironment regulated by the axis.
In recent years, emerging evidence has found abnormalities in the molecular mechanisms of GC, such as the abnormal regulators in the gene expression. The circRNAs were traditionally recognized as meaningless byproducts (34). Recent studies have proved circRNAs as an abundant and diverse type of RNA associated with various cellular processes and involved in the initiation and development of substantial diseases, such as cancer (35). The circRNAs that contain one or more MREs that could act as miRNA sponges and negatively modulate the miRNA activity. Therefore, the inhibitory effect on target genes by miRNAs is attenuated by the circRNAs. Though several circRNAs are found to be participating in the immune responses (36), such as binding and suppressing the kinase PKR (37); the immune regulatory function of circRNAs is awaiting deeper exploration (38).
In this study, we first described the regulatory function of hsa_circDYRK1A_017 (hsa_circ_0061695). Hsa_circFLNA_118 (hsa_circ_0091994) was recently reported as a cancer promotor in accordance with our results (39). We constructed the novel regulatory axis significantly correlated to the clinical outcomes. The results found that hsa_circDYRK1A_017 locates in +strand of chr21 (40), while hsa_circFLNA_118 locates in a strand of chrX (41). These circRNAs contribute to the malignant biological process in GC and shed light on the potent applications for early detection as well as novel therapeutic targets (28). Correspondingly, miR-6512-3p and miR-6270-5p were downregulated in the GC tissues and pointed out to be associated with skin reveals (42) and fibrosis (43). Herein, the circRNAs could modulate the GC progression through sponging miR-6512-3p and miR-6270-5p. Several studies focused on the impacts of VCAN on the GC initiation and progression (44, 45). Ye et al. reported that the upregulation of VCAN promotes GC proliferation, invasion, and migration (46). In another study, lncRNA VCAN-AS1 contributed to the progression of GC through regulation of the p53 expression (47). VCAN could serve as the novel therapeutic target or biomarker for GC (48–51). These results revealed the remarkable function of the ceRNA axis in tumorigenesis and the progression of GC.
In recent years, though great efforts were made to decipher the molecular basis of the heterogeneity, and significant progress has been made to understand the intrinsic pathogenesis of GC, the treatment made slow progression. Fluoropyrimidine and platinum-based chemotherapy as the first-line therapy and paclitaxel agents as second-line therapy are the standard treatment for advanced GC; however, the prognosis remained dismal (52–54). Involved in many cell biological behaviors, including cell adhesion, proliferation, migration, extracellular matrix assembly, and survival, a tumor immunosuppressive microenvironment plays an immense role in cell biology (55). The immunosuppressive molecules (e.g., PD-L1 and CTLA-4) and cells targeted therapies have revealed the prominent anticancer properties and suggested the possible approach to immunotherapy in regulating the circRNAs/miRNA/mRNA axis. A ceRNA was identified to be associated with the growth of tumors. However, the underlying mechanism remained obscure. In this study, we found that the hub ceRNA axis significantly promotes tumor progression via TME and immune cell reprogramming. The elevated CD1c+ dendritic cells were reported as the prognostic indicator for GC (56). Defected DCs CCR8, a chemokine receptor mainly expressed on the Treg cells plays a crucial role in the CCR8+ Treg-mediated immunosuppression and is uniquely upregulated in human tumor-resident Treg (31, 57). Another study indicated that Foxp3 in the tumor-infiltrating Treg cells serves as the immunosuppressive molecule to T-cell proliferation (58). HLA-G inhibits the function of immunocompetent cells, including natural killer (NK) and T cells, and represents a strategy employed by the tumors for the resistance to immune rejection (59). An immune cell infiltration analysis further confirms the immunosuppressive microenvironment. Apart from TAM and Treg, the upregulated mast cell is recognized. The recruited and co-localized Treg and mast cells in the tumor regions represented resistance to anti-PD-1 treatment (60). Overall, our data reinforced the association between the immune cell subtypes infiltration and VCAN expression. These results suggest that the circRNA/miRNA/mRNA axis may regulate the GC progression via tumor immune microenvironment and serve as a potential target for immunotherapy against GC.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
YD, WG, and SL designed the research. XL, ZL, and PL collected the data. SA, FS, and QH performed the data analysis. XL wrote the manuscript. XX, WG, and SL reviewed the manuscript. All the authors reviewed and approved the manuscript.
This work was supported by the National Natural Science Foundation of China (81602103 and 82172645), the Natural Science Foundation of Jiangsu Province (BK20200052), and the Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2021-LCYJ-MS-09 and 2021-LCYJ-PY-17).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.771206/full#supplementary-material
1. Arnold M, Ferlay J, Henegouwen MIV, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. (2020) 69:1564–71. doi: 10.1136/gutjnl-2020-321600
2. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Chen WQ, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F, et al. Cancer statistics in china, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Hamashima C. Update version of the Japanese guidelines for gastric cancer screening. Jap J Clin Oncol. (2018) 48:673–83. doi: 10.1093/jjco/hyy077
5. Fujitani K, Yang H, Mizusawa J, Kim Y, Terashima M, Han S, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. (2016) 17:309–18. doi: 10.1016/S1470-2045(15)00553-7
6. Kamiya S, Takeuchi H, Nakahara T, Niihara M, Nakamura R, Takahashi T, et al. Auxiliary diagnosis of lymph node metastasis in early gastric cancer using quantitative evaluation of sentinel node radioactivity. Gastric Cancer. (2016) 19:1080–7. doi: 10.1007/s10120-015-0572-2
7. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. (2011) 480:480–9. doi: 10.1038/nature10673
9. Finck A, Gill SI, June CH. Cancer immunotherapy comes of age and looks for maturity. Nat Commun. (2020) 11:4. doi: 10.1038/s41467-020-17140-5
10. Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. (2020) 20:662–80. doi: 10.1038/s41568-020-0285-7
11. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
12. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
13. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. (2018) 29:2052–60. doi: 10.1093/annonc/mdy264
14. Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. (2018) 392:123–33. doi: 10.1016/S0140-6736(18)31257-1
15. Shitara K, Van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370
16. Xie S, Chang Y, Jin H, Yang F, Xu Y, Yan X, et al. Non-coding RNAs in gastric cancer. Cancer Lett. (2020) 493:55–70. doi: 10.1016/j.canlet.2020.06.022
17. Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. (2020) 19:62. doi: 10.1186/s12943-020-01185-7
18. Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. (2019) 179:1033–55. doi: 10.1016/j.cell.2019.10.017
19. Li XW, Yang WH, Xu J. Circular RNA in gastric cancer. Chin Med J. (2020) 133:1868–77. doi: 10.1097/CM9.0000000000000908
20. Vo JN, Cieslik M, Zhang YJ, Shukla S, Xiao LB, Zhang YP, et al. The landscape of circular RNA in cancer. Cell. (2019) 176:869. doi: 10.1016/j.cell.2018.12.021
21. Chen SJ, Huang V, Xu X, Livingstone J, Soares F, Jeon J, et al. Widespread and functional RNA circularization in localized prostate cancer. Cell. (2019) 176:831. doi: 10.1016/j.cell.2019.01.025
22. Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, et al. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. (2017) 77:2339–50. doi: 10.1158/0008-5472.CAN-16-1883
23. Ding LX, Zhao YY, Dang SW, Wang Y, Li XL, Yu XT, et al. Circular RNA circ-DONSON facilitates gastric cancer growth and invasion via NURF complex dependent activation o transcription factor SOX4. Mol Cancer. (2019) 18:11. doi: 10.1186/s12943-019-1006-2
24. Zhu ZL, Rong ZY, Luo Z, Yu ZL, Zhang J, Qiu ZJ, et al. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306-3p/SIX1/vimentin axis. Mol Cancer. (2019) 18:20. doi: 10.1186/s12943-019-1054-7
25. Wang D, Jiang X, Liu Y, Cao G, Zhang X, Kuang Y. Circular RNA circ_HN1 facilitates gastric cancer progression through modulation of the miR-302b-3p/ROCK2 axis. Mol Cell Biochem. (2020) 476:199–212. doi: 10.1007/s11010-020-03897-2
26. Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. (2020) 19:112. doi: 10.1186/s12943-020-01208-3
27. Luo Z, Rong Z, Zhang J, Zhu Z, Yu Z, Li T, et al. Circular RNA circCCDC9 acts as a miR-6792-3p sponge to suppress the progression of gastric cancer through regulating CAV1 expression. Mol Cancer. (2020) 19:86. doi: 10.1186/s12943-020-01203-8
28. Yamamoto H, Watanabe Y, Sato Y, Maehata T, Itoh F. Non-invasive early molecular detection of gastric cancers. Cancers. (2020) 12:2880. doi: 10.3390/cancers12102880
29. Wang Z, Jiang Z, Zhou J, Liu Z. circRNA RNF111 regulates the growth, migration and invasion of gastric cancer cells by binding to miR-27b-3p. Int J Mol Med. (2020) 46:1873–85. doi: 10.3892/ijmm.2020.4709
30. Wei J, Wei W, Xu HF, Wang ZJ, Gao W, Wang TJ, et al. Circular RNA hsa_circRNA_102958 may serve as a diagnostic marker for gastric cancer. Cancer Biomark. (2020) 27:139–45. doi: 10.3233/CBM-182029
31. De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory Cells. Immunity. (2016) 45:1135–47. doi: 10.1016/j.immuni.2016.10.021
32. Gu P, Gao JF, D'Souza CA, Kowalczyk A, Chou K-Y, Zhang L. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell Mol Immunol. (2012) 9:136–46. doi: 10.1038/cmi.2011.62
33. Sukri A, Hanafiah A, Zin NM, Kosai NR. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS. (2020) 128:150–61. doi: 10.1111/apm.13034
34. Liu J, Liu T, Wang XM, He AL. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. (2017) 16:12. doi: 10.1186/s12943-017-0630-y
35. Arnaiz E, Sole C, Manterola L, Iparraguirre L, Otaegui D, Lawrie CH. CircRNAs and cancer: Biomarkers and master regulators. Semin Cancer Biol. (2019) 58:90–99. doi: 10.1016/j.semcancer.2018.12.002
36. Wilusz JE. Circle the wagons: circular RNAs control innate immunity. Cell. (2019) 177:797–9. doi: 10.1016/j.cell.2019.04.020
37. Liu CX, Li X, Nan F, Jiang S, Gao X, Guo SK, et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. (2019) 177:865. doi: 10.1016/j.cell.2019.03.046
38. Chen X, Yang T, Wang W, Xi WJ, Zhang TZ, Li Q, et al. Circular RNAs in immune responses and immune diseases. Theranostics. (2019) 9:588–607. doi: 10.7150/thno.29678
39. Xie Y, Liu Z, Zhu H. Knockdown of hsa_circ_0091994 constrains gastric cancer progression by suppressing the miR-324-5p/HMGA1 axis. Aging. (2021) 13:20598–608. doi: 10.18632/aging.203450
40. Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. (2015) 58:870–85. doi: 10.1016/j.molcel.2015.03.027
41. Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-Type Specific features of circular RNA expression. PLoS Genet. (2013) 9:15. doi: 10.1371/annotation/f782282b-eefa-4c8d-985c-b1484e845855
42. Joyce CE, Zhou X, Xia J, Ryan C, Thrash B, Menter A, et al. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet. (2011) 20:4025–40. doi: 10.1093/hmg/ddr331
43. Zhang K, Chen J, Zhang D, Wang L, Zhao W, Lin D, et al. microRNA expression profiles of scar and normal tissue from patients with posterior urethral stricture caused by pelvic fracture urethral distraction defects. Int J Mol Med. (2018) 41:2733–43. doi: 10.3892/ijmm.2018.3487
44. Ang L, Zhang Y, Cao L, Yang B, Young B, Kiani C, et al. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol. (1999) 58:597–605. doi: 10.1097/00005072-199906000-00004
45. Mitsui Y, Kato T, Maekawa S, Hashimoto Y, Shiina M, Sumida MI, et al. VCAN promotes clear cell renal cell carcinoma tumor progression and metastasis, and predicts poor prognosis. Cancer Res. (2017) 17:884–95. doi: 10.1158/1538-7445.AM2017-5749
46. Cheng Y, Sun H, Wu L, Wu F, Tang W, Wang X, et al. VUp-Regulation of VCAN promotes the proliferation, invasion and migration and serves as a biomarker in gastric cancer. Onco Targets Ther. (2020) 13:8665–75. doi: 10.2147/OTT.S262613
47. Feng L, Li J, Li F, Li HQ, Bei SH, Zhang XH, et al. Long noncoding RNA VCAN-AS1 contributes to the progression of gastric cancer via regulating p53 expression. J Cell Physiol. (2020) 235:4388–98. doi: 10.1002/jcp.29315
48. Chong X, Peng R, Sun Y, Zhang L, Zhang Z. Identification of key genes in gastric cancer by bioinformatics analysis. BioMed Res Int. (2020) 2020:7658230. doi: 10.1155/2020/7658230
49. Li Y, Wang JS, Zhang T, Wang HC, Li LP. Identification of new therapeutic targets for gastric cancer with bioinformatics. Front Genet. (2020) 11:17. doi: 10.3389/fgene.2020.00865
50. Zhang XL, Zheng PM, Li Z, Gao SJ, Liu GZ. The somatic mutation landscape and RNA prognostic markers in stomach adenocarcinoma. Onco Targets Ther. (2020) 13:7735–46. doi: 10.2147/OTT.S263733
51. Zhu ZP, Xu JH, Li LL, Ye WP, Chen BR, Zeng JJ, et al. Comprehensive analysis reveals CTHRC1, SERPINE1, VCAN and UPK1B as the novel prognostic markers in gastric cancer. Transl Cancer Res. (2020) 9:4093–110. doi: 10.21037/tcr-20-211
52. Bang Y, Van Cutsem E, Feyereislova A, Chung H, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
53. Boelke E, Peiper M, Budach W. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. (2008) 358:1965–1965. doi: 10.1056/NEJMc080178
54. Wilke H, Muro K, Van Cutsem E, Oh S, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. (2014) 15:1224–35. doi: 10.1016/S1470-2045(14)70420-6
55. Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. (2002) 14:617–23. doi: 10.1016/S0955-0674(02)00375-7
56. Liu WH, Zhao J, Li QQ, Wang QN, Zhou Y, Tong Z. Gastric cancer patients have elevated plasmacytoid and CD1c(+) dendritic cells in the peripheral blood. Oncol Lett. (2018) 15:5087–92. doi: 10.3892/ol.2018.7990
57. Villarreal DO, L'Huillier A, Armington S, Mottershead C, Filippova EV, Coder BD, et al. Targeting CCR8 induces protective antitumor immunity and enhances vaccine-induced responses in colon cancer. Cancer Res. (2018) 78:5340–8. doi: 10.1158/0008-5472.CAN-18-1119
58. Yuan X-L, Chen L, Li M-X, Dong P, Xue J, Wang J, et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. (2010) 134:277–88. doi: 10.1016/j.clim.2009.10.005
59. Rouas-Freiss N, Moreau P, Menier C, Carosella ED. HLA-G in cancer: a way to turn off the immune system. Semin Cancer Biol. (2003) 13:325–36. doi: 10.1016/S1044-579X(03)00023-3
Keywords: gastric cancer, Immunotherapy, novel circular RNA, ceRNA axis, immunosuppressive microenvironment
Citation: Li X, Li Z, Liu P, Ai S, Sun F, Hu Q, Dong Y, Xia X, Guan W and Liu S (2021) Novel CircRNAs in Hub ceRNA Axis Regulate Gastric Cancer Prognosis and Microenvironment. Front. Med. 8:771206. doi: 10.3389/fmed.2021.771206
Received: 06 September 2021; Accepted: 08 October 2021;
Published: 08 November 2021.
Edited by:
Meng Xue, Zhejiang University, ChinaReviewed by:
Minhong Shen, Princeton University, United StatesCopyright © 2021 Li, Li, Liu, Ai, Sun, Hu, Dong, Xia, Guan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxiang Dong, eXV4aWFuZ2RvbmdAbmptdS5lZHUuY24=; Wenxian Guan, Z3Vhbl93ZW54aWFuQHNpbmEuY29t; Song Liu, bGl1c29uZ0BuamdseXkuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.