- 1Department of Ophthalmology, Peking University Third Hospital, Beijing, China
- 2Beijing Ophthalmology and Visual Sciences Key Laboratory, Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 3Department of Physical Examination, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 4Department of Ophthalmology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 5Medical Faculty Mannheim, Department of Ophthalmology, Heidelberg University, Mannheim, Germany
- 6Institute of Clinical and Scientific Ophthalmology and Acupuncture Jonas & Panda, Heidelberg, Germany
- 7Institute of Molecular and Clinical Ophthalmology, Basel, Switzerland
Aims: To examine the prevalence of primary epiretinal membranes (ERMs) and associated systemic factors.
Methods: The cross-sectional, community-based Tongren Health Care Study enrolled participants who received regular health examinations in the Beijing Tongren Hospital from 2017 to 2019. Using fundus photographs, retinal specialists assessed the presence of ERMs and their systemic associations.
Results: Primary ERMs were detected in 841/22820 individuals, with a prevalence of 3.7% [95% confidence intervals (CI): 3.4–3.9%] in the total study population (mean age: 44.5 ± 13.8 years) and 6.5% (95% CI: 6.1–7.0%) in individuals aged 40+ years. In multivariable analysis, a higher ERMs prevalence was associated with older age [odds ratio (OR): 1.10; P < 0.001], higher serum cholesterol concentration (OR: 1.14; P = 0.003) and higher serum sodium concentration (SSC) (OR: 1.12; P < 0.001). In women, a higher SSC, even within the normal range, was associated with an increased risk of ERMs (OR: 1.19; P < 0.001). Female participants with an SSC of 144–145mmol/L as compared with those with an SSC of 135–137 mmol/L had a 5-fold increased odds of having ERMs (All women: OR: 5.33; P < 0.001; Women aged 40+years: OR: 4.63; P < 0.001).
Conclusion: Besides older age and higher serum cholesterol concentration, a higher SSC, even if within the normal range, was independently associated with a higher ERM prevalence in women.
Introduction
Epiretinal membranes (ERMs) are a common cause of visual impairment in the elderly population (1–5), with the pooled prevalence from population-based studies to be 9.7% (6). While intraocular causes including retinal diseases, previous intraocular surgeries, hyperopia, or myopia were reported to be associated with secondary ERMs, to mention a few (1, 3, 5, 7, 8), idiopathic ERMs present in eyes without evident abnormality, and their causes remain to be elusive (9). Systemic factors associated with the presence of ERMs were older age, female sex, ethnic background, hyperlipidemia, smoking, diabetes mellitus, and a lower serum concentration of urine acid (1, 3, 4, 7, 10, 11), however, none of the above-mentioned factors were consistently reported besides the increasing age.
Most ERMs remain relatively stable and treatment is not required, while vitrectomy surgery is indicated for ERMs patients associated with symptoms that severely affect their activities of daily living, including decreased visual acuity, metamorphopsia, double vision, or difficulty using their eyes together (2, 12). Of particular note is that even a very successful vitrectomy does not always ensure a desirable visual outcome (2). Our knowledge on the pathophysiology of ERM has been improved greatly by histopathological studies and advanced image technologies, however, there're no preventive measures since the exact pathological mechanisms of ERMs remain unknown.
The current community-based large-scale study was conducted in 20,000+ participants with comprehensive general medical examinations, with the aim to explore systemic factors associated with the primary ERMs.
Methods
The cross-sectional, community-based Tongren Health Care Study included individuals who attended regular health care check-up examinations in the Beijing Tongren Hospital from July 2017 to December 2019. The study population has been described in detail recently (13). The eligibility criterion for inclusion into the current study was an age of 18+ years. The study was conducted in adherence to the Declaration of Helsinki and approved by the Medical Ethics Committee of Beijing Tongren Hospital.
The examinations included an interview in which general demographic data and information about the medical history and other health-related events were obtained. The physical examinations included the assessment of anthropometric parameters such as body height and weight and circumferences of the waist and hip, measurement of blood pressure and electrocardiography, and biochemical examinations of samples of blood. Arterial hypertension was defined as a systolic blood pressure of 140 mmHg or more, a diastolic blood pressure of 90 mmHg or more, or a physician's-based diagnosis of hypertension or use of antihypertensive medication. Diabetes mellitus was defined based on the history of a previously diagnosed diabetes or receiving a glucose-lowering therapy and/or fasting blood glucose concentration of ≥7.0 mmol/L. The estimated glomerular filtration rate (eGFR) was calculated based on the serum creatinine concentration and using the Chronic Kidney Disease Epidemiology Collaboration equation (14).
The ophthalmological examinations, performed by experienced ophthalmologists, consisted of the measurement of best-corrected visual acuity, non-contact tonometry, slit lamp-based biomorphometry of the anterior and posterior segment of the eye, and 45° non-mydriatic fundus photography (Topcon TRG-NW7SF, Topcon, Tokyo, Japan; or Cannon, CR6-45NM, Canon, Tokyo, Japan). Based on the assessment of the fundus photographs, an ERM was classified into either a less severe subtype termed as cellophane macular reflex (CMR) or a more severe form termed as preretinal macular fibrosis (PMF) (3, 15). Eyes with the coexistence of CMR and PMF were classified as having PMF. A secondary ERM was defined as being associated with intraocular diseases including diabetic retinopathy, late-stage age-related macular degeneration, other retinopathy, and previous intraocular surgery including cataract surgery or vitreous retinal surgery (3). All ERMs without any associated intraocular disorder were classified as primary ERMs.
The data were analyzed using the statistical software SPSS 26.0 (SPSS Inc., Chicago, IL, USA) and the Statistical Package for the R (version 1.4.1103). Continuous variables were presented as mean ± standard deviation and categorical variables as the number of cases/percentages. In the univariable analysis, a binary logistic regression analysis was performed to assess associations between the ERM prevalence and other systemic parameters, without and with adjustment for age, the odds ratios (ORs) and their 95% confidence intervals (CI) were calculated. The multivariable analysis was then performed with the ERM prevalence as the dependent variable, and all those parameters with P-values ≤ 0.10 in the age-adjusted analysis as the independent variables, in all participants, in men and in women, respectively. The collinearity was considered when a variance inflation factor (VIF) of more than 5 was observed. After detecting the association between serum sodium concentration (SSC) and ERM, we re-assessed our findings by excluding individuals with hypernatremia (SSC > 145 mmol/L) and hyponatremia (SSC <135 mmol/L) to eliminate their confounding effects. We compared the SSCs among different age groups in the whole participants, in men and women respectively, using one-way analysis of variance (ANOVA) and adjusted by Bonferroni when a significant difference was detected. We stratified the SSC into four groups (135.0–137.0, 138.0–140.0, 141.0–143.0, and 144.0–145.0 mmol/L) and assessed its association with the ERM prevalence. A three-dimensional surface plot was created to visualize the associations among age, SSC, and the presence of ERM for women aged 40+ years, as most epidemiologic studies included individuals aged 40+ years old. A two-tailed P-value of < 0.05 was considered statistically significant.
Results
Out of 22,945 individuals (12,574 men, 54.8%) who were examined during the study period, 125 (0.54%) participants were excluded due to unreadable fundus photographs. The study eventually included 22,820 participants (12,514 women, 54.8%) with a mean age of 44.5 ± 13.8 years (range: 18–97 years). The mean uncorrected visual acuity, best-corrected visual acuity, and intraocular pressure were 0.40 ± 0.40 logarithm of the minimal angle of resolution (logMAR), 0.04 ± 0.10 logMAR, and 14.3 ± 3.0 mmHg, respectively.
Prevalence of Epiretinal Membranes
ERMs were found in 988 participants (4.3 ± 0.1%; 95% CI: 4.1–4.6%), among whom 147 subjects (0.6 ± 0.1%; 95% CI: 0.5–0.7%) with diabetic retinopathy (19/12.9%), retinal vascular diseases (7/4.8%), late-stage age-related macular degeneration (6/4.1%), other types of retinopathies (20/13.6%), history or signs of vitreous or retinal surgery (7/4.7%), and previous cataract surgery (88/59.9%) were classified as secondary ERMs and were excluded.
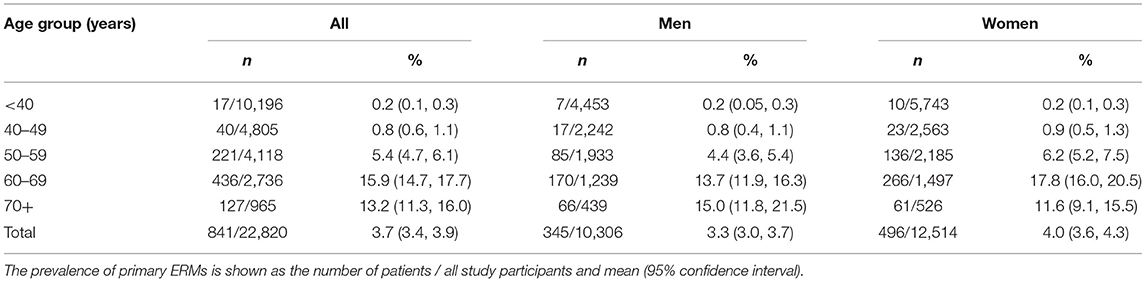
Primary ERMs (described as “ERMs” in the following paragraphs) were detected in 841 [345 (41.0%) men] out of the 22,820 individuals, with a prevalence of 3.7 ± 0.1% (95% CI: 3.4–3.9%). Unilateral ERMs and bilateral ERMs were found in 618 participants (73.5%) and 223 subjects (26.5%), respectively. For individuals aged 40+ years, the prevalence of ERMs was 6.5 ± 0.2% (95% CI: 6.1–7.0%), 5.8 ± 0.3% (95% CI: 5.2–6.4%), and 7.2 ± 0.3% (95% CI: 6.6–7.9%) for all individuals, for men, and for women, respectively (Table 1).
Serum Sodium Concentration as a Risk Factor for Epiretinal Membranes
In the univariable analysis, a higher ERM prevalence was associated with older age, female sex, higher prevalence of hypertension and diabetes, higher body mass index, waist-hip circumference ratio, systolic and diastolic blood pressure, SSC, higher serum concentrations of potassium, blood urea nitrogen, glucose, high sensitive C-reactive protein, lipoprotein a, triglycerides, total cholesterol, and low-density lipoprotein, and lower serum concentrations of calcium, phosphate and lower eGFR (all P ≤ 0.05) (Table 2). In the multivariable analysis, a higher ERM prevalence remained to be significantly associated with older age (OR: 1.10; P < 0.001), higher SSC (OR: 1.12; P < 0.001), higher total cholesterol (OR: 1.14; P = 0.003), and borderline with eGFR (OR: 1.01; P = 0.05) for all participants. A higher ERM prevalence was consistently associated with increasing age, in men and women, respectively, whereas its association with higher SSC and higher total cholesterol only remained statistically significant in women but not men (Table 3).
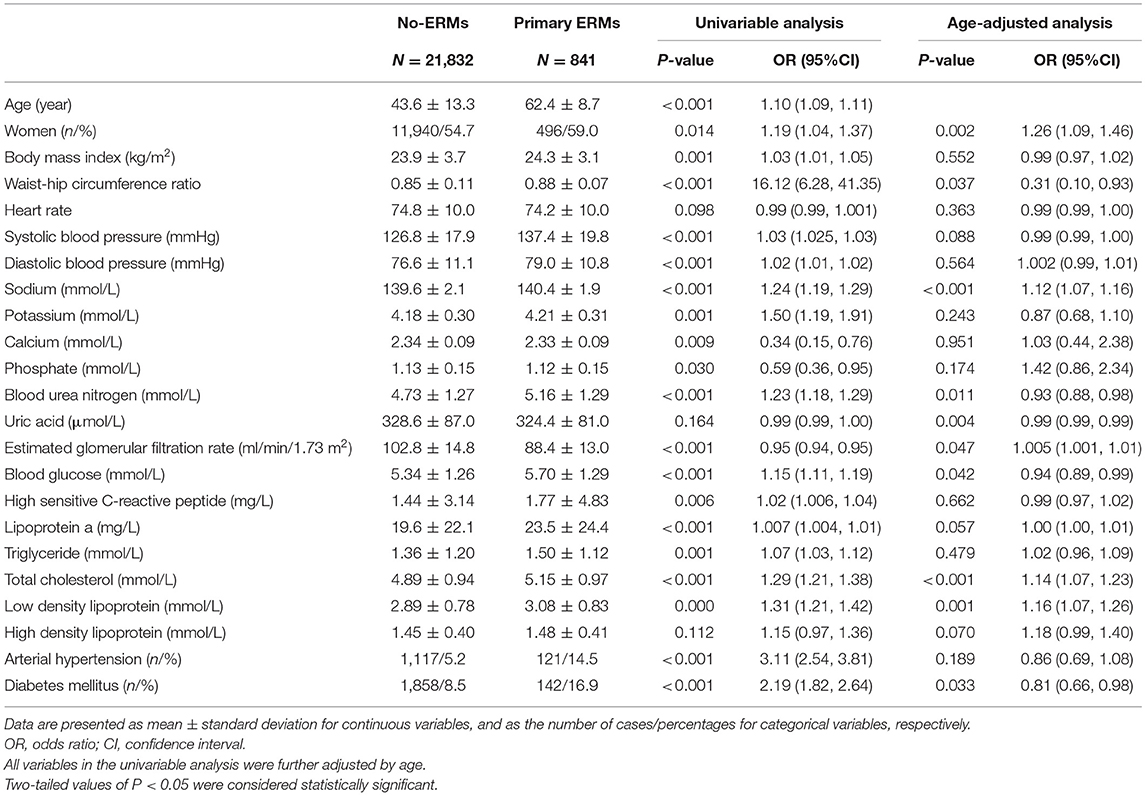
Table 2. Factors associated with primary epiretinal membranes (ERMs) by univariable logistic analysis and age-adjusted logistic analysis.
The mean SSC was 139.6 ± 2.1 mmol/L (range: 120–150 mmol/L) measured in 21,054 participants, with 30 (0.1%) individuals with hypernatremia and 250 (1.2%) participants with hyponatremia. In individuals with normal SSC (135–145 mmol/L), a positive correlation between higher SSC and older age was found in women (r = 0.33, P < 0.001, Pearson correlation analysis) but not in men (P = 0.35). In women, SSC increased continuously from the age of 50 years to the age of 70 years (one-way ANOVA, P < 0.05), after which it dropped slightly. The SSC did not differ significantly among the age groups in men (one-way ANOVA, P = 0.18) (Table 4; Figure 1).
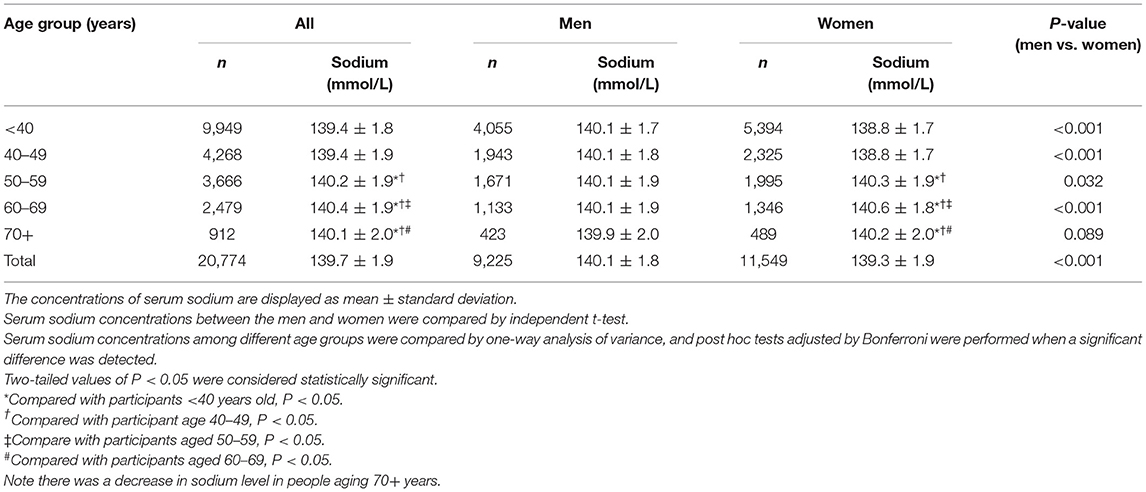
Table 4. Serum sodium concentration in the study population, stratified by age and sex (with serum sodium concentration within the normal range).
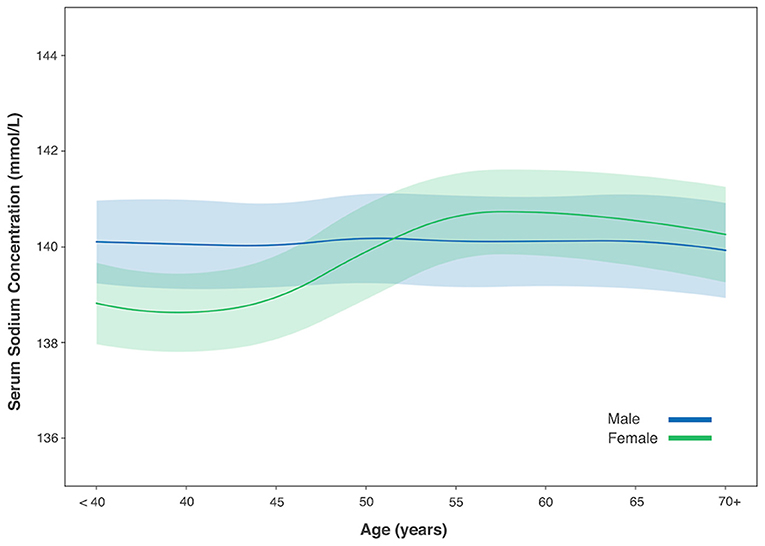
Figure 1. Serum sodium concentration with age in male and female gender. Figure was shown with mean value (solid line) and 1/2 standard deviation (shadow).
The association between SSC and ERM was modified by sex in the univariable analysis (P for interaction <0.001). In multivariable analysis, the association between SSC and ERMs remained statistically significant in the whole study population and in women (both P < 0.001), but not in men (both P > 0.05) (Tables 3, 5). In women, the ERM prevalence significantly increased from 0.9% in the subgroup with SSC of 135–137 mmol/L, to 3.0% (SSC: 138–140 mmol/L), 7.1% (SSC: 141–143 mmol/L) and to 11.0% (SSC: 144–145 mmol/L) (linear-by-linear association chi-square test, X2 = 160.04, P < 0.001). Female participants with an SSC of 144–145 mmol/L as compared with women with an SSC of 135–137 mmol/L had a 5.3-fold increased risk of ERMs (OR: 5.33; P < 0.001) in the multivariable analysis (Figure 2; Table 6).
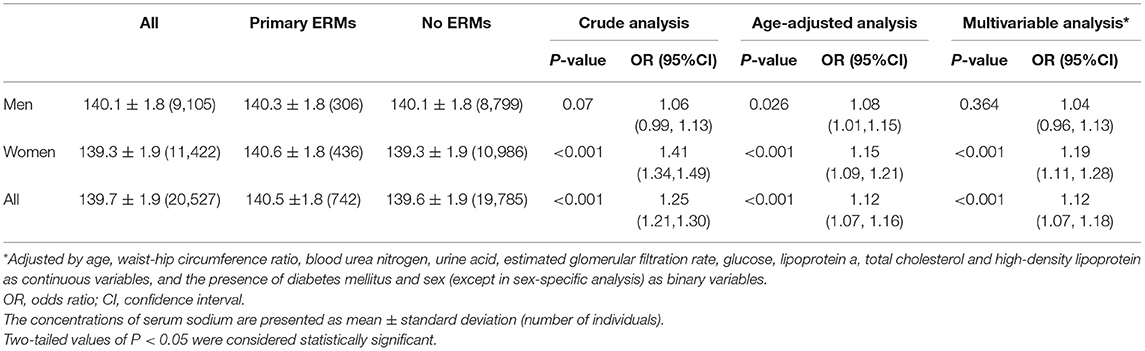
Table 5. Serum sodium concentration in study participants with vs. without primary epiretinal membranes (ERMs) (with serum sodium concentration within the normal range).
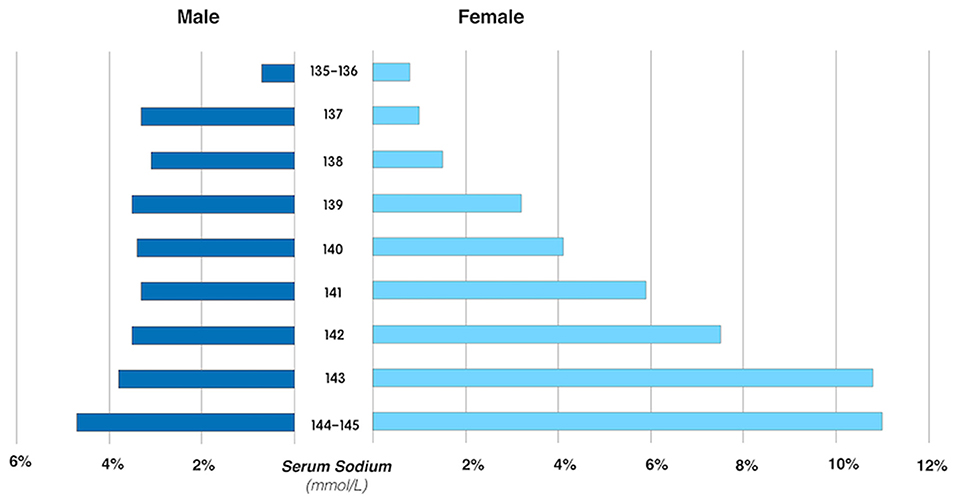
Figure 2. The prevalence of primary epiretinal membrane in male and female participants, stratified by the serum sodium concentration.
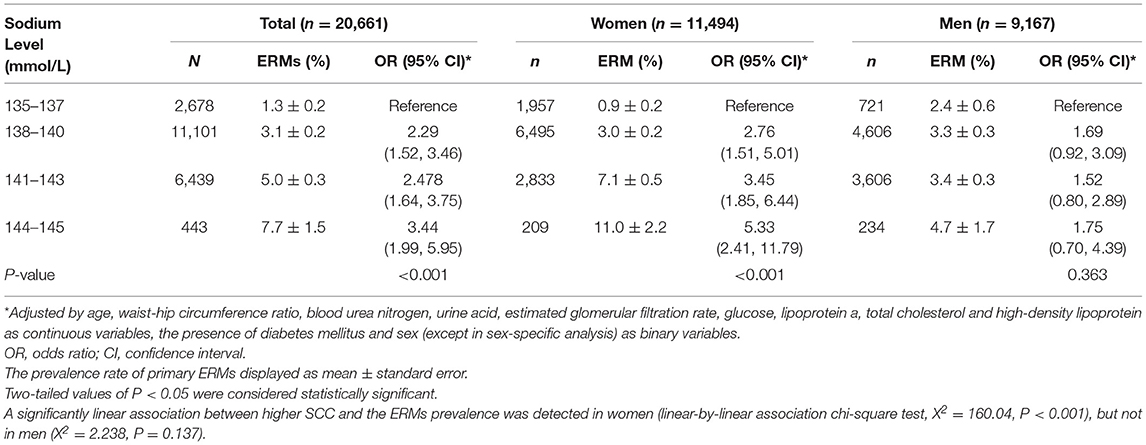
Table 6. Association between the prevalence of primary epiretinal membranes (ERMs) and the serum sodium concentration by multivariable analysis, stratified by sex.
If only participants aged 40+ years were included, the SSC was again significantly (P < 0.001) higher in women with primary ERMs (140.6 ± 1.8 mmol/L) as compared with women without ERMs (139.7 ± 2.0 mmol/L). Women with SSC of 144 mmol/l to 145 mmol/L as compared to women with an SSC of 135–137 mmol/L had 4.6-folds increased risk of ERMs (OR: 4.63; 95% CI: 2.10–10.21; P < 0.001) (Figure 3).
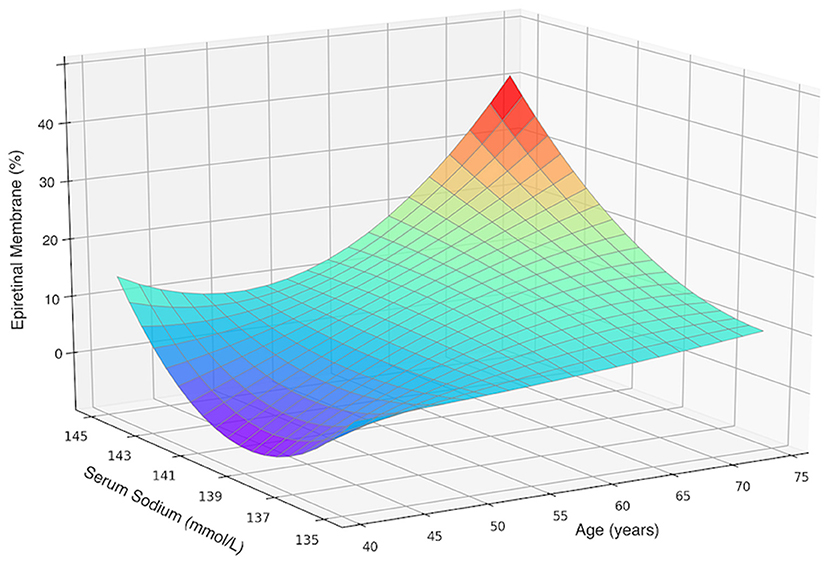
Figure 3. Three-dimensional surface plot visualizing the functional associations between the presence of primary epiretinal membrane and two dependent variables including age and serum sodium concentration in female participants aged 40 and over. An increase in epiretinal membrane prevalence with an increased sodium level was not dependent on age.
In a next step, all female patients with ERM were compared with 1:1 age-matched female controls, for a sensitivity analysis. A significant higher SSC was found in ERM patients (n = 436, age: 62.0 ± 8.4 years, SSC: 140.6 ± 1.8 mmol/L) in comparing with age-matched controls (n = 436, age: 62.0 ± 8.5 years, SSC: 137.5 ± 1.3 mmol/L) after multivariable analysis (OR: 6.39; 95% CI: 4.61–8.88; P < 0.001).
When comparing the subtypes of ERMs, female participants with PMFs as compared to those with CMR also show a slightly but significantly higher SSC (140.8 ± 1.8 vs. 140.3 ± 2.0 mmol/L; P = 0.01).
Inter-observer agreement was assessed by examining the photographs of 100 participants by both examiners (CCX, XBZ), with a kappa value of 0.851 (P < 0.001).
Discussion
The prevalence of primary ERMs in our study population was 3.7% and increased with older age. Factors associated with a higher ERM prevalence were, besides older age, a higher serum concentration of total cholesterol and a higher SSC. If the study population was stratified by sex, the correlation between a higher SSC, even within the normal range, with a higher ERM prevalence, was valid for women but not for men. Women with an SSC of 144–145 mmol/L as compared with those with an SSC of 135–137 mmol/L had a 5.3-fold increased odds of having an ERM for the whole age group, and 4.6-fold increased odds of having an ERM for those aged 40+ years.
The prevalence of primary ERMs as found in our study population was higher than the ERM frequency observed in other Asian populations (4, 5, 7, 16), but was very comparable to the pooled data of a recent meta-analysis (6).
Increasing age is a well-established risk factor for ERM development, as consistently reported by previous population-based studies and ours (1, 5, 17–19). One may discuss that vitreoretinal interface changes caused by a posterior vitreous detachment may be causally associated with the age-related increase of the ERM prevalence.
The association between female gender and higher ERM prevalence was inconsistently reported. While in a recent meta-analysis pooled 13 studies with 49,696 participants, female individuals tended to have a higher ERM prevalence than men with an OR of 1.34 (6), many investigations as well as ours did not find a sex-specific difference in the ERM prevalence (5, 7, 20, 21). The discrepancies in the association between sex and ERM may be due to variations of the ethnicities, study designs, and techniques to detect ERM.
The new finding in our study was the relationship between a higher SSC and a higher primary ERM prevalence, including or excluding participants with an abnormal SSC. In a case series study, sodium was detected in 10 out 20 ERM samples and tended to be more frequent in the thicker ERM (22), which is consistent with the significantly higher SSC in PMF patients than CMR patients in our study. In a study involving 27 participants with a macular hole or ERM, the sodium concentration in the vitreous was significantly higher than that in the fasting serum samples obtained perioperatively (146.7 ± 3.3 vs. 139.7 ± 3.4 mmol/L; P < 0.0001) (23).
Our findings combined with these studies suggest that sodium may be a factor involved in the pathogenesis of ERM, perhaps by modulating the glial activity and cell migration (22–25), whereas the exact mechanism remains to be explored. A cross-sectional study from Korea observed no difference in sodium intake between participants with and without ERM (16). One may take into account, however, that their data of the sodium intake were self-reported and not equivalent to the SSC.
Sodium (Na+) is a major electrolyte in the serum and extracellular fluids. Both higher and lower SSC, even within the normal range (135–145 mmol/L), are associated with primary cardiovascular events (26). A higher SSC is related to elevated serum lipid concentrations and elevated blood pressure, and extracellular sodium directly affects lipid metabolism by increasing the lipid accumulation in cultured adipocytes (27). Thus, the association between higher cholesterol and ERM prevalence observed in our study and previous similar findings (4, 8, 10, 18) may also help to explain the relationship between a higher SSC and higher ERM prevalence.
A positive correlation between SSC and age was found in women but not in men. The SSC in women was lower than that in men up to the age of 50 years, while beyond 50 years the association even reversed. This tendency has also been previously observed (28, 29). Besides, the female gender was also found to be an independent risk factor for hyponatremia (30–32). Possible explanations for these sex-specific differences may be the impact of female sex hormones in regulating serum sodium, sex differences in regulation of arginine vasopressin, renal sodium secretion, and absorption (28, 29, 33, 34). Interestingly, menopause among Chinese women occurs at approximately the age of 50 years, consisting with the age when the difference in SSC between women and men started to reverse.
Notably, though the age-related variations in SSC might have functioned as confounding factors, the significant association between SSC and ERM prevalence remained after age and multivariable adjustment, in all female participants and in those age 40+ years. It was also shown in a three-dimensional surface plot in which the ERM prevalence increases with the SSC, after stratification by age (Figure 3). Our findings may help to explain the higher ERM prevalence in women than men detected by other groups.
The strengths of our study include its large sample size, and the relatively large number of systemic parameters included in the statistical analysis. These parameters included the blood pressure, glucose, lipids and kidney function, and presence of hypertension and diabetes, which may be potential confounding factors for any association with SSC. Our study has several limitations. First, a selection bias may exist since our results were not derived from a population-based sample. However, most of our participants were employees or retirees from fixed organizations and were consecutively enrolled without considering their health conditions or other factors. Second, the lack of optical coherence tomography might have led to an underestimation of the ERM prevalence. However, fundus photography, as also adopted by other groups (1, 3, 4), has the advantage of a wider angle of imaging so that the identification of ERMs at the temporal vascular arcades became easier. In addition, the primary ERM prevalence in our study was comparable to that reported in a recent meta-analysis (6). Third, the medication and dietary information were not available for the current study, thus we are not sure about the causes of high SSC in individuals with ERM. Whether a low-salt diet would prevent the development or progression of ERM might be of interest to explore in the future. Fourth, our study is a cross-sectional investigation that could assess only factors associated with the prevalence of ERM but not risk factors for the incidence of ERM.
In conclusion, besides older age and higher total cholesterol, a higher serum sodium level may be an independent factor associated with the presence of ERM.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available on reasonable request from the corresponding authors.
Ethics Statement
The Institutional Review Board of Beijing Tongren Hospital approved the study design and waived the informed consent, under the measures for ethical reviews of biomedical research involving humans initiated by the Chinese government and consistent with the FDA guidance.
Author Contributions
YW and JJ: design of the study and critical review of manuscript. CX, JC, XZ, and JX: conduct of the study and collection and management of the data. CX, JC, CZ, DC, and YW: analysis and interpretation of data. CX, JC, and YW: preparation of manuscript. All authors provided important feedback on the methods and results and approved the final version of the manuscript.
Funding
The work was funded by the Research Development Fund of Beijing Municipal Health Commission (2019–4). The funder of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ERM, epiretinal membrane; SSC, serum sodium concentration; CMR, cellophane macular reflex; PMF, pre-macular fibrosis.
References
1. Cheung N, Tan SP, Lee SY, Cheung GCM, Tan G, Kumar N, et al. Prevalence and risk factors for epiretinal membrane: the Singapore Epidemiology of Eye Disease study. Br J Ophthalmol. (2017) 101:371–6. doi: 10.1136/bjophthalmol-2016-308563
2. Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Idiopathic epiretinal membrane and vitreomacular traction preferred practice pattern (R). Ophthalmology. (2020) 127:P145–83. doi: 10.1016/j.ophtha.2019.09.022
3. Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study. Aust Ophthalmol. (1997) 104:1033–40. doi: 10.1016/S0161-6420(97)30190-0
4. Miyazaki M, Nakamura H, Kubo M, Kiyohara Y, Iida M, Ishibashi T, et al. Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayama study. Graefes Arch Clin Exp Ophthalmol. (2003) 241:642–6. doi: 10.1007/s00417-003-0723-8
5. You Q, Xu L, Jonas JB. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing eye study. Eye. (2008) 22:874–9. doi: 10.1038/sj.eye.6702786
6. Xiao W, Chen X, Yan W, Zhu Z, He M. Prevalence and risk factors of epiretinal membranes: a systematic review and meta-analysis of population-based studies. BMJ Open. (2017) 7:e014644. doi: 10.1136/bmjopen-2016-014644
7. Duan XR, Liang YB, Friedman DS, Sun LP, Wei WB, Wang JJ, et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. (2009) 50:2018–23. doi: 10.1167/iovs.08-2624
8. Koh V, Cheung CY, Wong WL, Cheung CM, Wang JJ, Mitchell P, et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. (2012) 53:1018–22. doi: 10.1167/iovs.11-8557
9. Bu SC, Kuijer R, Li XR, Hooymans JM, Los LI. Idiopathic epiretinal membrane. Retina. (2014) 34:2317–35. doi: 10.1097/IAE.0000000000000349
10. Ye H, Zhang Q, Liu X, Cai X, Yu W, Yu S, et al. Prevalence and associations of epiretinal membrane in an elderly urban Chinese population in China: the Jiangning Eye Study. Br J Ophthalmol. (2015) 99:1594–7. doi: 10.1136/bjophthalmol-2015-307050
11. Zhu XB, Yang MC, Wang YX, Qian W, Yan YN, Yang JY, et al. Prevalence and risk factors of epiretinal membranes in a Chinese population: The Kailuan Eye Study. Invest Ophthalmol Vis Sci. (2020) 61:37. doi: 10.1167/iovs.61.11.37
12. Forlini M, Date P, D'Eliseo D, Rossini P, Bratu A, Volinia A, et al. Limited vitrectomy versus complete vitrectomy for epiretinal membranes: a comparative multicenter trial. J Ophthalmol. (2020) 2020:6871207. doi: 10.1155/2020/6871207
13. Xue CC, Cui J, Gao LQ, Zhang C, Dou HL, Chen DN, et al. Peripheral monocyte count and age-related macular degeneration. The Tongren Health Care Study. Am J Ophthalmol. (2021) 227:143–53. doi: 10.1016/j.ajo.2021.03.010
14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
15. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. (1994) 92:403–25; discussion 425–430.
16. Kim JM, Lee H, Shin JP, Ahn J, Yoo JM, Song SJ, et al. Epiretinal membrane: prevalence and risk factors from the Korea National Health and Nutrition Examination Survey, 2008 through 2012. Korean J Ophthalmol. (2017) 31:514–23. doi: 10.3341/kjo.2016.0098
17. Aung KZ, Makeyeva G, Adams MK, Chong EW, Busija L, Giles GG, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. (2013) 33:1026–34. doi: 10.1097/IAE.0b013e3182733f25
18. Bae JH, Song SJ, Lee MY. Five-year incidence and risk factors for idiopathic epiretinal membranes. Retina. (2019) 39:753–60. doi: 10.1097/IAE.0000000000002024
19. Yang Y, Yan YN, Wang YX, Xu J, Ren J, Xu L, et al. Ten-year cumulative incidence of epiretinal membranes assessed on fundus photographs. The Beijing Eye Study 2001/2011. PLoS ONE. (2018) 13:e0195768. doi: 10.1371/journal.pone.0195768
20. Kawasaki R, Wang JJ, Sato H, Mitchell P, Kato T, Kawata S, et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata study. Eye. (2009) 23:1045–51. doi: 10.1038/eye.2008.238
21. Ng CH, Cheung N, Wang JJ, Islam AF, Kawasaki R, Meuer SM, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. (2011) 118:694–9. doi: 10.1016/j.ophtha.2010.08.009
22. Romano MR, Cennamo G, Montorio D, Del Prete S, Ferrara M, Cennamo G. Correlation between various trace elements and ultramicroscopic structure of epiretinal macular membranes and glial cells. PLoS ONE. (2018) 13:e0204497. doi: 10.1371/journal.pone.0204497
23. Kokavec J, Min SH, Tan MH, Gilhotra JS, Newland HS, Durkin SR, et al. Biochemical analysis of the living human vitreous. Clin Exp Ophthalmol. (2016) 44:597–609. doi: 10.1111/ceo.12732
24. Rose CR, Chatton JY. Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience. (2016) 323:121–34. doi: 10.1016/j.neuroscience.2015.03.002
25. Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia. (2016) 64:1611–27. doi: 10.1002/glia.22964
26. Cole NI, Suckling RJ, Swift PA, He FJ, MacGregor GA, Hinton W, et al. The association between serum sodium concentration, hypertension and primary cardiovascular events: a retrospective cohort study. J Hum Hypertens. (2019) 33:69–77. doi: 10.1038/s41371-018-0115-5
27. Gao S, Cui X, Wang X, Burg MB, Dmitrieva NI. Cross-sectional positive association of serum lipids and blood pressure with serum sodium within the normal reference range of 135-145 mmol/L. Arterioscler Thromb Vasc Biol. (2017) 37:598–606. doi: 10.1161/ATVBAHA.116.308413
28. Goldstein P, Leshem M. Dietary sodium added salt, and serum sodium associations with growth and depression in the US general population. Appetite. (2014) 79:83–90. doi: 10.1016/j.appet.2014.04.008
29. Lu ZH, Zhu XX, Tang ZQ, Yang GQ, Du J, Wang XL, et al. Female sex hormones are associated with the reduction of serum sodium and hypertension complications in patients with aldosterone-producing adenoma. Endocr J. (2013) 60:1261–8. doi: 10.1507/endocrj.EJ13-0123
30. Berghuis B, van der Palen J, de Haan GJ, Lindhout D, Koeleman BPC, Sander JW, et al. Carbamazepine- and oxcarbazepine-induced hyponatremia in people with epilepsy. Epilepsia. (2017) 58:1227–33. doi: 10.1111/epi.13777
31. Druschky K, Bleich S, Grohmann R, Engel RR, Kleimann A, Stubner S, et al. Use and safety of antiepileptic drugs in psychiatric inpatients-data from the AMSP study. Eur Arch Psychiatry Clin Neurosci. (2018) 268:191–208. doi: 10.1007/s00406-017-0827-5
32. Filippone EJ, Ruzieh M, Foy A. Thiazide-associated hyponatremia: clinical manifestations and pathophysiology. Am J Kidney Dis. (2020) 75:256–64. doi: 10.1053/j.ajkd.2019.07.011
33. Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab. (2002) 283:E711–21. doi: 10.1152/ajpendo.00192.2002
Keywords: epiretinal membrane, associated factors, serum sodium, prevalence, epidemiology
Citation: Xue CC, Cui J, Zhu XB, Xu J, Zhang C, Chen DN, Wang YX and Jonas JB (2021) Serum Sodium Concentration and Increased Risk for Primary Epiretinal Membrane. Front. Med. 8:770362. doi: 10.3389/fmed.2021.770362
Received: 03 September 2021; Accepted: 02 December 2021;
Published: 24 December 2021.
Edited by:
Shaochong Zhang, Sun Yat-sen University, ChinaReviewed by:
Yi-Ting Hsieh, National Taiwan University Hospital, TaiwanAlessandro Meduri, University of Messina, Italy
Copyright © 2021 Xue, Cui, Zhu, Xu, Zhang, Chen, Wang and Jonas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya Xing Wang, eWF4aW5nd0BnbWFpbC5jb20=; Dong Ning Chen, dHIxMzUwMTA4Mjk2NEAxNjMuY29t; Chun Zhang, emhhbmdjMUB5YWhvby5jb20=
†These authors have contributed equally to this work and share first authorship
 Can Can Xue1,2†
Can Can Xue1,2† Jie Xu
Jie Xu Chun Zhang
Chun Zhang Ya Xing Wang
Ya Xing Wang Jost B. Jonas
Jost B. Jonas