- 1Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque, NM, United States
- 2Department of Medicine, George Washington University, Washington, DC, United States
- 3Department of Medicine, Stritch School of Medicine, Loyola University Chicago, Maywood, IL, United States
- 4Research Service, Raymond G. Murphy Veteran Affairs (VA) Medical Center, Albuquerque, NM, United States
- 5Medicine Service, Division of Nephrology, Raymond G. Murphy Veteran Affairs (VA) Medical Center, Albuquerque, NM, United States
The decreased ability of the kidney to regulate water and monovalent cation excretion predisposes patients with chronic kidney disease (CKD) to dysnatremias. In this report, we describe the clinical associations and methods of management of dysnatremias in this patient population by reviewing publications on hyponatremia and hypernatremia in patients with CKD not on dialysis, and those on maintenance hemodialysis or peritoneal dialysis. The prevalence of both hyponatremia and hypernatremia has been reported to be higher in patients with CKD than in the general population. Certain features of the studies analyzed, such as variation in the cut-off values of serum sodium concentration ([Na]) that define hyponatremia or hypernatremia, create comparison difficulties. Dysnatremias in patients with CKD are associated with adverse clinical conditions and mortality. Currently, investigation and treatment of dysnatremias in patients with CKD should follow clinical judgment and the guidelines for the general population. Whether azotemia allows different rates of correction of [Na] in patients with hyponatremic CKD and the methodology and outcomes of treatment of dysnatremias by renal replacement methods require further investigation. In conclusion, dysnatremias occur frequently and are associated with various comorbidities and mortality in patients with CKD. Knowledge gaps in their treatment and prevention call for further studies.
Introduction
Dysnatremias, defined usually as serum sodium concentration ([Na]) either below 135 mmol/L or above 145 mmol/L, represent the most frequently encountered electrolyte disorder in a variety of clinical settings (1), such as chronic kidney disease (CKD) (2, 3). The major clinical manifestations of dysnatremias result from disturbances of the intracellular volume of brain cells secondary to abnormalities in effective osmolarity (tonicity). Tonicity is the property of any fluid to reduce, not change, or increase the intracellular volume of cells bathed in it through osmotic fluid transfers (4). [Na] is the key clinical indicator of serum tonicity (4).
Hyponatremia can be associated with osmotic cell swelling, osmotic cell shrinking, or no change in the intracellular volume of cells. Hyponatremia causing osmotic swelling of cells (hypotonic or true hyponatremia) (5) is typically associated with a low serum osmolality but may be associated with normal or high serum osmolality in patients with low [Na] values and excessive loads of a solute with total body water (TBW) distribution, e.g., in patients with hyponatremic CKD and high serum urea levels. Hypertonic (or translocational) hyponatremia results from an excess of solutes with extracellular distribution, other than sodium salts, e.g., glucose or mannitol, causing osmotic exit of fluid from the intracellular compartment, hyponatremia, and elevated serum tonicity and osmolality (5). Isotonic hyponatremia with normal cell volume (pseudohyponatremia, or spurious hyponatremia, or artifactual hyponatremia) is encountered when low [Na] values are reported by methods requiring pre-measurement dilution of the serum sample, including flame emission spectrophotometry or indirect ion-specific electrode, and plasma solid content is abnormally high due to hyperlipidemia or hyperproteinemia; and [Na] measured by the direct ion-specific electrode is within the normal range (4, 5).
Dysnatremias result from a single or combined disturbances in the external balances of water, sodium, and potassium (6, 7). The kidney regulates [Na] through the excretion of sodium and potassium, and mainly through the production of dilute or concentrated urine under the direction of vasopressin (8). Abnormalities in urinary diluting or concentrating ability result in dysnatremias (8, 9). Patients with dysnatremia in the setting of CKD should be investigated for disturbances in the external balances of water, sodium, and potassium, and in urinary dilution or concentration (2, 10). This review addresses the pathophysiology, epidemiology, clinical manifestations, mortality, and management of dysnatremias in patients with CKD not on dialysis, and those on maintenance hemodialysis or peritoneal dialysis. Identification of aspects of dysnatremias in patients with CKD that need further studies will also be addressed in this review.
Review
Pathophysiology of Dysnatremias in Patients With CKD
Healthy human kidneys have the capacity to produce several liters of urine daily and can dilute the urine to a minimal osmolality of 50 mOsm/kg or to concentrate the urine to a maximal osmolality of 1,200 mOsm/kg in response to plasma vasopressin levels (9, 11). In CKD, anatomic derangements of the tubular and vascular structures of the diluting and concentrating nephron segments, disturbances in the interstitial hypertonicity of the renal medulla, impaired response of the principal cells of the concentrating nephron segments to vasopressin, and osmotic diuresis of the remaining functioning nephrons curtail the concentrating and diluting abilities of the kidneys (4, 9, 12).
In CKD, the impairment of the urinary concentrating ability outpaces that of the diluting ability (12, 13). Osmotic diuresis of the remaining functioning nephrons, primarily due to urea loads, plays a role in the impairment of the renal concentrating ability (14, 15). However, poor response to vasopressin of the principal epithelial cells in the concentrating segment of the nephron represents the major cause of loss of the concentrating ability. Defects in several stages of this response have been identified in studies of experimental CKD. Fine et al. (16) reported the impaired water permeability and the response of adenylate cyclase activity to vasopressin in a study of isolated perfused cortical collecting tubules from uremic rabbits (16). Teitelbaum and McGuinnes (17) found low levels of mRNA of the V2 receptor in principal cells of the inner medullary tubule of uremic rats. Suzuki et al. (18) reported decreased response of aquaporin 2 expressions in the inner medulla of uremic rats after water restriction, which caused a rise in plasma vasopressin levels with a blunted rise in urine osmolality.
Patients with early stages of CKD have the capability of excreting normal ingested loads of sodium and potassium salts and azotemic compounds in the urine, thus achieving steady states of sodium and potassium balance and serum concentrations of azotemic indices (i.e., creatinine and urea). The decreased renal concentrating ability in CKD obligates the larger volumes of urine in the early stages of CKD than in the healthy stage in order to excrete these solute loads (9). However, the renal ability to excrete water loads becomes progressively limited as the glomerular filtration rate declines and, consequently, the range of water intake that allows normal [Na] values becomes progressively narrower (9). Hyposthenuria or isosthenuria, which occurs in advanced stages of CKD, predispose to both hyponatremia and hypernatremia (2). Evaluation of dysnatremias in these patients should address gains or losses in sodium, potassium, and water through the kidneys, but also through the respiratory and gastrointestinal systems, and the skin (19).
Patients treated by maintenance hemodialysis or peritoneal dialysis have limited margins of water intake allowing a normal [Na] range. In addition, these patients may develop dysnatremias due to prescription errors. Hypernatremia has been reported both after hemodialysis sessions (20–22) and in peritoneal dialysis (23–25). Patients on peritoneal dialysis treated with icodextrin-containing dialysis fluids may develop hypertonic hyponatremia secondary to extracellular accumulation of icodextrin metabolites (26).
Incidence and Prevalence of Dysnatremias in Patients With CKD and Those on Dialysis
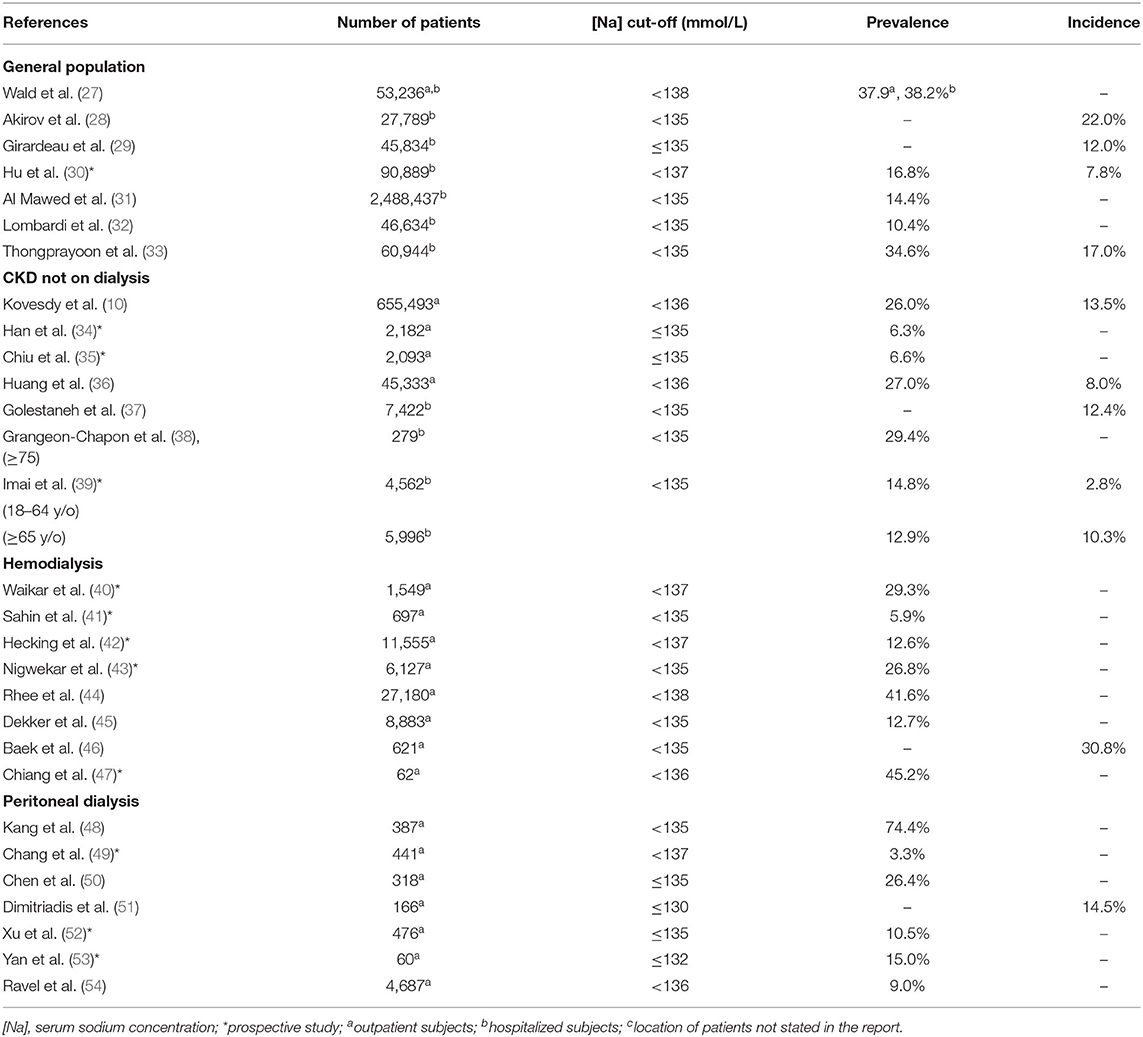
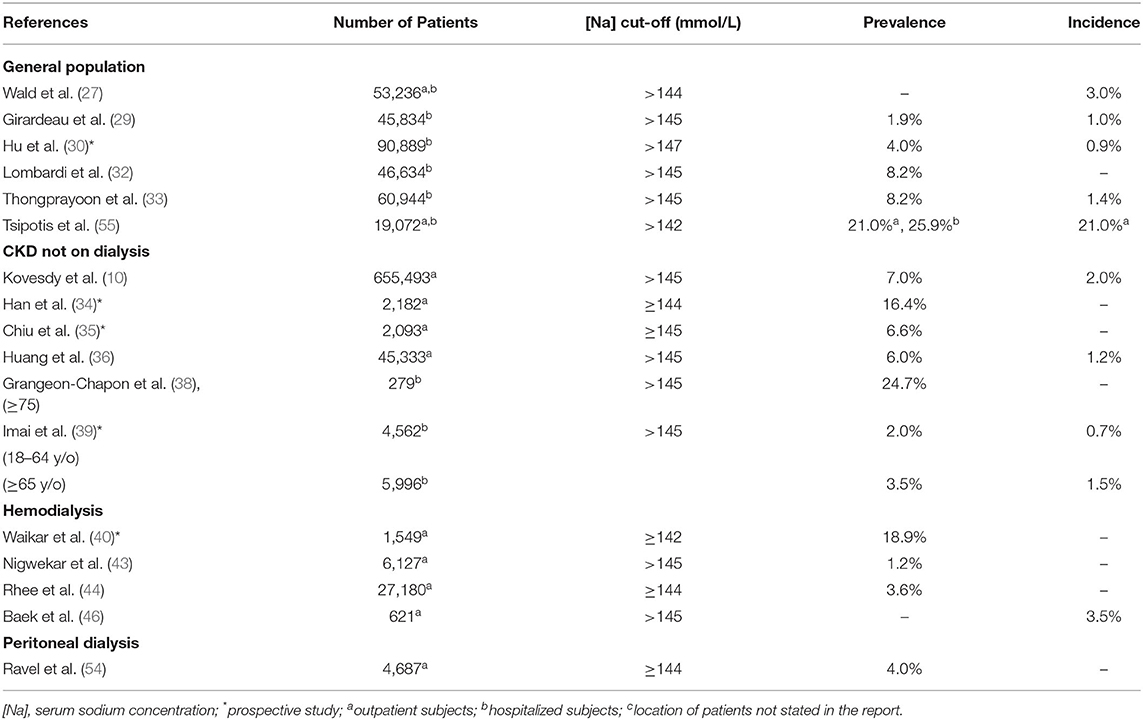
Table 1 shows the incidence and prevalence of hyponatremia in the studies of the general population, CKD not on dialysis, hemodialysis, and peritoneal dialysis (10, 27–54). Table 2 shows the incidence and prevalence of hypernatremia in the same population segments (10, 28–30, 32–36, 38–40, 47, 53–55). The values for prevalence and incidence related to dysnatremias vary widely in each of the four categories of patients in Tables 1, 2 and overlap substantially among the four patient categories. The incidence of hyponatremia in a study of hospitalized patients was 9% in patients without CKD and increased progressively within CKD stages up to 18.1% in patients with CKD stage 5 (37). In another study, which compared adult (18–64 years) and elderly patients (>65 years) with and without CKD at presentation to the emergency department (39), the prevalence of hyponatremia was 2.8% in patients without CKD and 10.3% in those with CKD in the adult group and 14.8% in patients without CKD and 12.9% in those with CKD in the elderly group. In the same study, the prevalence of hypernatremia was 0.7% in patients without CKD and 2.0% in those with CKD in the adult group and 1.5% in patients without CKD, and 3.5% in those with CKD in the elderly group. All differences in prevalence were statistically significant. The findings of these two studies (37, 39) suggest that patients with CKD, at least those who are not elderly, are at higher risk for dysnatremias than those without CKD.
Several characteristics of the studies in Tables 1, 2 contributed both to the overlapping of the incidence and prevalence values between the categories of patients analyzed and to the wide range of these values in each category of patients. Important characteristics, which should be addressed in future studies in this area, are discussed below:
(a) Confounding conditions could have influenced the reported incidence and prevalence of dysnatremias. The studies of dysnatremias in the general population included varying percentages of patients with CKD. Conversely, a fraction of patients in studies involving patients with CKD also had other conditions associated with hyponatremia, e.g., congestive heart failure (10).
(b) The cut-off [Na] values for hyponatremia suggested by various authors ranged between ≤130 mmol/L and <138 mmol/L (Table 1), while the cut-off values for hypernatremia ranged between 142 mmol/L (55) and >147 mmol/L (Table 2). These ranges in [Na] cut-off values were responsible for the substantial differences in incidence and prevalence values.
(c) Many patients featured in Tables 1, 2 had diabetes mellitus, a condition that affects the calculations of incidence or prevalence of hyponatremia if [Na] values are not corrected for the degree of hyperglycemia. Hyperglycemia causes hypertonic hyponatremia. Not accounting for the degree of hyperglycemia in studies of dysnatremias, i.e., using the measured [Na], provides false information about the relationship among body sodium, potassium, and water. The corrected [Na], i.e., a predicted [Na] value after the correction of hyperglycemia, provides an appropriate estimate of this relationship (56). Katz (57) calculated theoretically a decrease in [Na] equal to 1.6 mmol/L for each 5.6 mmol/L rise in serum glucose concentration. Subsequently, the proposed range of coefficients for the calculation of the corrected [Na] was from 1.35 to 4.00 mmol/L reduction in [Na] for every 5.6 mmol/L rise in serum glucose (58). A review of this topic concluded that Katz's coefficient should be used for calculating the corrected [Na], with exceptions that make monitoring of [Na] and serum glucose during the treatment of hyperglycemia mandatory (56). The general form of the Al-Kudsi formula (59), which uses the Katz's coefficient to calculate the corrected [Na], is as follows where both sodium and glucose concentrations are in mmol/L (56):
Several studies on dysnatremias calculated the corrected [Na] using the Al-Kudsi formula (30, 34, 36, 41–43, 46, 47, 49), and one study excluded from statistical analysis serum glucose levels >7.5 mmol/L (51). This last study was also the only one to address spurious hyponatremia by excluding [Na] values in patients with significant hyperlipidemia. The remaining studies in Tables 1, 2 either used coefficients different from Katz's coefficient to calculate the corrected [Na] or did not calculate it. The second influence of hyperglycemia, unique to diabetic patients on dialysis, who develop limited or no osmotic diuresis from glycosuria, pertains to thirst and consumption of water which is retained and reduces the value of [Na] after the correction of hyperglycemia (60). For these reasons, the incidence and prevalence of dysnatremias should be calculated separately in diabetic and non-diabetic CKD populations. Only one small study calculated the prevalence of hyponatremia in non-diabetic peritoneal dialysis patients (53). This study, which assessed changes in TBW and extracellular volume by repeated bioimpedance measurements, identified factors associated with the development of hyponatremia during peritoneal dialysis. These factors include large peritoneal ultrafiltration volumes causing excessive loss of sodium, and changes in nutritional status. Based on these findings, the authors of the study proposed a scheme for addressing hyponatremia in this patient population.
Clinical Manifestations, Associations With Comorbidities, and Mortality of Dysnatremias in Patients With CKD
Hypotonic Hyponatremia
The clinical manifestations of hypotonic hyponatremia result from cerebral edema with the degree, rapidity of development, and duration of hyponatremia determining severity (5, 61). Brain cells undergo intracellular volume adaptation to hyponatremia. This adaptation determines the chronicity of hyponatremia. Within up to 7 h of hyponatremia, brain cells, mainly astrocytes which express aquaporin-4 in abundance (62), lose water to the cerebrospinal fluid through hydrostatic forces (63) and lose electrolytes, such as potassium, sodium, and chloride (5, 64). Subsequently, further intracellular brain cell volume reduction occurs through the loss of organic osmolytes, which is completed by 48 h (5). Hyponatremia lasting <48 h is considered acute, while hyponatremia of 48 h or longer is considered chronic.
Severe clinical manifestations of hyponatremia include seizures, coma, hypoxia secondary to noncardiogenic pulmonary edema and/or hypercapnic respiratory failure (65), and death from cerebral herniation; moderate manifestations include lethargy, disorientation, and confusion; and mild manifestations include fatigue, nausea, and headache (5). Even mild hyponatremia (130–135 mmol/L) is associated with attention deficits, which may require directed testing to be detected, gait disturbances, osteoporosis, and a high risk of fractures (5, 66–68). In patients with advanced CKD, the neurological manifestations of uremia can be confused with the manifestations of dysnatremias, and changes in serum urea concentration affect the treatment of dysnatremias. A paucity of studies exists regarding clinical manifestations related to dysnatremias in patients with CKD. One study reported depressed mental function in patients on peritoneal dialysis with hyponatremia (52).
Certain conditions increase the risk for hyponatremia in patients with CKD not on dialysis, and those on hemodialysis or peritoneal dialysis. These conditions include women gender (34, 36, 41, 44, 48, 54), race other than African American (34, 40, 42, 44, 54), low body weight or body mass index (34, 35, 40, 42–45, 49), diabetes mellitus (35, 40, 41, 43–45, 50, 54), and low serum albumin (10, 34, 35, 40–44, 46–50, 52, 54). In both hemodialysis and peritoneal dialysis patients, hyponatremia was found to be associated with a low residual renal function (43, 44, 47, 49, 51, 54) and excessive weight, most probably fluid, gains (40–42, 44, 45, 47, 48). Hyponatremia in CKD populations increases the risk for several adverse outcomes, such as hospitalization for infections (69), protein-energy malnutrition (70) and impaired cognitive function (71) in hemodialysis patients, and poor peritonitis outcomes (72) plus the higher incidence of new cardiovascular events in peritoneal dialysis patients (73).
Hyponatremia represents a risk for all-cause mortality in the general population (5) and in patients with CKD not on dialysis (10, 34–36), in whom it represents also a risk factor for the mortality from cardiovascular disease or malignancies (36). In hemodialysis patients, hyponatremia was found to be a risk factor for all-cause mortality (40, 42–47), for all-cause mortality only among patients with diabetes mellitus (41), and cardiovascular mortality (40). In peritoneal dialysis patients, hyponatremia was found to be a risk factor for all-cause mortality (49, 54, 72, 74). One study found no association between hyponatremia and 2-year mortality in peritoneal dialysis patients (50). The pathophysiological mechanisms by which hyponatremia increases the risk for mortality in patients with CKD are not well-understood. One study concluded that mortality in hyponatremic peritoneal dialysis patients is most probably due to co-morbidities (48).
Hospitalized patients with COVID-19 have a high prevalence of acute kidney injury with increased death risk (75) and dysnatremias (76–79). The syndrome of inappropriate antidiuretic hormone secretion (SIADH) was identified as the cause of hyponatremia in two cases of COVID-19 (80, 81). Both hyponatremia and hypernatremia represent mortality risks in patients with COVID-19 (75–79).
Hypernatremia
Acute hypernatremia causes lesions in the brain, such as cell shrinkage, petechial and subarachnoid hemorrhages, hematomas, subdural fluid collections, vascular congestion, and venous thrombosis (82). Children with hypernatremia develop irritability, restlessness, muscular twitching, hyperreflexia, and seizures. Elderly patients with hypernatremia rarely develop seizures, but manifest lethargy, delirium, and coma. Less frequent manifestations of hypernatremia include fever, nausea, and vomiting. Alert hypernatremic patients have intense thirst (82). Higher [Na] values are associated with the progression of established CKD independently of other risk factors (83).
Conditions presenting a risk for hypernatremia in patients with CKD not on dialysis include men's gender, older age, heart failure, low estimated glomerular filtration rate, and high levels of body mass index, systolic blood pressure, and serum albumin (34). Conditions predisposing patients on hemodialysis or peritoneal dialysis to hypernatremia will need further investigation.
Hypernatremia increases the risk for mortality in the general population (82) and in patients with CKD not on dialysis (10, 34–36, 38). In hemodialysis patients, hypernatremia was observed to be a risk factor for all-cause mortality (44) and mortality risk for causes other than cardiovascular disease or malignancy in a second study (46). Whether hypernatremia is associated with mortality in peritoneal dialysis patients has not been studied.
Treatment of Dysnatremias in Patients With CKD Not on Dialysis
Treatment of Hyponatremia in Patients With CKD Not on Dialysis
Guidelines (84, 85) and other reports (5, 19, 86, 87) address treatment of hyponatremia in the general population. Figure 1, based on Musso and Bargman's report (88), presents the steps of management of hyponatremia in all patients, such as those with CKD. The last step, evaluation of extracellular volume (ECV) is critical in determining the mechanism and guiding the management of hyponatremia in the general population (5, 19, 86, 87) and all categories of patients with CKD.
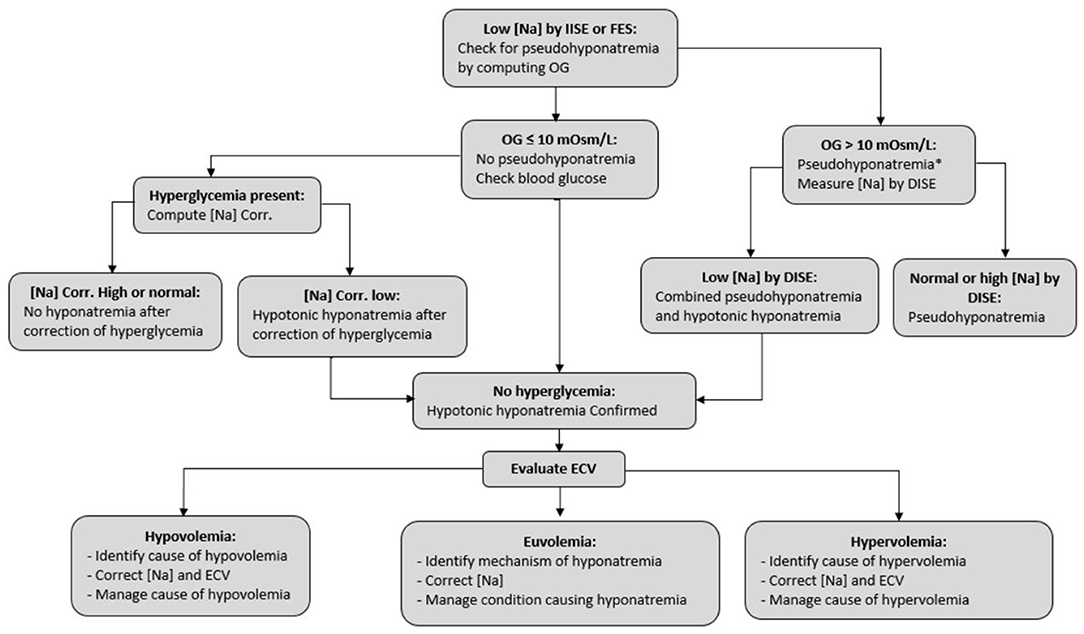
Figure 1. Steps in the management of hyponatremia. [Na], serum sodium concentration; IISE, indirect ion-specific electrode; FES, flame emission spectrophotometry; DISE, direct ion-specific electrode; OG, osmol gap, computed as measured serum osmolality minus the sum 2×[Na] + serum glucose + serum urea, where both glucose and urea concentrations are in mmol/L; ECV, extracellular volume. *Hyponatremia combined with a wide osmol gap and usually high serum osmolality can also be encountered when there is an excess of an exogenous solute with extracellular distribution, e.g., mannitol. Diagnosis of this case of hypertonic hyponatremia is obtained by history.
Table 3 summarizes the approaches to the treatment of hyponatremia in the general population (5). Infusion of hypertonic saline and restriction of fluid intake represent key general measures for treating hyponatremia in all patients with CKD, such as patients with anuria, while administration of loop diuretics or oral urea are effective means only in patients with significant renal function. Oral urea administration as the sole treatment of hyponatremia is safe and effective (89). Urea administration produces osmotic diuresis and increased free water clearance in the general population (90). Whether urea administration is also safe and effective in the early stages of CKD is not known currently. Even mild hyponatremia should be treated. Rondon-Berrios and Berl (91) proposed treating mild chronic hyponatremia with water intake limitation based on the electrolyte-free water clearance and oral administration of compounds increasing urinary water excretion such as sodium salts, urea, loop diuretics, and vasopressin inhibitors.

Table 3. Methods of treating hyponatremia in the general population (5).
We will address the correction of hyponatremia by saline infusion. In patients with CKD, the importance of an accurate estimate of TBW—in the calculation of the volume of infused saline, as well as in the overall management of these patients, acquires even greater importance than in patients with preserved renal function.
The aims of correcting hyponatremia by saline infusion include prevention of brain herniation, improvement of symptoms, and prevention of osmotic demyelination (5). The risk of osmotic demyelination from the rapid rise in [Na] is higher in chronic than in acute hyponatremia. During the rapid correction of chronic hyponatremia, organic osmolyte accumulation in the intracellular compartment of brain cells occurs slowly leading to shrinkage of their intracellular volume. These changes are thought to be linked to the development of osmotic myelinolysis (92). The European guidelines recommend rapid correction of hyponatremia with severe symptoms, regardless of whether the hyponatremia is acute or chronic, by the infusion of hypertonic saline, frequent measurements of [Na] in the first hour of treatment, and evaluation of the symptoms after the increase of [Na] by 5 mmol/L; if symptoms improve, the guidelines recommend stopping the hypertonic saline and limiting the rise in [Na] to a total of 10 mmol/L within the first 24 h and then managing the condition that caused the hyponatremia and increasing [Na] by 8 mmol/L every 24 h thereafter until [Na] reaches 130 mmol/L: if symptoms do not improve, the guidelines suggest continuing the infusion of hypertonic saline with an increase in [Na] by 1 mmol/L hourly until a total rise of 10 mmol/L or [Na] reaches 130 mmol/L (85). Tandukar et al. (93) summarized the findings of 19 publications reporting 21 patients who developed osmotic demyelination syndrome after the correction of hyponatremia by ≤10 mmol/L per 24 h. Sterns and coauthors recommend a change in [Na] during treatment of symptomatic chronic hyponatremia up to 6 mmol/L in the first 6 h, unless symptoms persist when even higher rates of increase in [Na] are indicated, 6–8 mmol/L in the first 24 h, 12–14 mmol/L in 48 h, and 14–16 mmol/L in 72 h (94). Overcorrection should be prevented rigorously (95) and corrected promptly with hypotonic infusions if it occurs. Acute hyponatremia may be treated faster. Whether the same rates of correction of hyponatremia should be applied to patients with early stages of CKD and those treated by dialysis will need to be investigated. In the absence of new information, it would be prudent to apply these guidelines along with clinical judgment to patients with hyponatremic CKD.
Changes in [Na] resulting from the change in body sodium content are determined by the total change in body sodium and by TBW (6). Consequently, TBW, which changes in the same direction and by the same magnitude as ECV in isotonic ECV disturbances, is used when calculating the volume of saline-infused to correct hyponatremia. The Adrogué–Madias formula, which has been used extensively in the management of hyponatremias, calculates the rise in [Na] after infusion of 1 L of saline hypertonic to the serum as follows (86):
Where [Na]Initial is the pre-infusion and [Na]Final is the post-infusion [Na], [Na]Infusate is the sodium concentration in the infused saline, and TBWInitial is the pre-infusion TBW. Determination of the volume of infused saline (VInfused) required to produce the desired rise in [Na] using this formula requires one more calculation. For example, in a patient with [Na]Initial = 110 mmol/L and TBWInitial = 40 L, the Adrogué–Madias formula calculates that infusion of 1 L of 513 mmol/L (3%) saline will result in a 9.8 mmol/L rise in [Na]. If the desired rise in [Na] is 6 mmol/L, VInfused = 1 × 6/9.8 = 0.6 L. A formula published subsequently, and based on the same principles as the Adrogué–Madias formula, calculates directly VInfused, as follows (19):
In the above example, Equation(3) calculates also a VInfused of 0.6 L.
One potential source of variance between [Na]Final values predicted by Equations (2) or (3) and observed relates to the fact that these formulas do not include urinary excretions of water, sodium, and potassium. The urinary excretion may vary considerably during treatment between various categories of hyponatremia (19). Renal excretion of water and monovalent cations during treatment may be less of an issue in patients with advanced CKD.
Another source of variance may be due to an inaccurate estimate of TBWInitial (19). Hyponatremias in both the general and CKD populations can develop in the settings of hypovolemia, euvolemia, or hypervolemia (5, 19). Several conditions and pathogenic mechanisms are responsible for the development of hyponatremia in patients with normal, low, or high ECV, and consequently abnormal TBWInitial values. The importance of the estimate of TBWInitial entered in formulas 2 or 3 is greater in patients with CKD, because of the limited capacity of their kidney to correct errors. Whether ECV and TBW are normal, i.e., whether patients are at their dry weight, is critical in advanced CKD not only for the evaluation of the mechanism and for the management of dysnatremias, but also other clinical reasons, e.g., prevention of cardiovascular complications of hypervolemia or management of hypertension.
Several methods have been proposed for estimating TBW in hyponatremic patients. The older method calculates TBW as an arbitrary fraction of body weight, the difference between women and men, and the difference between older and younger individuals in each gender (86). This method may provide estimates with error because it does not account for body composition at dry weight, in particular about the body fat content, or changes in TBW accompanying the hyponatremia. In addition, the use of sex differences poses a challenge among transgender patients.
Another method for estimating TBW is based on the anthropometric formulas, which represent statistical regression analyses in populations with euvolemia. These statistical analyses compared the TBW measurements by isotopic dilution methods and gender, age, body weight, height, and, in some formulas, the race of the studied subjects. The formulas provided by these studies appropriately account for weight change secondary to a change in body fat, but not for weight change caused by a change in body water. Water gain increases body weight and the fraction of TBW/body weight, while the gain in body fat increases body weight and TBW but decreases the fraction of TBW/body weight (96). The anthropometric formulas always calculate a decrease in the fraction TBW/body weight as weight increases (96). In patients with disorders of TBW, TBW should be estimated as the TBW at dry weight calculated by an anthropometric formula plus or minus the difference between actual and dry weights (19, 96, 97). The drawbacks of this method are that anthropometric formulas, which have large margins of error, and that dry weight is not known in many cases and even when known it may have been miscalculated.
Various other methods, which have been applied for evaluating ECV and TBW in dialysis patients, have limitations (98, 99). ECV associated with optimal perfusion of vital organs, i.e., with the effective arterial blood volume, may vary from the normal value in disease states, e.g., in congestive heart failure (100). Measurement of TBW and ECV by bioimpedance and simultaneous performance of lung ultrasonography to evaluate the extravascular lung ECV is considered a promising method for estimating the dry weight (98, 101, 102). Careful clinical evaluation of the fluid status of patients on hemodialysis is imperative (103, 104). In the future, bioimpedance may become the method of choice for the TBW value used to calculate the volume of the saline infusate in hyponatremic CKD patients. The management of ECV in these patients is best done by combining clinical evaluation, bioimpedance, and lung ultrasonography.
Another source of imprecision in Equations 2 and 3 is that they do not account for exchanges of sodium between the extracellular compartment and the osmotically inactive sodium stored in polyanionic proteoglycans, mainly glycosaminoglycan found in skin, cartilage, and other tissues when [Na] is changing (7). In a study of infusion of hypertonic saline in normal individuals, the rise in [Na] at the end of infusion was almost identical to the rise predicted by the Adrogué–Madias formula, but [Na] decreased 4 h later (105); this decrease in [Na] was not explained by the urinary losses of sodium, potassium, and water. The authors of this study proposed that it was due to the uptake of sodium by proteoglycans. For all these reasons, monitoring of [Na] during and after saline infusion for correction of hyponatremia is critical in patients with or without CKD (19). Measuring urine volume and monovalent cation concentrations in urine will not be needed in every case, but will be useful in some cases with early stages of CKD, foremost in cases of hypovolemic hyponatremia in which infusion of hypertonic saline not only will raise [Na] but also at some point will remove the volume stimulus for vasopressin release, which will result in the production of large volumes of dilute urine. Monitoring urine volume and measuring sodium and potassium concentrations in this urine volume when treatment started and after urine volume starts increasing may provide important therapeutic information.
Treatment of Hypernatremia in Patients With CKD Not on Dialysis
Hypernatremia may also develop in the setting of hypovolemia, euvolemia, or hypervolemia (82, 106). The proper management of hypernatremia requires the identification of the underlying cause and careful correction (107). The prevention of cerebral edema during treatment is of paramount importance. If hypernatremia is chronic (≥48 h) or of unknown duration, correction of [Na] should not exceed 0.5–1.0 mmol/L per hour or 8–10 mmol/L in the first 24 h, to prevent cerebral edema, permanent neurologic damage, or death (107). Asymptomatic chronic hypernatremia may be corrected over 48 h or longer. More rapid correction (up to 1 mmol/L per hour, up to 8–15 mmol/L over the first 8 h) may be appropriate if the onset of hypernatremia is acute (<48 h), e.g., in accidental sodium loading, and unstable patients (107). In these settings, rapid correction improves the prognosis without increasing the risk of cerebral edema (107). The volume of hypotonic saline needed to produce the desired decrease in [Na] can be computed by modification of Equation 3 as follows (82):
Hypernatremia can be corrected by infusion intravenously of sterile water (108). When infusion of sterile water is used, Equation 4 should be modified as follows (82):
Monitoring of [Na] is also imperative during treatment and for a period of at least 24 h after the treatment of hypernatremia with the infusion of hypotonic fluid.
Treatment of Dysnatremias by Dialytic Methods
Dangoisse et al. (109) and Rosner and Connor (110) summarized the principles of management of dysnatremias by continuous renal replacement therapy (CRRT): this management requires customizing either the CRRT circuit or the dialysis solution. The CRRT circuit in continuous venovenous hemofiltration or hemodiafiltration can be customized by computing the sodium concentration at the end of the circuit and then adding a post-filter replacement volume of hypertonic or hypotonic fluid calculated to bring the sodium concentration of the circuit to the desired level. The CRRT dialysis solutions for treating severe dysnatremias are customized by adding to the dialysis solution an appropriate volume of hypertonic saline for hypernatremia or sterile water for hyponatremia calculated by a nomogram to bring the sodium concentration of the dialysis fluid to the desired level. The drawbacks of this approach are that the concentrations of other important ingredients of the dialysis fluid, e.g., potassium, calcium, and magnesium are decreased and there are increased chances of error (110).
Dysnatremias are treated by hemodialysis by changing the sodium concentration of the dialysis fluid. The treatment of dysnatremias by peritoneal dialysis will be presented in the peritoneal dialysis section. Attention is required for the prevention of errors during the treatment of severe dysnatremia by dialytic methods (110–113). These procedures require performance in intensive care units by well-trained personnel. During the procedure, the patient should be clinically monitored, and [Na] should be determined frequently, e.g., every 1–2 h. Clinical monitoring should continue and blood chemistries and concentration of sodium in the dialysis fluid should be repeated after the procedure. Point-of-care chemistry measurements are required. Careful application of multidisciplinary protocols for managing dysnatremias by dialytic methods may improve patient outcomes (114).
Treatment of Hyponatremia by Dialytic Methods
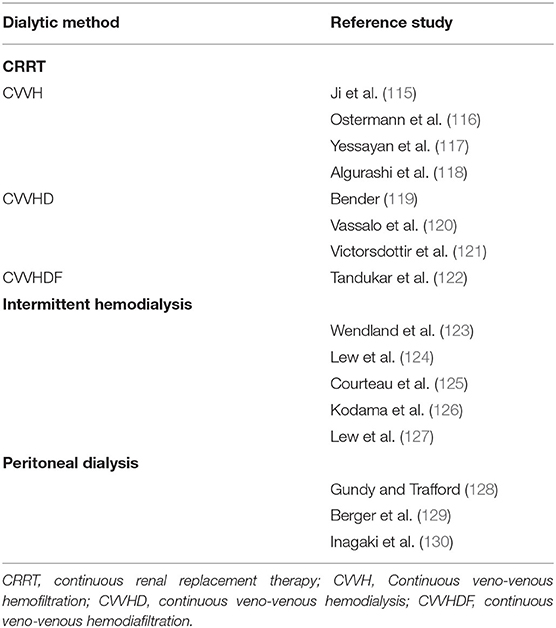
Dialytic methods employed in the treatment of hyponatremia include CRRT methods, intermittent hemodialysis, and peritoneal dialysis. Table 4 contains reports of management of hyponatremia by dialytic methods (115–130). One issue that requires further study in hyponatremia treated by hemodialysis is the effect of a decrease in plasma urea concentration during the procedure. Experimental studies have demonstrated that a urea concentration gradient between the brain intracellular and extracellular compartments at the end of a hemodialysis session causes osmotic entry of fluid into the brain cells and can contribute to the dialysis disequilibrium syndrome: following hemodialysis, urea concentration in the brain cells of azotemic rats was higher than the corresponding plasma concentration and the brain cells exhibited water gain (131). In addition, the accumulation of organic osmolytes, in particular myoinositol and taurine, was higher in brain cells of azotemic than non-azotemic rats 24 h after correction of chronic hyponatremia (132).
On the basis of rapid correction of severe hyponatremia by hemodialysis with no adverse consequences in one patient, it was suggested that azotemia protects from such consequences when hyponatremia is treated by hemodialysis (133). However, osmotic demyelination syndrome developed after the correction of [Na] from 100 to 121 mmol/L over 2.5 h during the first hemodialysis of a patient with pre-dialysis blood urea of 36.4 mmol/L or 218 mg/dl (134). Sirota and Berl (135) suggested that the risk of osmotic demyelination from rapid correction of hyponatremia by hemodialysis may be low in patients who have been on a regular chronic hemodialysis schedule but is substantially higher in patients starting hemodialysis. The treatment of hyponatremia by hemodialysis using the correction rates of [Na] recommended by existing guidelines for the general population should be combined with a slow rate of reduction of blood urea level by the use of shorter dialysis times, lower blood or dialysis fluid flow rates, less efficient dialyzers, or combinations of these measures. More frequent hemodialysis sessions can compensate for the slow removal of azotemic substances.
The methodology used for treating hyponatremia by peritoneal dialysis requires either the addition to the peritoneal dialysis solution of a volume of hypotonic saline calculated to bring the sodium concentration of this solution to the desired level (130), or performance of hourly exchanges of dialysis solution containing 4.25% dextrose (9). In this last method, free water transfers from the blood compartment to the peritoneal cavity during the early phase of a peritoneal dialysis exchange when using hypertonic dialysis fluid because of sodium sieving (136). One azotemic patient who was treated for the first time with peritoneal dialysis for a [Na] of profound 101 mmol/L developed osmotic myelinolysis with a fatal outcome (137). [Na] of this patient was 126 mmol/L on day 2 and 138 mmol/L on day 3.
Treatment of Hypernatremia by Dialytic Methods
Dialytic methods for treating hypernatremia include CRRT (138–142), intermittent hemodialysis (124, 143, 144), and peritoneal dialysis (25, 130, 145). Management of hypernatremia by CRRT is done by customizing the dialysis fluid (139, 141, 142), or infusing hypertonic sodium solutions when there is an urgent need for the very slow rate of reduction in [Na], e.g., in patients with hypernatremia and cerebral edema (140). The rate of reduction of [Na] in hypernatremic patients treated with CRRT should be monitored. In a retrospective study, the hourly rate of reduction in [Na] > 1 mmol/L and dependency on vasopressors were shown to be risk factors for mortality (146). In contrast, a prospective study found no relation between the rate of change in [Na] during CRRT and mortality in dysnatremic patients (147).
Hypernatremia is corrected by hemodialysis by modifying the sodium concentration in the hemodialysis fluid (124, 127, 144). The rate of correction of hypernatremia should be the same as in the section of treatment in patients with CKD not on dialysis. For this reason, the sodium concentration in the dialysis fluid should not differ from [Na] greatly and dialysis should be slow as detailed in the section on the treatment of hyponatremia by hemodialysis. A proposed novel method of producing dialysis fluid allows a larger range of sodium concentration in the dialysis fluid without altering the concentrations of other important ingredients, e.g., potassium and calcium, in this fluid (127). Correction of hypernatremia by peritoneal dialysis is done by lowering the sodium concentration of the dialysis fluid (25) or by using the commercial dialysis solution in documented acute cases in which hypernatremia can be treated at a fast rate (145).
Needs for Future Studies
There are important deficits in our knowledge about the treatment of dysnatremias in patients with CKD not on dialysis and by dialytic methods, particularly by peritoneal dialysis. These deficits include the targets of change in [Na], the best method to achieve these targets, the problems encountered during treatment, and particularly the outcomes of the treatments (113, 148). Whether treatment of dysnatremias in CKD improves mortality is another important question that needs to be studied.
Finally, the prevention of dysnatremias is an important factor in the management of patients with CKD. The range of fluid intake that allows maintenance of [Na] within the normal range in this patient group remains limited. Careful instruction to all patients about fluid intake and identification of patients who have experienced or may be prone to dysnatremias because of dietary habits, associated diseases, or medications affecting thirst, should be part of the routine management of patients with CKD. A recent review of thirst in hemodialysis patients identified differences between these patients and individuals with normal kidney function and areas where our curiosity remains unfulfilled (149). Studies of thirst in patients with CKD may constitute a critical step in the prevention of dysnatremias.
Conclusions
Dysnatremias occur frequently in patients with CKD and are associated with adverse outcomes. The aim of the treatment of dysnatremias is the same in patients with CKD and the general population. Whether azotemia permits faster rates of correction of dysnatremias in patients with CKD and whether treatment of these conditions by renal replacement methods improves patient outcomes are questions that need to be studied. Prevention of dysnatremias in patients with CKD requires careful patient education about fluid intake and further research on thirst regulation and on conditions affecting thirst in this patient population.
Author Contributions
SA and MU: conceptualization. SA, SL, TI, AT, and MU: literature review. SA, AT, and MU: methodology. SA and AT: writing – original draft preparation. SL, TI, and MU: writing – review and editing. All the authors contributed to the article and approved the submitted version.
Funding
This study has been supported and funded by Dialysis Clinics Inc., (DCI). Grant ID: 3RGX8-FP00007518. IRB ID: 19-429.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. (2006) 119(7 Suppl. 1):S30–S5. doi: 10.1016/j.amjmed.2006.05.005
2. Covesdy CP. Significance of hypo- and hypernatremia in chronic kidney disease. Nephrol Dial Transplant. (2012) 27:891–8. doi: 10.1093/ndt/gfs038
3. Sun L, Hou Y, Xiao Q, Du Y. Association of serum sodium and risk of all-cause mortality in patients with chronic kidney disease: a meta-analysis and systematic review. Sci Rep. (2017) 7:15949. doi: 10.1038/s41598-017-16242-3
4. Rohrscheib M, Rondon-Berrios H, Argyropoulos C, Glew, R.H, Murata GH, et al. Indices of serum tonicity in clinical practice. Am J Med Sci. (2015) 349:537–44. doi: 10.1097/MAJ.0000000000000470
5. Rondon-Berrios H, Agaba EI, Tzamaloukas AH. Hyponatremia: pathophysiology, classification, manifestations and management. Int Urol Nephrol. (2014) 46:2153–65. doi: 10.1007/s11255-014-0839-2
6. Edelman IS, Leibman J, O'Meara MP, Birkenfeld L.W. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. (1958) 37:1236–56. doi: 10.1172/JCI103712
7. Sterns RH. Disorders of plasma sodium-causes, consequences and correction. N Engl J Med. (2015) 372:55–65. doi: 10.1056/NEJMra1404489
8. Schrier RW. Body water homeostasis: clinical disorders of urine dilution and concentration. J Am Soc Nephrol. (2006) 17:1820–32. doi: 10.1681/ASN.2006030240
9. Combs S, Berl T. Dysnatremias in patients with kidney disease. Am J Kidney Dis. (2014) 63:294–303. doi: 10.1053/j.ajkd.2013.09.017
10. Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. (2012) 125:677–84. doi: 10.1161/CIRCULATIONAHA.111.065391
11. Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. (1973) 52:2340–52. doi: 10.1172/JCI107423
12. Bricker NS, Dewey RR, Lubowitz H, Stokes J, Kirkensgaard T. Observations on the concentrating and diluting mechanisms of the diseased kidney. J Clin Invest. (1959) 38:516–23. doi: 10.1172/JCI103829
13. Tannen RL, Regal EM, Dunn MJ, Schrier RW. Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med. (1969) 280:1135–41. doi: 10.1056/NEJM196905222802101
14. Rapaport S, Brodsky WA, West, C.D, Makler B. Urinary flow, excretion of solutes, and osmotic work during diuresis of solute loading in hydropenia in man. Science. (1948) 108:630–2. doi: 10.1126/science.108.2814.630
15. Roumelioti ME, Ing TS, Rondon-Berrios H, Glew RH, Khitan ZJ, Sun Y, et al. Principles of quantitative water and electrolyte replacement of losses from osmotic diuresis. Int Urol Nephrol. (2018) 50:1263–70. doi: 10.1007/s11255-018-1822-0
16. Fine LG, Schlondorff D, Trizna W, Gilbert RM, Bricker NS. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclace responsiveness in the cortical collecting tubule to vasopressin. J Clin Invest. (1978) 61:1519–27. doi: 10.1172/JCI109072
17. Teitelbaum I, McGuinness S. Vasopressin resistance in chronic renal failure. Evidence for the role of decreased V2 receptor mRNA. J Clin Invest. (1995) 96:378–85. doi: 10.1172/JCI118044
18. Suzuki K, Hatano R, Michimata M, Kazama I, Suzuki M, Miyama N, et al. Residual urinary concentrating ability and AQP2 expression in a rat model for chronic renal failure. Nephron Physiol. (2005) 99:16–22. doi: 10.1159/000081798
19. Tzamaloukas AH, Malhotra D, Rosen BH, Raj DSC, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. (2013) 2:e005199. doi: 10.1161/JAHA.112.005199
20. Obialo CI, John S, Bashir K. Iatrogenic hypernatremia in hemodialysis patients: A result of erroneous online conductivity monitor and conductivity meter reading. Hemodial Int. (2017) 21:E73–E5. doi: 10.1111/hdi.12546
21. Lindner A, Moskovtchenko JF, Traeger J. Accidental mass hypernatremia during hemodialysis. Simultaneous observation in six cases. Nephron. (1972) 9:99–105. doi: 10.1159/000180139
22. Bhosale GP, Shah VR. Successful recovery from iatrogenic severe hypernatremia and severe metabolic acidosis resulting from accidental use of inappropriate bicarbonate concentrate for hemodialysis treatment. Saudi J Kidney Dis Transpl. (2015) 26:107–10. doi: 10.4103/1319-2442.148754
23. Smith RJ, Block MR, Arieff AI, Blumenkrantz MJ, Coburn JW. Hypernatremic, hyperosmolar coma complicating chronic peritoneal dialysis. Proc Clin Dial Transplant Forum. (1974) 4:96–9.
24. Truniger B, Colombi A. Peritoneal dialysis, ultrafiltration and hyperosmolarity. Schweiz Med Wochenschr. (1976) 106:1102–9.
25. Moritz ML, del Rio M, Grooke GA, Singer LP. Acute peritoneal dialysis as both cause and treatment of hypernatremia in an infant. Pediatr Nephrol. (2001) 16:697–700. doi: 10.1007/s004670100644
26. Posthuma N, ter Wee PM, Donker AJ, Oe PL, van Dorp W, Peers EM, et al. Serum disaccharides and osmolality in CCPD patients using icodextrin or glucose as daytime dwell. Perit Dial Int. (1997) 17:602–7. doi: 10.1177/089686089701700613
27. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. (2010) 170:294–302. doi: 10.1001/archinternmed.2009.513
28. Akirov A, Diker-Cohen T, Steinmetz T, Amitai O, Shimon I. Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur J Intern Med. (2017) 46:25–9. doi: 10.1016/j.ejim.2017.07.017
29. Girardeau Y, Jannot AS, Chatellier G, Saint-Jean O. Association between borderline dysnatremia and mortality insight into a new data mining approach. MBV Med Inform Decis Mak. (2017) 17:152. doi: 10.1186/s12911-017-0549-7
30. Hu J, Wang Y, Geng X, Chen R, Zhang P, Lin J, et al. Dysnatremia is an independent indicator of mortality in hospitalized patients. Med Sci Monit. (2017) 23:2408–25. doi: 10.12659/MSM.902032
31. Al Mawed S, Pankratz VS, Chong K, Sandoval M, Roumelioti ME, Unruh M. Low serum sodium levels at hospital admission: Outcomes among 2.3 million hospitalized patients. PLoS One. (2018) 13:e0194379. doi: 10.1371/journal.pone.0194379
32. Lombardi G, Ferraro PM, Calvaruso L, Naticchia A, D'Alonzo S, Gambaro G. Sodium fluctuations and mortality in a general hospitalized population. Kidney Blood Press Res. (2019) 44:604–14. doi: 10.1159/000500916
33. Thongprayoon C, Cheungpasitporn W, Yap, J.Q, Qian Q. Increased mortality risk associated with serum sodium variations and borderline hypo- and hypernatremia in hospitalized adults. Nephrol Dial Transplant. (2020) 35:1746–52. doi: 10.1093/ndt/gfz098
34. Han S-W, Tilea A, Gillespie BW, Finkelstein FO, Kiser MA, Eisele G, et al. Serum sodium levels and patient outcomes in an ambulatory clinic-based chronic kidney disease cohort. Am J Nephrol. (2015) 41:200–9. doi: 10.1159/000381193
35. Chiu DYY, Kalra PA, Sinha S, Green D. Association of serum sodium levels with all-cause and cardiovascular mortality in chronic kidney disease: results from a prospective observational study. Nephrology (Carlton). (2016) 21:476–82. doi: 10.1111/nep.12634
36. Huang H, Jolly SE, Airy M, Arrigain S, Schold JD, Nally JV, et al. Associations of dysnatremias with mortality in chronic kidney disease. Nephrol Dial Transplant. (2017) 32:1204–10. doi: 10.1093/ndt/gfw209
37. Golestaneh L, Neugarten J, Kaskel F, McGinn AP. Progressive kidney disease may not alter the association of hyponatremia with mortality. Clin Exp Nephrol. (2018) 22:889–97. doi: 10.1007/s10157-018-1536-8
38. Grangeon-Chapon C, Dodoi M, Esnault VL, Favre G. Osmotic stress and mortality in elderly patients with kidney failure: a retrospective study. Clin Interv Aging. (2019) 14:225–9. doi: 10.2147/CIA.S158987
39. Imai N, Shibagaki Y. The prevalence of dysnatremia in the elderly patients without CKD. Am J Emerg Med. (2019) 37:499–501. doi: 10.1016/j.ajem.2018.12.004
40. Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med. (2011) 124:77–84. doi: 10.1016/j.amjmed.2010.07.029
41. Sahin OZ, Asci G, Kircelli F, Yilmaz M, Duman S, Ozkahya M, et al. The impact of low serum sodium level on mortality depends on glycemic control. Eur J Clin Invest. (2012) 42:534–40. doi: 10.1111/j.1365-2362.2011.02613.x
42. Hecking M, Karaboyas A, Saran R, Sen A, Hörl WH, Pisoni RL, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. (2012) 59:238–48. doi: 10.1053/j.ajkd.2011.07.013
43. Nigwekar SU, Wenger J, Thadhani R, Bhan I. Hyponatremia, mineral metabolism, and mortality in incident maintenance hemodialysis patients: a cohort study. Am J Kidney Dis. (2013) 62:755–62. doi: 10.1053/j.ajkd.2013.02.367
44. Rhee CM, Ravel VA, Ayus JC, Sim JJ, Streja E, Mehrotra R, et al. Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol Dial Transplant. (2016) 31:992–1001. doi: 10.1093/ndt/gfv341
45. Dekker MJ, Marcelli D, Canaud B, Konings CJAM, Leunissen KM, Levin NW, et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr. (2016) 70:779–84. doi: 10.1038/ejcn.2016.49
46. Baek SH, Kim S, Na KY, Chin HJ. Predialysis hyponatremia and mortality in elderly patients beginning to undergo hemodialysis. Korean J Intern Med. (2018) 33:970–9. doi: 10.3904/kjim.2016.296
47. Chiang WF, Hsiao PJ, Wu KL, Chan JS. Association of predialysis serum sodium level with fluid status in patients on maintenance hemodialysis. Int Urol Nephrol. (2020) 52:1571–9. doi: 10.1007/s11255-020-02521-y
48. Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Characteristics and clinical outcomes of hyponatraemia in peritoneal dialysis patients. Nephrology (Carlton). (2013) 18:132–7. doi: 10.1111/nep.12013
49. Chang TI, Kim YL, Kim H, Ryu GW, Kang EW, Park JT, et al. Hyponatremia as a predictor of mortality in peritoneal dialysis patients. PLoS One. (2014) 9:e111373. doi: 10.1371/journal.pone.0111373
50. Chen K-H, Chen C-Y, Lee C-C, Weng C-M, Hung, C-C. Baseline hyponatremia does not predict two-year mortality in patients with chronic peritoneal dialysis. Ren Fail. (2014) 36:1371–5. doi: 10.3109/0886022X.2014.945182
51. Dimitriadis C, Sekercioglu N, Pipili C, Oreopoulos D, Bargman JM. Hyponatremia in peritoneal dialysis: epidemiology in a single center and correlation with clinical and biochemical parameters. Perit Dial Int. (2014) 34:260–70. doi: 10.3747/pdi.2012.00095
52. Xu R, Pi H-c, Xiong Z-y, Liao J-I, Hao L, Liu G-l, et al. Hyponatremia and cognitive impairment in patients treated with peritoneal dialysis. Clin J Am Soc Nephrol. (2015) 10:1806–13. doi: 10.2215/CJN.02240215
53. Yan M-T, Cheng C-J, Wang H-Y, Yang C-S, Peng S-J, Lin S-H. Evaluating hyponatremia in non-diabetic uremic patients on peritoneal dialysis. Perit Dial Int. (2016) 36:196–204. doi: 10.3747/pdi.2014.00239
54. Ravel VA, Streja E, Mehrotra R, Sim JJ, Harley K, Ayus JC, et al. Serum sodium and mortality in a national peritoneal dialysis cohort. Nephrol Dial Transplant. (2017) 32:1224–33. doi: 10.1093/ndt/gfw254
55. Tsipotis E, Price LL, Jaber BL, Madias NE. Hospital-associated hypernatremia spectrum and clinical outcomes in an unselected cohort. Am J Med. (2018) 131:72–82.e1. doi: 10.1016/j.amjmed.2017.08.011
56. Ing TS, Ganta K, Bhave G, Lew SQ, Agaba EI, Argyropoulos C, et al. The corrected serum sodium concentration in hyperglycemic crises: computation and clinical applications. Front Med (Lausanne). (2020) 7:477. doi: 10.3389/fmed.2020.00477
57. Katz MA. Hyperglycemia-induced hyponatremia: calculation of expected serum sodium depression. N Engl J Med. (1973) 289:843–44. doi: 10.1056/NEJM197310182891607
58. Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med. (2015) 373:548–59. doi: 10.1056/NEJMra1503102
59. Al-Kudsi RR, Daugirdas JT, Ing TS, Kheirbeck AO, Popli S, Hano JE, et al. Extreme hyperglycemia in dialysis patients. Clin Nephrol. (1982) 17:228–31.
60. Ramdeen G, Tzamaloukas AH, Malhotra D, Leger A, Murata GH. Estimates of interdialytic sodium and water intake based on the balance technique: differences between nondiabetic and diabetic subjects on hemodialysis. ASAIO J. (1998) 44:812–7. doi: 10.1097/00002480-199811000-00009
61. Kheetan M, Ogu I, Shapiro JI, Khitan, ZJ. Acute and chronic hyponatremia. Front Med (Lausanne). (2021) 8:693738. doi: 10.3389/fmed.2021.693738
62. Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. (2007) 22:778–84. doi: 10.1007/s00467-006-0411-0
63. Nase G, Helm PJ, Enger R, Ottersen OP. Water entry into astrocytes during brain edema formation. Glia. (2008) 56:895–902. doi: 10.1002/glia.20664
64. Melton JE, Patlak CS, Pettigrew KD, Cserr HF. Volume regulatory loss of Na, Cl and K from rat brain during acute hyponatremia. Am J Physiol. (1987) 252:F661–F9. doi: 10.1152/ajprenal.1987.252.4.F661
65. Ayus JC, Arieff AI. Pulmonary complications of hyponatremic encephalopathy. Noncardiogenic pulmonary edema and hypercapnic respiratory failure. Chest. (1995) 107:517–21. doi: 10.1378/chest.107.2.517
66. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, attention deficits. Am J Med. (2006) 119:71.e1–8. doi: 10.1016/j.amjmed.2005.09.026
67. Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. (2010) 25:554–63. doi: 10.1359/jbmr.090827
68. Kinsella S, Moran S, Sullivan MO, Molloy MGM, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. (2010) 5:275–80. doi: 10.2215/CJN.06120809
69. Mandai S, Kuwahara M, Kasagi Y, Kusaka K, Tanaka T, Shikuma S, et al. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol. (2013) 14:276. doi: 10.1186/1471-2369-14-276
70. Poulikakos D, Marks V, Lelos N, Banerjee D. Low serum sodium is associated with protein energy wasting and increased interdialytic weight gain in haemodialysis patients. Clin Kidney J. (2014) 7:156–60. doi: 10.1093/ckj/sft170
71. Odagiri G, Sugawara N, Kikuchi A, Takahashi I, Umeda T, Saitoh H, et al. Cognitive function among hemodialysis patients in Japan. Ann Gen Psychiatry. (2011) 10:20. doi: 10.1186/1744-859X-10-20
72. Tseng M-H, Cheng C-J, Sung C-C, Chou Y-C, Chu P, Chen GS, et al. Hyponatremia is a surrogate marker of poor outcome in peritoneal dialysis-related peritonitis. BMC Nephrol. (2014) 15:113. doi: 10.1186/1471-2369-15-113
73. Kim HW, Ryu GW, Park CH, Kang EW, Park JT, Han SH, et al. Hyponatremia predicts new-onset cardiovascular events in peritoneal dialysis patients. PLoS One. (2015) 10:e0129480. doi: 10.1371/journal.pone.0129480
74. Al-Chidadi A, Nitsch D, Davenport A. The effect of serum sodium on survival in patients treated by peritoneal dialysis in the United Kingdom. Perit Dial Int. (2017) 37:70–7. doi: 10.3747/pdi.2015.00305
75. Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. (2021) 77:204–15. doi: 10.1053/j.ajkd.2020.09.002
76. Atila C, Sailer CO, Basseti S, Tschudin-Sutter S, Bingisser R, Siegemund M, et al. Prevalence outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. (2021) 184:409–18. doi: 10.1530/EJE-20-1374
77. Hirsch JS, Uppal NN, Sharma P, Khanin Y, Shah HH, Malieckal DA, et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. (2021) 36:1135–8. doi: 10.1093/ndt/gfab067
78. Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, et al. Prognostic impact of hyponatremia hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) Registry analysis. Front Endocrinol. (2021) 11:599255. doi: 10.3389/fendo.2020.599255
79. Tzoukis P, Wang JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab. (2021) 106:1637–48. doi: 10.1210/clinem/dgab107
80. Kleybolte J, Storek B, Hegner B. SARS-CoV-2-induced SIADH: a novel cause of hyponatremia. Z Gerontol Geriatr. (2021) 54:301–4. doi: 10.1007/s00391-021-01863-1
81. Sherazi A, Bedi P, Udenbulu E, Rubin V, Alasadi L, Spitalewitz S. Hyponatremia and encephalopathy in a 55-year-old woman with syndrome of inappropriate antidiuretic hormone secretion as an isolated presentation of SARF-Cov-2 infection. Am J Case Rep. (2021) 22:e930135. doi: 10.12659/AJCR.930135
82. Rondon-Berrios H, Argyropoulos C, Ing TS, Raj DS, Malhotra D, Agaba EI, et al. Hypertonicity: clinical entities, manifestations and treatment. World J Nephrol. (2017) 6:1–13. doi: 10.5527/wjn.v6.i1.1
83. Cole NI, Suckling RJ, Desilva V, He FJ, MacGregor GA, Swift PA. Serum sodium concentration and the progression of established chronic kidney disease. J Nephrol. (2019) 32:259–64. doi: 10.1007/s40620-018-0541-z
84. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. (2013) 126(10 Suppl. 1):S1–S42. doi: 10.1016/j.amjmed.2013.07.006
85. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant. (2014) 29(Suppl. 2):i1–i39. doi: 10.1093/ndt/gfu040
86. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. (2000) 342:1581–9. doi: 10.1056/NEJM200005253422107
87. Lien YHH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. (2007) 120:653–8. doi: 10.1016/j.amjmed.2006.09.031
88. Musso CG, Bargman JM. Asymptomatic hyponatremia in peritoneal dialysis patients: an algorithmic approach. Int Urol Nephrol. (2014) 46:2239–41. doi: 10.1007/s11255-014-0817-8
89. Rondon-Berrios H, Tandukar S, Mor MK, Ray EC, Bender FH, Kleyman TR, et al. Urea for the treatment of hyponatremia. Clin J Am Soc Nephrol. (2018) 13:1627–32. doi: 10.2215/CJN.04020318
90. Popli S, Tzamaloukas AH, Ing TS. Osmotic diuresis-induced hypernatremia: better explained by solute-free water clearance or electrolyte-free water clearance. Int Urol Nephrol. (2014) 46:207–10. doi: 10.1007/s11255-012-0353-3
91. Rondon-Berrios H, Berl T. Mild chronic hyponatremia in the ambulatory setting: significance and management. Clin J Am Soc Nephrol. (2015) 10:2268–78. doi: 10.2215/CJN.00170115
92. Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest. (1991) 88:303–9. doi: 10.1172/JCI115292
93. Tandukar S, Sterns RH, Rondon-Berrios H. Osmotic demyelination syndrome following correction of hyponatremia by ≤ 10 mEq/L per day. Kidney 360. (2012) 2:1415–23. doi: 10.34067/KID.0004402021
94. Sterns RH, Nigwekar SU, Hix, JK. The treatment of hyponatremia. Semin Nephrol. (2009) 29:282–99. doi: 10.1016/j.semnephrol.2009.03.002
95. Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. (1987) 317:1190–5. doi: 10.1056/NEJM198711053171905
96. Tzamaloukas AH. Effect of edema on urea kinetic studies in peritoneal dialysis patients. Perit Dial Int. (1994) 14:398–401. doi: 10.1177/089686089401400416
97. Sun Y, Mills D, Ing TS, Shapiro JI, Tzamaloukas AH. Body sodium, potassium and water in peritoneal dialysis-associated hyponatremia. Perit Dial Int. (2014) 34:253–9. doi: 10.3747/pdi.2012.00201
98. Alexandrou ME, Balafa O, Sarafidis P. Assessment of hydration status in peritoneal dialysis patients: validity, prognostic value, strengths, and limitations of available techniques. Am J Nephrol. (2020) 51:589–612. doi: 10.1159/000509115
99. Flythe JE, Chang TI, Gallagher MP, Lindley E, Madero M, Sarafidis PA, et al. Blood pressure and volume management in dialysis: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. (2020) 97:861–76. doi: 10.1016/j.kint.2020.01.046
100. Roumelioti ME, Glew RH, Khitan ZJ, Rondon-Berrios H, Argyropoulos CP, Malhotra D, et al. Fluid balance concepts in medicine: principles and practice. World J Nephrol. (2018) 7:1–28. doi: 10.5527/wjn.v7.i1.1
101. Siriopol D, Onofriescu M, Voroneanu L, Apetrii M, Nistor I, Hogas S, et al. Dry weight assessment by combined ultrasound and bioimpedance monitoring in low cardiovascular risk hemodialysis patients: a randomized controlled trial. Int Urol Nephrol. (2017) 49:143–53. doi: 10.1007/s11255-016-1471-0
102. Ekinci C, Karabork M, Siriopol D, Dincer N, Covic A, Kanbay M. Effects of volume overload and current techniques for assessment of fluid status in patients with renal disease. Blood Purif. (2018) 46:34–47. doi: 10.1159/000487702
103. Vasko R, Müller GA, Ratliff BB, Jung K, Gauczinski S, Koziolek MJ. Clinical judgment is the most important element in overhydration assessment of chronic hemodialysis patients. Clin Exp Nephrol. (2013) 17:563–8. doi: 10.1007/s10157-012-0745-9
104. Sommerer C, Felten P, Toernig J, Zeier M, Dikow R. Bioimpedance analysis is not superior to clinical assessment in determining hydration status: a prospective randomized-controlled trial in a Western dialysis population. Hemodial Int. (2021) 11:2021. doi: 10.1111/hdi.12919
105. Olde Engberink RHG, Rorije NMG, van den Born BJH, Vogt L. Quantification of nonosmotic sodium storage capacity following acute hypertonic saline infusion in healthy individuals. Kidney Int. (2017) 91:738–45. doi: 10.1016/j.kint.2016.12.004
106. Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. (2000) 342:1493–99. doi: 10.1056/NEJM200005183422006
107. Dhondup T, Qian Q. Acid-base and electrolyte disorders in patients with and without chronic kidney disease: an update. Kidney Dis (Basel). (2017) 3:136–48. doi: 10.1159/000479968
108. Ramaswamykanive H, Greaves J. Intravenous infusion of sterile water for the treatment of hypernatraemia. Anaesth Intensive Care. (2014) 42:258–62. doi: 10.1177/0310057X1404200215
109. Dangoisse C, Dickie H, Tovey L, Ostermann M. Correction of hyper- and hyponatremia during continuous renal replacement therapy. Nephron Clin Pract. (2014) 128:394–8. doi: 10.1159/000369347
110. Rosner MH, Connor MJ Jr. Management of severe hyponatremia with continuous renal replacement therapies. Clin J Am Soc Nephrol. (2018) 13:787–9. doi: 10.2215/CJN.13281117
111. Gul A, Miskulin DC, Paine SS, Narsipur SS, Arbeit LA, Harford AM, et al. Comparison of prescribed and measured dialysate sodium: a quality improvement project. Am J Kidney Dis. (2016) 67:439–45. doi: 10.1053/j.ajkd.2015.11.004
112. Neyra JA, Ortiz-Soriano VM, Ali D, Morris PE, Johnston CM. A multidisciplinary approach for the management of severe hyponatremia in patients requiring continuous renal replacement therapy. Kidney Int Rep. (2018) 4:59–66. doi: 10.1016/j.ekir.2018.09.001
113. Tinawi M, Bastani B. A mathematical approach to severe hyponatremia and hypernatremia in renal replacement therapies. Semin Dial. (2021) 34:42–50. doi: 10.1111/sdi.12918
114. Pirklbauer M. Hemodialysis treatment in patients with severe electrolyte disorders: management of hyperkalemia and hyponatremia. Hemodial Int. (2020) 24:282–9. doi: 10.1111/hdi.12845
115. Ji DX, Gong DH, Xu B, Tao J, Ren B, Zhang YD, et al. Continuous veno-venous hemofiltration in the treatment of acute severe hyponatremia: a report of 11 cases. Int J Artif Organs. (2007) 30:176–80. doi: 10.1177/039139880703000213
116. Ostermann M, Dickie H, Tovey L, Treacher D. Management of sodium disorders during continuous haemofiltration. Crit Care. (2010) 14:418. doi: 10.1186/cc9002
117. Yessayan L, Yee J, Frinak S, Szamofalvi B. Treatment of severe hyponatremia in patients with kidney failure: role of continuous venovenous hemofiltration with low-sodium replacement fluid. Am J Kidney Dis. (2014) 64:305–10. doi: 10.1053/j.ajkd.2014.01.451
118. Algurashi BY, Gramish JA. Safe correction of severe hyponatremia in patient with severe renal failure using continuous venovenous hemofiltration with modified content in the replacement fluid. Saudi J Kidney Dis Transplant. (2018) 29:1470–4. doi: 10.4103/1319-2442.248308
119. Bender FH. Successful treatment of severe hyponatremia in a patient with renal failure using continuous venovenous hemodialysis. Am J Kidney Dis. (1998) 32:819–31. doi: 10.1016/S0272-6386(98)70141-6
120. Vassalo D, Camilleri D, Moxham V, Ostermann M. Successful management of severe hyponatraemia during continuous renal replacement therapy. Clin Kidney J. (2012) 5:155–7. doi: 10.1093/ckj/sfr170
121. Victorsdottir O, Indridason OS, Palsson R. Successful treatment of extreme hyponatremia in an anuric patient using continuous venovenous hemodialysis. Blood Purif. (2013) 36:274–9. doi: 10.1159/000355397
122. Tandukar S, Kim C, Kalra K, Verma S, Palevsky PM, Puttarajappa C. Severe hyponatremia and continuous renal replacement therapy: safety and effectiveness of low-sodium dialysate. Kidney Med. (2020) 2:437–49. doi: 10.1016/j.xkme.2020.05.007
123. Wendland EM, Kaplan AA. A proposed approach to the dialysis prescription in severely hyponatremic patients with end-stage renal disease. Semin Dial. (2012) 25:82–5. doi: 10.1111/j.1525-139X.2011.00981.x
124. Lew SQ, Kohn OF, Cheng YL, Kjellstrand CM, Ing TS. Three-stream, bicarbonate-based hemodialysis solution delivery system revisited: with an emphasis on some aspects of acid-base principles. Artif Organs. (2017) 41:509–18. doi: 10.1111/aor.12947
125. Courteau C, Al Khoury A, Michel RP, Weber CL. Acute hemodialysis in a young man with severe symptomatic hyponatremia and kidney injury. Hemodial Int. (2018) 22:E45–E8. doi: 10.1111/hdi.12636
126. Kodama M, Hirai D, Tsuji S, Shim J, Koizumi U, Seta K, et al. Successful sodium level correction with a 3% saline bolus before intermittent hemodialysis in a patient with severe hyponatremia accompanied by acute kidney injury. Intern Med. (2021) 60:2645–9. doi: 10.2169/internalmedicine.6667-20
127. Lew SQ, Cheng YL, Tzamaloukas AH, Ing TS. A new approach to individualize dialysis fluid sodium concentration by using a four-stream, bicarbonate-based fluid delivery system. Artif Organs. (2021) 45:779–83. doi: 10.1111/aor.13929
128. Gundy T, Trafford JA. Efficacy of peritoneal dialysis in severe thiazide-induced hyponatraemia. Postgrad Med J. (1981) 57:734–5. doi: 10.1136/pgmj.57.673.734
129. Berger A, Carty I, Tirosh E. Peritoneal dialysis in a premature infant with hyponatremia, hypervolemia and anuria. Isr J Med Sci. (1983) 19:51–2.
130. Inagaki Y, Miyazaki T, Amano I. Peritoneal dialysis as therapy for electrolyte and acid-base disorders. Int J Artif Organs. (1989) 12:632–7. doi: 10.1177/039139888901201006
131. Silver SM, Desimone JA, Jr, Smith DA, Sterns RH. Dialysis disequilibrium syndrome (DDS) in the rat: role of the “reverse urea effect”. Kidney Int. (1992) 42:161–6. doi: 10.1038/ki.1992.273
132. Soupart A, Silver S, Schroöeder B, Sterns R, Decaux G. Rapid (24-hour) reaccumulation of brain organic osmolytes (particularly myo-inositol) in azotemic rats after correction of chronic hyponatremia. J Am Soc Nephrol. (2002) 13:1433–41. doi: 10.1097/01.ASN.0000017903.77985.CD
133. Oo TN, Smith CL, Swan SK. Does uremia protect against the demyelination syndrome in severely hyponatremic patients with end-stage renal disease? Semin Dial. (2003) 16:68–71. doi: 10.1046/j.1525-139X.2003.03015.x
134. Huang W-Y, Weng W-C, Peng T-I, Ro L-S, Yang C-W, Chen K-H. Central pontine myelinolysis and extrapontine myelinolysis after rapid correction of hypernatremia by hemodialysis in a uremic patient. Ren Fail. (2007) 29:635–8. doi: 10.1080/08860220701392314
135. Sirota JC, Berl T. Is osmotic demyelination a concern dialyzing hyponatremic patients? Semin Dial. (2011) 24:407–9. doi: 10.1111/j.1525-139X.2011.00899.x
136. Smit W, Struijk DG, Ho-Dak-Pannekeet MM, Krediet RT. Quantification of free water transport in peritoneal dialysis. Kidney Int. (2004) 66:849–54. doi: 10.1111/j.1523-1755.2004.00815.x
137. Loo CS, Lim TO, Fan KS, Murad Z, Suleiman AB. Pontine myelinolysis following correction of hyponatraemia. Med J Malaysia. (1995) 50:180–2.
138. Park HS, Hong YA, Kim HG, Choi SR, Sun IO, Chung BH, et al. Usefulness of continuous renal replacement therapy for correcting hypernatremia in a patient with severe congestive heart failure. Hemodial Int. (2012) 16:559–63. doi: 10.1111/j.1542-4758.2011.00650.x
139. Paquette F, Goupil R, Madore F, Troyanov S, Bouchard J. Continuous venovenous hemofiltration using customized replacement fluid for acute kidney injury with severe hypernatremia. Clin Kidney J. (2016) 9:540–2. doi: 10.1093/ckj/sfw036
140. Fülöp T, Zsom L, Rodríguez RD, Chabrier-Rosello JO, Hamrahian M, Koch CA. Therapeutic hypernatremia management during continuous renal replacement therapy with elevated intracranial pressures and respiratory failure. Rev Endocr Metab Disord. (2019) 20:65–75. doi: 10.1007/s11154-019-09483-2
141. Huang C, Liu Y, Li L, Liu H, Zhang P. Continuous veno-venous hemofiltration in the treatment of severely burned patients with acute hypernatremia: a retrospective study of 13 cases. Int J Artif Organs. (2020) 43:416–21. doi: 10.1177/0391398819893381
142. Lippold C, Patel A. Correction of hypernatremia by infusing D5W (5% dextrose in water) prefilter in patients receiving continuous renal replacement therapy: a case series. Hemodial Int. (2020) 24:E27–E32. doi: 10.1111/hdi.12819
143. Pazmiño PA, Pazmiño, B.P. Treatment of acute hypernatremia with hemodialysis. Am J Nephrol. (1993) 13:260–5. doi: 10.1159/000168630
144. Nur S, Khan Y, Nur S, Boroujerdi H. Hypernatremia: correction rate and hemodialysis. Case Rep Med. (2014) 2014:736073. doi: 10.1155/2014/736073
145. el-Dhar S, Gomez RA, Campbell FG, Chevalier RL. Rapid correction of acute salt poisoning by peritoneal dialysis. Pediatr Nephrol. (1983) 1:602–4. doi: 10.1007/BF00853595
146. Ma E, Liu Y, Bai M, Li Y, Yu Y, Zhou M, et al. The reduction rate of serum sodium and mortality in patients undergoing continuous venovenous hemofiltration for acute severe hypernatremia. Am J Med Sci. (2016) 352:272–9. doi: 10.1016/j.amjms.2016.06.002
147. Han SS, Bae E, Kim DK, Kim YS, Han JC, Joo KW. Dysnatremia, its correction, and mortality in patients undergoing continuous renal replacement therapy: a prospective observational study. BMC Nephrol. (2016) 17:2. doi: 10.1186/s12882-015-0215-1
148. Rhee CM, Ayus JC, Kalantar-Zadeh K. Hyponatremia in the dialysis population. Kidney Int Rep. (2019) 4:769–80. doi: 10.1016/j.ekir.2019.02.012
Keywords: dysnatremia, hyponatremia, hypernatremia, chronic kidney disease, hemodialysis, peritoneal dialysis
Citation: Arzhan S, Lew SQ, Ing TS, Tzamaloukas AH and Unruh ML (2021) Dysnatremias in Chronic Kidney Disease: Pathophysiology, Manifestations, and Treatment. Front. Med. 8:769287. doi: 10.3389/fmed.2021.769287
Received: 01 September 2021; Accepted: 04 November 2021;
Published: 06 December 2021.
Edited by:
Michael L. Moritz, University of Pittsburgh, United StatesReviewed by:
Fabio Rosario Salerno, Western University, CanadaMassimo Torreggiani, Centre Hospitalier Le Mans, France
Copyright © 2021 Arzhan, Lew, Ing, Tzamaloukas and Unruh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark L. Unruh, bWx1bnJ1aEBzYWx1ZC51bm0uZWR1
 Soraya Arzhan
Soraya Arzhan Susie Q. Lew
Susie Q. Lew Todd S. Ing
Todd S. Ing Antonios H. Tzamaloukas
Antonios H. Tzamaloukas Mark L. Unruh
Mark L. Unruh