- 1Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Institute of Pathology, Fudan University, Shanghai, China
Lymphoepithelioma-like carcinoma is a rare type of tumor that is histologically identical to lymphoepithelial carcinoma of the nasopharynx. Lymphoepithelioma-like carcinomas (LELCs) are closely associated with viral infections. Human papillomavirus (HPV)-associated LELCs have been reported in a variety of anatomic sites. We reported an extremely rare case of a 25-year-old woman with LELC derived from the anal canal, which is the second case reported at this site. The tumor was diffusely positive for p16 staining, and was correlated with high-risk HPV-16; Epstein-Barr virus-encoded small RNA was negative; PD-L1 positivity and abundant CD8+ T cell infiltration were observed, indicating a “hot” immune microenvironment. In reporting this case, we highlight the potential for misdiagnosis and suggested an association of HPV infection with LELC in the anal canal.
Introduction
Lymphoepithelioma refers to a syncytial growth pattern of undifferentiated malignant cells with prominent non-malignant lymphoplasmacytic stromal infiltration, which was originally described as a neoplasm of the nasopharynx. Tumors with morphology similar to that of nasopharyngeal lymphoepithelioma have been termed lymphoepithelioma-like carcinomas (LELCs). LELCs have been previously identified in several anatomic sites other than the nasopharynx, with a relatively low incidence, such as in the salivary gland (1), lung (2), thymus (3), stomach (4), urinary tract (5), uterine cervix (6), oral cavity (7), breast (8), and skin (9).
Lymphoepithelioma-like carcinomas have been reported to be closely related to viral infections. Epstein-Barr virus (EBV) infections have been purported to be involved in the etiology of lymphoepithelioma of the nasopharynx (10). It was previously described that EBV infection was also associated with most LELCs in foregut-derived organs, such as the salivary gland (11), thymus (12), stomach (4), intra-hepatic biliary epithelium (cholangiocarcinoma) (13), and lung (2), and, rarely but occasionally, in hindgut-derived organs, such as the colon (14). However, in some other so-called non-foregut organs, EBV infection may be less important. LELCs of the liver were reported to be associated with hepatitis B virus (HBV) and hepatitis C virus (HCV) infections (15, 16), while some LELCs derived from the gynecologic tract (17) were found to be human papillomavirus (HPV)-related.
Human papillomavirus-associated LELC is rarely derived from anal canal. To our knowledge, only one case of anal canal-derived LELC has been described previously, which is by Scott et al. (18). Herein, we report a new case of HPV-associated LELC originating from the anal canal with high PD-L1 expression and abundant CD8+ T cell infiltration.
Case Description
A 25-year-old woman was admitted for intermittent hematochezia. A digital rectal examination revealed a polyp 20 mm above the pectinate line. A full clinical investigation revealed no tumors elsewhere. A liquid-based cytology test combined with a human HPV-DNA test was performed to exclude cervical disease. The patient underwent a transanal resection, and the sessile rectal polyp (25 mm) was removed.
Histologically, a poorly differentiated submucosal tumor with relatively clear boundaries was discovered beneath the non-neoplastic rectal mucosa. The submucosal tumor showed a lymphoepithelioma-like growth pattern (Figure 1). The tumor was composed of sheets of large tumor cells with oval or round vesicular nuclei and prominent nucleoli, and had a syncytial cytoplasmic appearance. The intratumoral stroma was densely infiltrated with lymphocytes and plasma cells. The tumor invaded the submucosa to a depth of 11 mm, and the deep and radial margins were clear.
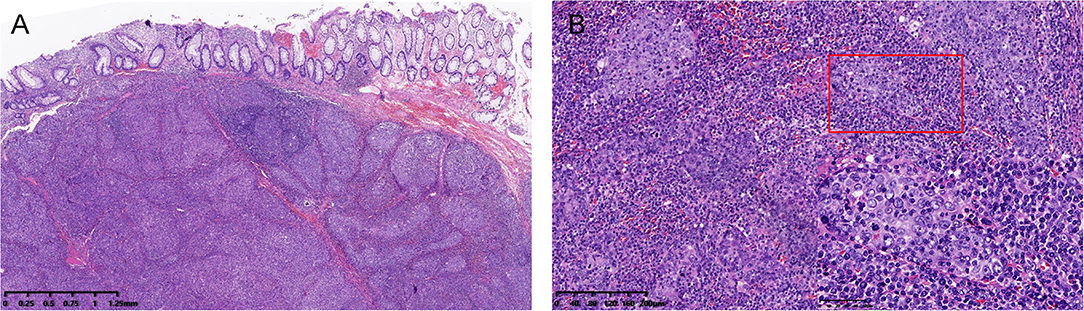
Figure 1. (A) Low-power view showing a submucosal tumor with relatively clear boundaries (hematoxylin and eosin, H&E, 2× magnification). (B) High-power view of the tumor showing a syncytial cytoplasmic appearance and abundant intratumoral immune infiltration (H&E. 10× and 40× magnification).
Immunohistochemically, the tumor cells were positive for pan-CK, P63, P40, and CK5/6 (Figure 2A), and negative for SATB2, Villin, CDX2, and CK20 (Figure 2B). Diffuse block-type immunoreactivity (diffuse nuclear and cytoplasmic staining) of p16 was shown (Figure 2C). Therefore, a diagnosis of poorly differentiated squamous carcinoma with lymphoepithelioma-like morphology was made.
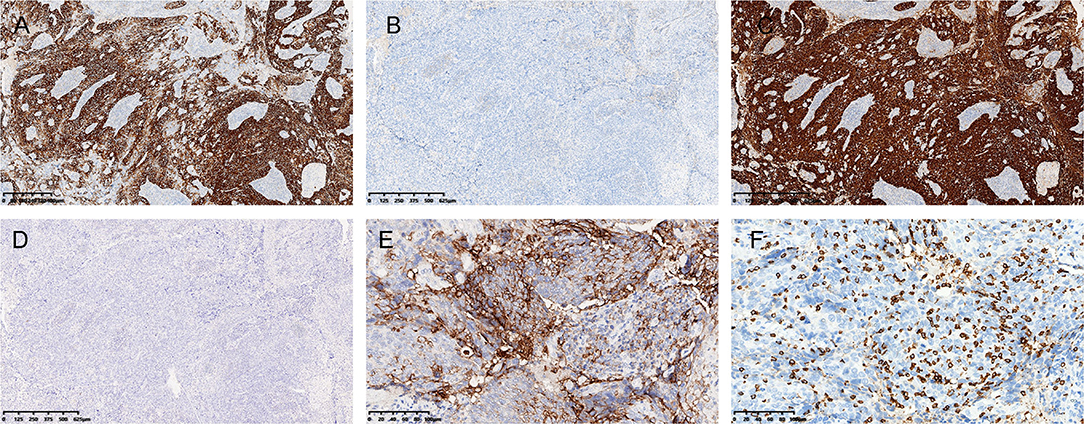
Figure 2. Immunohistochemistry and ISH. (A) The tumor was diffusely positive for CK5/6 (A), and negative for CK20 (B). (C) Immunostaining for p16 was diffusely positive, and (D) in situ hybridization (ISH) for Epstein-Barr virus-encoded small RNA was negative (D). (E) Strong PD-L1 positivity and (F) abundant CD8+ T cell infiltration were observed. [(A–D), 4× magnification; (E,F), 20× magnification].
Since LELCs have been previously reported to be closely related to EBV infection, in situ hybridization for EBV-encoded small RNA (EBER) was performed. The tumor cells were negative for EBER, while strongly positive staining was observed in the external positive control (Figure 2D). Linear array HPV genotyping (Yaneng Bio, Shenzhen, China) was performed for the detection of 23 HPV types, namely, 17 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51–53, 56, 58, 59, 66, 68, 73, and 82) and 6 low-risk HPV types (6, 11, 42, 43, 81, and 83). High-risk HPV-16 was detected in this case. Therefore, this was a case of HPV-associated LELC of the anal canal.
Given that there was a strong local immune infiltration in the tumor microenvironment, immunostaining was performed for DNA mismatch repair (MMR) proteins, PD-L1, and CD8. The tumor showed intact nuclear staining of four MMR proteins (MLH1, PMS2, MSH2, and MSH6). Normal colonic mucosa (adjacent to the carcinoma) and lymphocytes served as positive internal controls. The immunostaining intensity of PD-L1 was evaluated by the combined positive score (CPS) (19), and a strong immunoreaction was observed with a CPS > 70 (Figure 2E). We also analyzed the presence of intratumoral CD8+ T cells and found that abundant tumor-infiltrating lymphocytes (TILs) were CD8-positive (Figure 2F).
The patient subsequently underwent further endoscopic evaluation and mapping biopsies. Neither endoscopic nor microscopic examination revealed any abnormalities. Thereafter, the patient received three cycles of chemotherapy (paclitaxel + Cisplatin + capecitabine) combined with concurrent radiotherapy. The patient continued to be asymptomatic with no signs of recurrence or metastasis 8 months post-operation.
Discussion
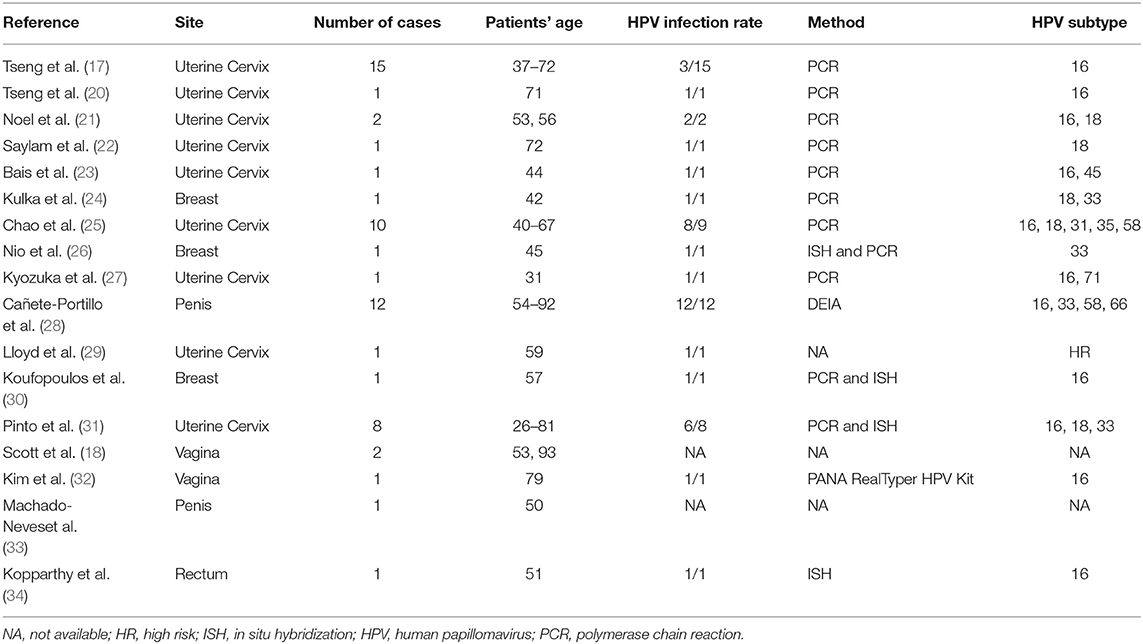
Lymphoepithelioma-like carcinoma is an extremely rare type of tumor. Histologically, it mimics nasopharyngeal carcinoma. Viral infections may have an oncogenic role in the tumorigenesis of LELC. Several research groups have found a strong association between HPV and LELCs originating from so-called non-foregut sites, which are summarized in Table 1. Generally, the tumors tend to occur in late adulthood (range is from 26 to 93 years; median age is 57 years). The uterine cervix was the most frequently reported site. The penis, breast, vagina, rectum, and anal canal were also involved in a minority of cases. Regarding the subtype of HPV, high-risk HPV-16 and−18 were the most common types of HPV (detection rate: 63.4 and 14.6%, respectively), which were also the most common types of HPV associated with invasive squamous cell carcinomas (up to 41% for HPV-16 and 22% for HPV-18) (35, 36). It is reasonable to postulate that HPV may play a role in LELC that is similar to that in invasive squamous cell carcinomas.
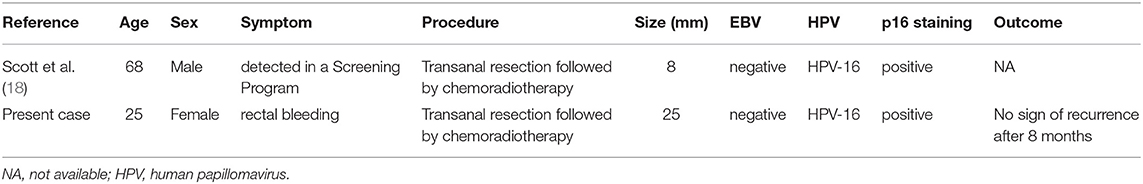
Human papillomavirus -associated LELC originating from the anal canal is extremely rare. To date, only two cases have been reported in English literature, including the present case (Table 2). The patients included one female and one male. The age of the patients was 68 and 25 at diagnosis, respectively. The male patient had no clinical symptoms and was diagnosed in a bowel cancer screening program, while the female patient was initially admitted because of rectal bleeding. The tumor sizes were relatively small, ranging from 8 to 25 mm. The presence of high-risk type HPV-16, not EBV, was observed in both cases. Both of the patients presented with localized neoplasms but with no evidence of metastasis. Follow-up of the present case revealed no evidence of recurrence after 8 months, whereas the prognostic data of the male patient were not available.
Although LELC of the anal canal appears to be extremely rare, knowledge of this variant is critical to properly classify analogous tumors. The diagnosis of LELC depends on a comprehensive morphological examination along with immunohistochemical and molecular analyses. Tumors with a syncytial cytoplasmic appearance, as well as a dense stromal inflammatory infiltrate, are deemed to show a lymphoepithelioma-like growth pattern. Further immunostaining and molecular analyses would help prevent misdiagnosis, particularly of lymphoma, melanoma, neuroendocrine carcinoma, poorly differentiated carcinoma, medullary carcinoma, etc. In our case of anal canal LELC, diffuse positive cytoplasmic staining of pan-CK would help to prevent the misdiagnosis of lymphoma and melanoma. Neuroendocrine carcinoma constantly expresses neuroendocrine markers that are not normally expressed in LELCs. Abundant lymphocytic infiltration and the absence of gland formation prevent the tumor from being misdiagnosed as poorly differentiated adenocarcinoma. As for rectal medullary carcinoma, morphologic overlap does exist between this rare subtype of colorectal adenocarcinoma and LELC (37). The loss of expression of glandular epithelial cell markers, such as CDX2 and CK20; positive expression of squamous cell carcinoma markers, such as P40, P63, CK5/6; intact nuclear staining of MMR proteins and the HPV-related biomarker p16 help to determine the classification of the tumor type. In female patients, metastatic cervical cancer should also be taken into consideration. The differential diagnosis relies on comprehensive history-taking and gynecological examination. Accurate diagnosis and correct categorization should be made based on morphology, immunocytochemistry, and virology, as well as adequate history-taking. The characteristic morphology of syncytial growth pattern in the background consisted of prominent lymphoid stroma, and the propensity for tumor cells to be infected with HPV, warrant classification as HPV-related LELC.
There are no established treatment guidelines for LELC. Both patients with anal canal LELC were treated with transanal resection followed by concurrent chemoradiotherapy. Considering the rapid advancement and remarkable survival benefits for various tumor types, immunotherapy is now considered to be a promising cancer treatment, including for LELCs. It has been reported that a few unresectable, advanced LELCs originating from the liver and lung responded favorably to immune checkpoint inhibitors (38, 39). The expression of PD-L1 and cytotoxic T cells expressing cell-surface CD8 has emerged as a potential predictive biomarker for immunotherapy response (40, 41). Positive PD-L1 expression had been previously reported to be high in LELCs of the uterine cervix (31) and thymus (3). In the present case, we also found a relatively high PD-L1 expression level, with a CPS > 70. Abundant intratumoral CD8+ cytotic T-cells were also observed, suggesting a “hot” immune microenvironment. Therefore, we postulate that this patient could potentially benefit from immune checkpoint inhibitors, such as nivolumab or pembrolizumab.
Given its low incidence, the prognosis of LELC is poorly understood. Similar to what has been reported previously, patients with cervical or pulmonary LELCs have more favorable outcomes and significantly prolonged progression-free survival and overall survival times (17, 25, 31, 42). In LELC of the salivary glands, despite frequent locoregional lymph node and distant metastases, the 5-year survival rate is still high (43). LELCs tend to exhibit an indolent behavior (8). Complete resection may be curative for a particular type of LELC (44). Inflammatory infiltrates in the stroma reflect both the humoral and cell-mediated immune responses of the patients to the tumor, resulting in decreased lymph node metastases, suggesting a favorable prognostic indicator (6). The number of LELCs, especially those originating from the anal canal, is still limited; more studies with longer follow-up periods should be carried out to better define the clinical behavior of these tumors.
Conclusion
In summary, we reported a rare case of LELC of the anal canal and reviewed the previous literature on HPV-related LELCs. The tumor was associated with high-riskHPV-16. Strong PD-L1expression and abundant CD8+ T cell infiltration suggested that the tumor may benefit from immunotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WW analyzed and interpreted the patient data and was a major contributor to the writing of the manuscript. WS interpreted the pathological data. LW interpreted the clinical data. LW and WS substantively revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ma H, Lin Y, Wang L, Rao H, Xu G, He Y, et al. Primary lymphoepithelioma-like carcinoma of salivary gland: sixty-nine cases with long-term follow-up. Head Neck. (2014) 36:1305–12. doi: 10.1002/hed.23450
2. Chen J, Gu C, Chen X, Dai C, Zhao S, Xie H, et al. Clinicopathological and prognostic analyses of 86 resected pulmonary lymphoepithelioma-like carcinomas. J Surg Oncol. (2021) 123:544–52. doi: 10.1002/jso.26276
3. Suster D, Pihan G, Mackinnon AC, Suster S. Expression of PD-L1/PD-1 in lymphoepithelioma-like carcinoma of the thymus. Mod Pathol. (2018) 31:1801–6. doi: 10.1038/s41379-018-0097-4
4. Liang P, Ren XC, Gao JB, Chen KS. CT findings and clinical features of Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Medicine. (2019) 98:e14839. doi: 10.1097/MD.0000000000014839
5. Lopez-Beltran A, Paner G, Blanca A, Montironi R, Tsuzuki T, Nagashima Y, et al. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Arch. (2017) 470:703–9. doi: 10.1007/s00428-017-2117-z
6. Hasumi K, Sugano H, Sakamoto G, Masubuchi K, Kubo H. Circumscribed carcinoma of the uterine cervix, with marked lymphocytic infiltration. Cancer. (1977) 39:2503–7.
7. Evans AT, Guthrie W. Lymphoepithelioma-like carcinoma of the uvula and soft palate: a rare lesion in an unusual site. Histopathology. (1991) 19:184–6. doi: 10.1111/j.1365-2559.1991.tb00013.x
8. Trihia H, Siatra H, Gklisty H, Diamantopoulos P, Arapantoni-Dadiotis P, Kalogerakos K. Lymphoepithelioma-like carcinoma of the breast: cytological and histological features and review of the literature. Acta Cytol. (2012) 56:85–91. doi: 10.1159/000330677
9. Carr KA, Bulengo-Ransby SM, Weiss LM, Nickoloff BJ. Lymphoepitheliomalike carcinoma of the skin. A case report with immunophenotypic analysis and in situ hybridization for Epstein-Barr viral genome. Am J Surg Pathol. (1992) 16:909–13. doi: 10.1097/00000478-199209000-00010
10. Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal Carcinoma (NPC, Lymphoepithelioma). StatPearls. Copyright, Stat. Treasure Island, FL: StatPearls Publishing (2020).
11. Kuo T, Hsueh C. Lymphoepithelioma-like salivary gland carcinoma in Taiwan: a clinicopathological study of nine cases demonstrating a strong association with Epstein-Barr virus. Histopathology. (1997) 31:75–82. doi: 10.1046/j.1365-2559.1997.5830814.x
12. Chen PC, Pan CC, Yang AH, Wang LS, Chiang H. Detection of Epstein-Barr virus genome within thymic epithelial tumours in Taiwanese patients by nested PCR, PCR in situ hybridization, and RNA in situ hybridization. J Pathol. (2002) 197:684–8. doi: 10.1002/path.1141
13. Ding Y, Sun Z, You W, Zhang S, Chang C, Yan S, et al. Lymphoepithelioma-like intrahepatic cholangiocarcinoma with Epstein-Barr virus infection: report of a rare case. Ann Transl Med. (2019) 7:497. doi: 10.21037/atm.2019.08.105
14. Kon S, Kasai K, Tsuzuki N, Nishibe M, Kitagawa T, Nishibe T, et al. Lymphoepithelioma-like carcinoma of rectum: possible relation with EBV. Pathol Res Pract. (2001) 197:577–82. doi: 10.1078/0344-0338-00130
15. Zhang K, Tao C, Tao Z, Wu F, An S, Wu J, et al. Lymphoepithelioma-like carcinoma in liver not associated with Epstein-Barr virus: a report of 3 cases and literature review. Diagn Pathol. (2020) 15:115. doi: 10.1186/s13000-020-01035-6
16. Emile JF, Adam R, Sebagh M, Marchadier E, Falissard B, Dussaix E, et al. Hepatocellular carcinoma with lymphoid stroma: a tumour with good prognosis after liver transplantation. Histopathology. (2000) 37:523–9. doi: 10.1046/j.1365-2559.2000.00952.x
17. Tseng CJ, Pao CC, Tseng LH, Chang CT, Lai CH, Soong YK, et al. Lymphoepithelioma-like carcinoma of the uterine cervix: association with Epstein-Barr virus and human papillomavirus. Cancer. (1997) 80:91–7.
18. Scott K, Trainor J, McVeigh G, Jamison J, Loughrey MB, Kelly PJ, et al. Human Papillomavirus (HPV)-associated lymphoepithelioma-like carcinoma of the vagina and anal canal: a rare variant of squamous cell carcinoma. Int J Gynecol Pathol. (2019) 38:183–8. doi: 10.1097/PGP.0000000000000483
19. Ott PA, Piha-Paul SA, Munster P, Pishvaian MJ, van Brummelen EMJ, Cohen RB, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol. (2017) 28:1036–41. doi: 10.1093/annonc/mdx029
20. Tseng LH, Tseng CJ, Soong YK, Hsueh S, Pao CC. Evidence of Epstein-Barr virus in lymphoepithelioma-like carcinoma of the uterine cervix: a case report. Changgeng Yi Xue Za Zhi. (1998) 21:184–8.
21. Noel J, Lespagnard L, Fayt I, Verhest A, Dargent J. Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: report of two cases and review of the literature. Hum Pathol. (2001) 32:135–8. doi: 10.1053/hupa.2001.20901
22. Saylam K, Anaf V, Fayt I, Noel JC. Lymphoepithelioma-like carcinoma of the cervix with prominent eosinophilic infiltrate: an HPV-18 associated case. Acta Obstet Gynecol Scand. (2002) 81:564–6. doi: 10.1034/j.1600-0412.2002.810616.x
23. Bais AG, Kooi S, Teune TM, Ewing PC, Ansink AC. Lymphoepithelioma-like carcinoma of the uterine cervix: absence of Epstein-Barr virus, but presence of a multiple human papillomavirus infection. Gynecol Oncol. (2005) 97:716–8. doi: 10.1016/j.ygyno.2005.01.016
24. Kulka J, Kovalszky I, Svastics E, Berta M, Füle T. Lymphoepithelioma-like carcinoma of the breast: not Epstein-Barr virus-, but human papilloma virus-positive. Hum Pathol. (2008) 39:298–301. doi: 10.1016/j.humpath.2007.08.006
25. Chao A, Tsai CN, Hsueh S, Lee LY, Chen TC, Huang SL, et al. Does Epstein-Barr virus play a role in lymphoepithelioma-like carcinoma of the uterine cervix? Int J Gynecol Pathol. (2009) 28:279–85. doi: 10.1097/PGP.0b013e31818fb0a9
26. Nio Y, Tsuboi K, Tamaoki M, Tamaoki M, Maruyama R. Lymphoepithelioma-like carcinoma of the breast: a case report with a special analysis of an association with human papilloma virus. Anticancer Res. (2012) 32:1435–41.
27. Kyozuka H, Watanabe T, Furukawa S, Soeda S, Kiko Y, Fujimori K. Lymphoepithelioma-like carcinoma of the uterine cervix: a case report. Eur J Gynaecol Oncol. (2017) 38:459–61.
28. Cañete-Portillo S, Clavero O, Sanchez DF, Silvero A, Abed F, Rodriguez IM, et al. Medullary carcinoma of the penis: a distinctive HPV-related neoplasm: a report of 12 cases. Am J Surg Pathol. (2017) 41:535–40. doi: 10.1097/PAS.0000000000000779
29. Lloyd I, Chadwick B. Lymphoepithelioma-like carcinoma of the uterine cervix: cytomorphologic features and diagnostic pitfalls by liquid-based cytology. Diagn Cytopathol. (2018) 46:443–6. doi: 10.1002/dc.23873
30. Koufopoulos N, Syrios J, Papanikolaou A, Misitzis I, Kapatou KA, Dimas D, et al. Lymphoepithelioma-like breast carcinoma. Pol J Pathol. (2018) 69:98–104. doi: 10.5114/pjp.2018.75344
31. Pinto A, Huang M, Nadji M. Lymphoepithelioma-like carcinoma of the uterine cervix: a pathologic study of eight cases with emphasis on the association with human papillomavirus. Am J Clin Pathol. (2019) 151:231–9. doi: 10.1093/ajcp/aqy130
32. Kim Y, Kim SW. Lymphoepithelioma-like carcinoma of the vagina: a rare case report and review of the literature. J Obstet Gynaecol Res. (2021) 47:1917–21. doi: 10.1111/jog.14722
33. Machado-Neves R, Teixeira B, Fonseca E, Valente P, Lindoro J, Vila F, et al. Penile lymphoepithelioma-like carcinoma: a rare case With PD-L1 expression. Int J Surg Pathol. (2021) 29:690–2. doi: 10.1177/1066896920988340
34. Kopparthy P, Chaffin J, Feely MM, Parekh HD. HPV-16-associated lymphoepithelial-like carcinoma mimicking rectal tonsil. Clin J Gastroenterol. (2021) 14:810–4. doi: 10.1007/s12328-021-01362-1
35. Bertoli HK, Rasmussen CL, Sand FL, Albieri V, Norrild B, Verdoodt F, et al. Human papillomavirus and p16 in squamous cell carcinoma and intraepithelial neoplasia of the vagina. Int J Cancer. (2019) 145:78–86. doi: 10.1002/ijc.32078
36. Bertoli HK, Baandrup L, Aalborg GL, Kjaer AK, Thomsen LT, Kjaer SK. Time trends in the incidence and survival of vaginal squamous cell carcinoma and high-grade vaginal intraepithelial neoplasia in Denmark - a nationwide population-based study. Gynecol Oncol. (2020) 158:734–9. doi: 10.1016/j.ygyno.2020.05.683
37. Ahadi M, Sokolova A, Brown I, Chou A, Gill AJ. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: an update and critical assessment. Pathology. (2021) 53:454–61. doi: 10.1016/j.pathol.2020.10.010
38. Wu Z, Xian X, Wang K, Cheng D, Li W, Chen B. Immune checkpoint blockade therapy may be a feasible option for primary pulmonary lymphoepithelioma-like carcinoma. Front Oncol. (2021) 11:626566. doi: 10.3389/fonc.2021.626566
39. Hermel DJ, Du EZ, Lin R, Frenette CT, Sigal DS. Checkpoint inhibition in the treatment of unresectable, advanced lymphoepithelioma-like hepatocellular carcinoma. J Clin Transl Hepatol. (2021) 9:265–8. doi: 10.14218/JCTH.2020.00094
40. Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. (2021) 124:359–67. doi: 10.1038/s41416-020-01048-4
41. Lee JS, Ruppin E. Multiomics prediction of response rates to therapies to inhibit programmed cell death 1 and programmed cell Death 1 Ligand 1. JAMA Oncol. (2019) 5:1614–8. doi: 10.1001/jamaoncol.2019.2311
42. Chen B, Chen X, Zhou P, Yang L, Ren J, Yang X, et al. Primary pulmonary lymphoepithelioma-like carcinoma: a rare type of lung cancer with a favorable outcome in comparison to squamous carcinoma. Respir Res. (2019) 20:262. doi: 10.1186/s12931-019-1236-2
43. Chiosea SI, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, Bishop JA, editors. Head and Neck Pathology. 3rd ed. Philadelphia, PA: Elsevier (2019). p. 284–362.e5. doi: 10.1016/B978-0-323-47916-5.00013-3
Keywords: human papillomavirus, lymphoepithelioma-like carcinoma, anal canal, immune microenvironment, case report
Citation: Weng W, Sheng W and Wang L (2021) Human Papillomavirus-Associated Lymphoepithelioma-Like Carcinoma of the Anal Canal: A Case Report and Literature Review. Front. Med. 8:766960. doi: 10.3389/fmed.2021.766960
Received: 30 August 2021; Accepted: 19 October 2021;
Published: 19 November 2021.
Edited by:
Ala-Eddin Al Moustafa, Qatar University, QatarReviewed by:
Zubair Khan, University of Texas Health Science Center at Houston, United StatesNan Lin, Trinity Partners, United States
Copyright © 2021 Weng, Sheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Wang, d2FuZ2xlaTA1NjcxM0AxNjMuY29t; Weiqi Sheng, c2hlbmd3ZWlxaTIwMDZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share last authorship
 Weiwei Weng
Weiwei Weng Weiqi Sheng
Weiqi Sheng Lei Wang
Lei Wang