
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 24 December 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.764472
Background: Both nonalcoholic fatty liver disease (NAFLD) and Helicobacter pylori (H. pylori) infection have high prevalence worldwide, and the relationship between both remains controversial. We try to investigate whether H. pylori infection is associated with NAFLD and increased liver fat deposition and stiffness in this cross-sectional study.
Methods: The physical examination data of 5,665 subjects were obtained from February 2018 to June 2019 in this study. Clinical and biochemical data were collected. NAFLD was diagnosed using abdominal color Doppler ultrasonography. Liver steatosis and stiffness were understood by two parameters of transient elastography (TE): fat attenuation parameter (FAP) and liver stiffness measurement (LSM). H. pylori infection was determined using the 13C urea breath tests.
Results: The total prevalence of NAFLD and H. pylori infection was 30.2 and 37.0%, respectively. In men, the prevalence of NAFLD and the levels of FAP and LSM in H. pylori-positive group were significantly higher than H. pylori-negative group (all p < 0.01), but no significant difference was found in women. In men, the infection rate of H. pylori in NAFLD group and LSM ≥ 7.4 kPa group was significantly higher than control group. Multivariate logistic regression analysis revealed that H. pylori infection was not independently associated with NAFLD and FAP ≥ 240 dB/m. However, H. pylori infection was associated with LSM ≥ 7.4 kPa in men.
Conclusions: Our study suggests that H. pylori infection is not significantly associated with NAFLD and elevated liver steatosis, whereas it may be the risk factor of elevated liver stiffness in men.
Non-alcoholic fatty liver disease (NAFLD) is a worldwide liver disease in the contemporary society, and the global prevalence of NAFLD is estimated around 25% (1); the situation is similar in China (2). Considering the high incidence rate of NAFLD and its potential risk of adverse effects such as liver fibrosis and cirrhosis, liver failure, and even hepatocellular carcinoma (HCC), it is necessary to explore the pathogenesis of NAFLD and intervene actively.
Although the etiology of NAFLD has not been fully elucidated, it is closely related to a variety of metabolic disorders caused by insulin resistance (IR) and is even considered to be the liver manifestation of metabolic syndrome (MetS) (3). Recently, the relationship between NAFLD and microbes has attracted much attention. Helicobacter pylori (H. pylori), which is estimated to infect approximately 50% of the world population, has been paid attention to its extragastric manifestations. It is also to be found that H. pylori infection plays a potential role in IR development, which makes researchers who are interested in exploring the correlation between H. pylori infection and NAFLD (4).
Some studies tried to discuss the relationship between H. pylori infection and NAFLD. In a meta-analysis, a significantly increased risk of NAFLD among the patients with H. pylori infection had been found (5). However, controversial results also remained (6). First, the prevalence of NAFLD and H. pylori infection may not be the same in different regions, races, social customs, and eating habits, which makes the results of their correlation inconsistent. The comprehensive analysis of data from multiple regions worldwide is needed. Second, NAFLD is usually diagnosed according to the color Doppler ultrasonography in previous studies, and there may be some artificial bias. Furthermore, for liver fibrosis, which is of great significance in the pathological process of NAFLD, whether H. pylori infection is related to its progression is still lacking relevant research. Considering the difficulty in obtaining a number of liver pathological specimens from the general population infected with H. pylori, it is feasible to obtain the relevant epidemiological data through a noninvasive examination that can quantitatively understand the steatosis and fibrosis of liver.
Transient elastography (TE) is such a detection technology that measures liver stiffness using mechanical and/or ultrasound shear wave propagation through hepatic parenchyma with high accuracy. According to liver stiffness measurement (LSM) and fat attenuation parameter (FAP) from TE, we can get the quantitative assessment of liver fibrosis and steatosis, which had a good consistency with liver biopsy (7, 8). Several guidelines recommend the use of TE to assess liver fat deposition and fibrosis (9–11).
Thus, we conducted this study not only according to abdominal color Doppler ultrasonography but also through TE, to investigate the prevalence of H. pylori infection in different NAFLD status and steatosis, stiffness levels of liver. To our knowledge, we have not found any similar study before. Quantitative analysis of NAFLD in this study may provide more helpful results to accurately understand the relationship between NAFLD and H. pylori infection.
The data were obtained from the Health Management Center, Sichuan Provincial People's Hospital. All the subjects were asked to complete a medical history questionnaire, followed by physical examination (height, body weight, blood pressure, and circumference of waist, hip, and neck), and laboratory examination (routine blood test, liver and kidney function, fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), uric acid, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and hepatitis B virus markers), abdominal ultrasonography, chest imaging (X-ray or CT), TE FibroTouch, and 13C urea breath test.
The metabolic syndrome (MetS) was defined by the presence of at least three of the following five metabolic abnormalities, based on the Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 edition): waist circumference ≥ 90 cm for men and ≥ 85 cm for women; FPG ≥ 6.1 mmol/L and/or type 2 diabetes mellitus (T2DM) was previously diagnosed and treated; blood pressure ≥ 130/85 mmHg and/or hypertension was previously diagnosed and treated; fasting triglyceride ≥ 1.7 mmol/L; fasting HDL cholesterol <1.04 mmol/L.
Subjects were excluded if they had (a) alcoholic abuse, had a daily consumption ≥ 30 g for men and ≥ 20 g for women, (b) evidence of suffered from hepatitis B virus, hepatitis C virus, or human immunodeficiency virus (HIV), (c) a history of using drugs that can cause hepatic steatosis, such as corticosteroids, amiodarone, estrogen, and so on, (d) severe malnutrition, parenteral nutrition, and other end-stage diseases or cancers, (e) contraindications for FibroTouch examination (i.e., ascites, pregnancy, heart pacemakers, and other implanted materials).
The study protocol was approved by the ethics committees of the Sichuan Provincial People's Hospital, according to Declaration of Helsinki (2013) and its later amendments or comparable ethical standards [approval number 177(2017)].
Nonalcoholic fatty liver disease was diagnosed using abdominal ultrasonography (Philips IU elite, Siemens, Germany), the probe frequency was 1–5 MHz, and the center frequency was 3.5 MHz, performed by a doctor at or above the rank of the attending physician from the imaging center of our hospital. According to the diagnostic criteria issued by Chinese Medical Association (12), hepatic steatosis was defined based on the presence of at least two of the three following findings: the near-field echo of liver was diffuse enhanced and stronger than that of kidney; poor visualization of intrahepatic duct structure; far-field echo of liver attenuated gradually.
The new generation of TE series FibroTouch (Wuxi Hisky Medical Technology, Beijing, China) was applied. Two trained physicians used FibroTouch according to the operations manual, blinded to the patients' clinical data. LSM analyses were expressed in kilopascals (kPa) and FAP was expressed in decibels per meter (dB/m). Only if 10 successful measurements were obtained, an IQR/M ratio of LSM and FAP <30% and a success rate of > 60% were considered reliable and then used for analysis. A cutoff value of FAP ≥ 240 dB/m and LSM ≥ 7.4 kPa was considered for elevated liver steatosis and stiffness, respectively.
Helicobacter pylori infection was determined using the 13C urea breath tests (Beijing Boran Pharmaceutical Co., Ltd. Beijing, China). We followed a standardized procedure for the sample collection. All subjects fasted overnight for more than 8 h, maintained normal breath, inserted the straw into the bottom of one sample tube, and exhaled slowly into the sample tube through the straw for 4 to 5 s. Thereafter, they pulled the straw out, tightened the cap immediately, and this was considered as the 0-min sample. Then, the subjects took another bottle with urea 13C granules and 80 to 100 mL cold drinking water, rested for 30 min, and then collected the breath sample again. The two collected gas samples were tested for 13CO2, and δ‰ was used to represent the determination result; δ‰ = (isotopic abundance of 13C for the test sample – isotopic abundance of 13C for reference sample) × 1000/the isotopic abundance 13C for reference sample. The detection value was defined as δ‰ measured at 30 min subtracted from that measured at 0 min. H. pylori infection was considered positive when the detection value was ≥ 4.0.
Statistical analysis was performed using IBM SPSS 21.0 (IBM Corp., NY, USA). Continuous data are expressed as mean ± standard deviation (SD) for normally distributed data and median with 25th and 75th percentile for non-normally distributed data. Categorical data are expressed in percentages. The significance of differences was tested using either Student's t-test (for continuous variables) or chi-squared test (for categorical variables). Univariable and multivariable regression models were performed using the logistic regression analysis, and the results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). A p-value < 0.05 was considered statistically significant.
The total clinical and laboratory baseline characteristics of the 5,665 subjects (3,089 men and 2,576 women) in the study are shown in Table 1, with a mean age of 49.07 ± 10.17 years. 36.4% had hypertension or SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg, 11.0% had T2DM or FPG ≥ 6.1 mmol/L, 32.7% had dyslipidemia, 35.5% had abdominal overweight, and 18.1% met the diagnostic criteria of MetS. The prevalence of the items mentioned above was higher in men than that in women, and the differences were statistically significant. The total infection rate of H. pylori was 37.0%, which was higher in men than that in women, (38.1 vs. 35.7%), but no significant difference was found (p = 0.057).
Subjects were categorized based on their sex to investigate the differences in the prevalence of NAFLD and levels of FAP and LSM in different H. pylori status. In men, the prevalence of NAFLD in H. pylori-positive group was significantly higher than that in H. pylori-negative group (44.5 vs. 39.5%, p <0.01). The levels of FAP and LSM in H. pylori-positive groups were also significantly higher than that in H. pylori-negative groups (all p < 0.01). However, in women, no significant differences were found in the above three indicators between H. pylori-positive and H. pylori-negative groups, as shown in Table 2.
In men, the prevalence of H. pylori infection was significantly higher in NAFLD and LSM ≥ 7.4 kPa groups than the corresponding control groups (41.0 vs. 36.1%, 44.9% vs. 36.6%, respectively, all p < 0.01), but it was not found in women. There was no significant difference in the prevalence of H. pylori infection between FAP ≥ 240 dB/m group and control group in both men and women (all p > 0.05), as shown in Figure 1.
As shown in Tables 3–5, stratified analyses were performed for age, sex, and dyslipidemia, to explore the association between H. pylori infection with NAFLD and increased levels of FAP and LSM. After adjusting for confounding factors in different models, H. pylori infection was not found to be a risk factor for NAFLD and FAP ≥ 240 dB/m. However, H. pylori infection was revealed to be the risk factor for LSM ≥ 7.4 kPa in men and above 50-year-old subjects.
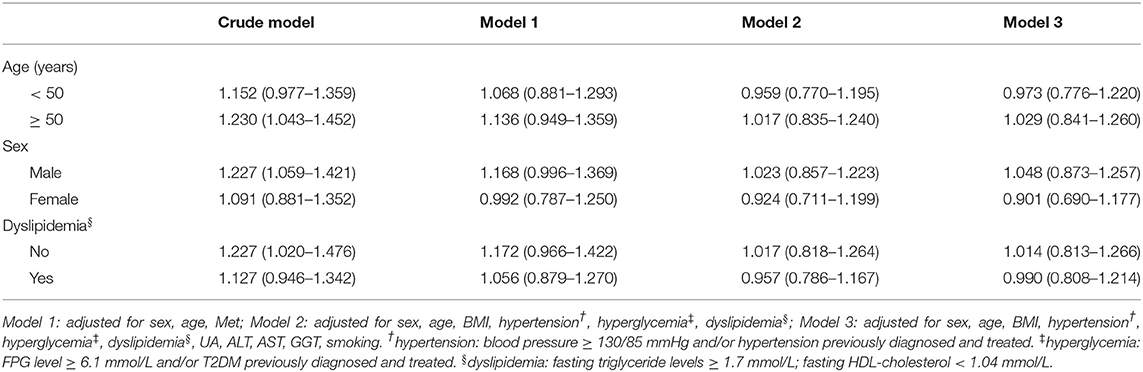
Table 3. Multivariate logistic regression analysis of the association between H. pylori infection and NAFLD, stratified by age, sex, and dyslipidemia.
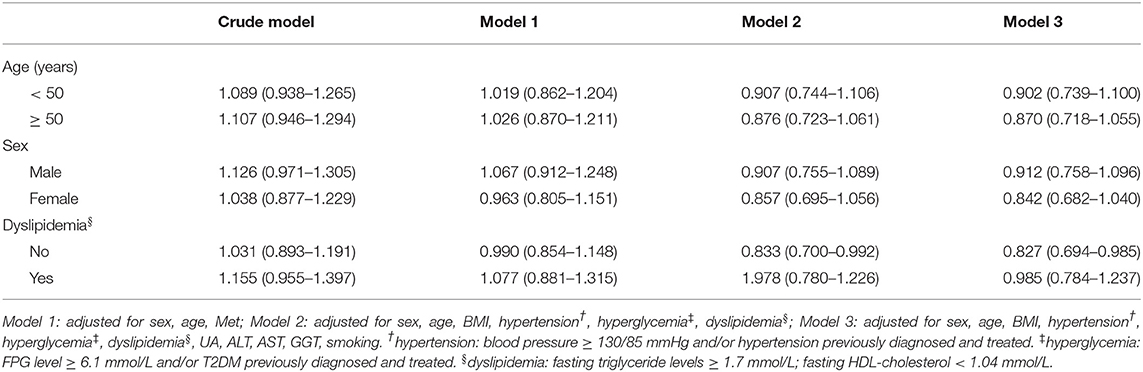
Table 4. Multivariate logistic regression analysis of the association between H. pylori infection and FAP ≥ 240 dB/m, stratified by age, sex, and dyslipidemia.
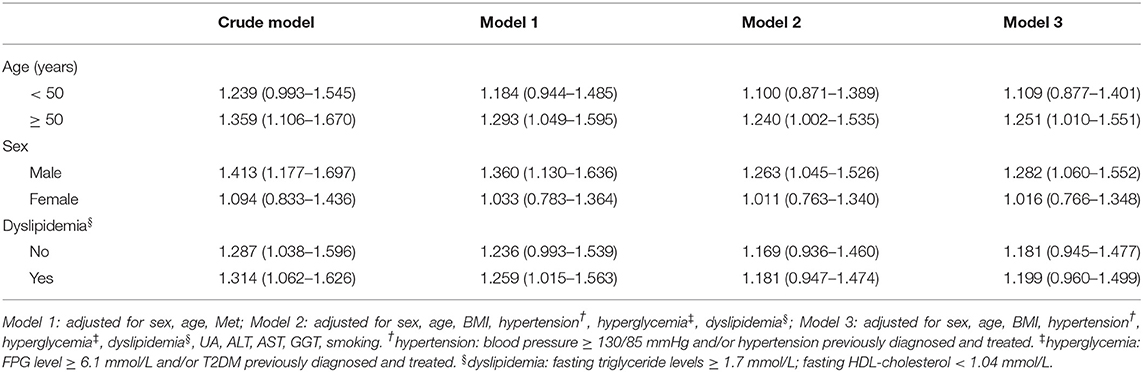
Table 5. Multivariate logistic regression analysis of the association between H. pylori infection and LSM ≥ 7.4 kPa, stratified by age, sex, and dyslipidemia.
As one of the main causes of chronic liver diseases, exploring the etiology of NAFLD has become a hot spot. The prevalent pathogenic model of NAFLD is that of “parallel multiple hit,” and H. pylori infection may be one of the “hit” (13). Cindoruk et. al found that H. pylori 16S rDNA was discovered from patient with NAFLD by liver biopsy (14). Since then, scholars had discussed the relationship between H. pylori and NAFLD and proposed some mechanisms between the both.
The pathogenetic link between NAFLD and H. pylori was in debate and clinical data were limited. IR was focused on to be the underlying mechanism between H. pylori and NAFLD. Polyzos et al. first reported that anti-H. pylori IgG levels were significantly higher in patients with liver biopsy-proven NAFLD, which indicate that H. pylori infection might contribute to NAFLD directly or indirectly via IR (13). The patients who suffered from H. pylori infection had increased expression of inflammatory factors such as IL-1, IL-6, and TNF-a, which reduced insulin sensitivity (4). Some hormones such as leptin, adiponectin, fetuin-A have also been found the changes in IR promotion in patients with H. pylori infection (6). Furthermore, NAFLD also presented increased intestinal permeability, and that liver might be damaged by bacteria and its toxins contained in the portal flow in patients with NAFLD. Based on this, it could be hypothesized that those hormones and pro-inflammatory cytokines induced by H. pylori may also gain into liver via portal vein and contribute to the pathogenesis of NAFLD (15). Additionally, researchers who reported the changes in homeostatic model assessment of IR, fatty liver index, and echographic liver pattern in patient with H. pylori had found that the metabolic profile improved after the treatment for H. pylori eradication (16). However, studies for an association between H. pylori infection and NAFLD had also produced contradictory results (17). Considering that both H. pylori infection and NAFLD were multifactorial pathogenesis, more data from multiple regions around the world were needed to explore the relationship between the both.
Previous studies usually used color Doppler ultrasound to diagnose NAFLD, and this qualitative diagnosis method might have artificial bias. To better understand the correlation between H. pylori infection and NAFLD, we used TE to evaluate the degree of liver adipose deposition while defining NAFLD by color ultrasound. In our study, the results showed that in men, the prevalence of NAFLD and FAP level in the H. pylori-positive groups were all higher than H. pylori-negative groups. In women, the H. pylori-positive rate in the NAFLD group was also higher than non-NAFLD group whereas there was no significant difference in the H. pylori-positive rate between groups of FAP ≥ 240 dB/m and <240 dB/m. According to multiple regression analysis, H. pylori was not a risk factor for NAFLD and FAP ≥ 240 dB/m in both men and women.
Our findings were consistent with some previous studies. Okushin et al. reported that no significant relationship between NAFLD and H. pylori seropositivity was found in a large Japanese cross-sectional study of 13,737 participants (18). Baeg et al. also failed to find a correlation between H. pylori and NAFLD by analyzing the data of 3,663 South Koreans (19). Even in studies that had positive results, the OR values were not significant (20–23). In our view, the results mentioned above may illustrate a problem, even if H. pylori infection does have an impact on the pathological development of NAFLD, its role may be limited, with relatively small weights compared with other risk factors of NAFLD.
Although we did not find positive correlation between H. pylori infection and NAFLD, H. pylori infection was found to be associated with high LSM value, suggesting that H. pylori infection may be a risk factor for increased liver stiffness. After further multivariate regression analysis stratified by age, sex, and dyslipidemia, the results revealed the association maintained in men, but not among women.
In 2009, Goo et al. reported that in the animal model of carbon tetrachloride (CCl4)-induced hepatic cirrhosis, significant increase in the fibrotic score and also in serum ALT and AST levels was shown in the CCl4+H. pylori group compared with that in the CCl4-treated group. In addition, immunohistochemical study against H. pylori shows positive antigen fragments in the liver of the infected group, suggested that H. pylori infection could be an important infectious factor contributing to the development of liver cirrhosis (24). Furthermore, liver fibrogenesis was due to an imbalance between fibrogenesis and fibrolysis, and hepatic stellate cells (HSCs) were the pivot in this process. The results of animal experiments by Ki et al. suggested that H. pylori endotoxins that transported in the portal vein to the liver, coupled with liver injury by CCl4, might accelerate hepatic fibrosis through increasing TGF-b1-inducing proinflammatory signaling mediated by ERK and NF-kB in HSCs (25). It had also been reported in humans supporting that H. pylori was associated with the progression of liver fibrosis. Polyzos et al. reported that thirteen adult patients with biopsy-proven non-alcoholic steatohepatitis underwent a 13C urea breath test, and the patients with H. pylori-positive received eradication therapy. The result shows that H. pylori eradication had no long-term effect on hepatic steatosis, but shows a trend toward improvement in NAFLD fibrosis score (26).
However, the studies mentioned above were either animal models or the sample size which was too small, and the animal models established by chemical drugs were also different from the natural course of H. pylori infection in general human population. Considering the difficulty of obtaining a large number of liver samples from the general population infected with H. pylori, it is feasible to obtain relevant epidemiological data first. Currently, there is a lack of large sample studies on the correlation between H. pylori with the progression of early liver fibrosis in the general population. Our results supported the positive correlation between H. pylori infection and liver fibrosis at the epidemiological level and suggested that H. pylori may have different pathogenic risks in liver fat deposition and liver fibrosis progression in men. It was particularly important to understand this, because for a series of pathological processes of NAFLD, fat deposition could be reversed. However, liver fibrosis was somewhat difficult to reverse, and its impact on the development of liver disease was more profound.
The limitation of this study was the lack of comparison of liver pathological findings. The definition of NAFLD was determined by ultrasonography but not by liver biopsy. However, to some extent, this was compensated by TE, which had a good consistency with liver biopsy. Second, our study was cross-sectional, lack of intervention comparison, and the results only reflected epidemiological links, but failed to draw pathological and clinical conclusions. Even though we have found that H. pylori infection may be associated with increased liver stiffness in men, considering the gender difference of this correlation, we could not distinguish whether this was directly related to the interaction between them, or only reflected the differences of social behavior and lifestyle between men and women. A well-designed prospective study is warranted to clarify whether there is a causative link between them in the future.
In conclusion, our study suggests that H. pylori infection is not significantly associated with NAFLD and elevated liver steatosis, whereas it may be the risk factor of elevated liver stiffness in men. If this correlation is confirmed at the pathophysiological level of clarify the causative link between them in the future, eradication of H. pylori may be considered in therapeutic strategies of the relevant male population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committees of the Sichuan Provincial People's Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YL and PS designed the study. YL and DL performed the data collection and analysis. YL and PS wrote the manuscript. YpL assisted in data collection and document writing. All authors read and approved the manuscript.
This work was supported by Key Research and Development Projects of the Ministry of Science and Technology, China, No. 2017YFC0113901.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
2. Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. (2012) 61:409–15. doi: 10.1136/gutjnl-2011-300342
3. Sookoian S, Pirola CJ. Nonalcoholic fatty liver disease and metabolic syndrome: Shared genetic basis of pathogenesis. Hepatology. (2016) 64:1417–20. doi: 10.1002/hep.28746
4. Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. (2011) 16:79–88. doi: 10.1111/j.1523-5378.2011.00822.x
5. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Manatsathit W, Jaruvongvanich V, Ungprasert P. Helicobacter pylori and Risk of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. J Clin Gastroenterol. (2018) 52:386–91. doi: 10.1097/MCG.0000000000000784
6. Zhou BG, Yang HJ, Xu W, Wang K, Guo P, Ai YW. Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Helicobacter. (2019) 24:e12576. doi: 10.1111/hel.12576
7. Yang WS, Va P, Bray F, Gao S, Gao J, Li HL, et al. The role of pre-existing diabetes mellitus on hepatocellular carcinoma occurrence and prognosis: a meta-analysis of prospective cohort studies. PLoS ONE. (2011) 6:e27326. doi: 10.1371/journal.pone.0027326
8. Ou X, Wang X, Wu X, Kong Y, Duan W, Zhou J, et al. [Comparison of FibroTouch and FibroScan for the assessment of fibrosis in chronic hepatitis B patients]. Zhonghua Gan Zang Bing Za Zhi. (2015) 23:103–6. doi: 10.3760/cma.j.issn.1007-3418.2015.02.006
9. European Association for the Study of the L. European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–1402. doi: 10.1016/j.jhep,0.2015.11.004
10. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
11. European Association for the Study of the Liver. Electronic address, e.e.e., and European Association for the Study of the L. EASL Clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. (2018) 69:154–181. doi: 10.1016/j.jhep.2018.03.018
12. Fan JG, Jia JD Li YM, Wang BY, Lu LG, Shi JP, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: Update 2010. J Dig Dis. (2011) 12:38–44. doi: 10.1111/j.1751-2980.2010.00476.x
13. Polyzos SA, Kountouras J, Papatheodorou A, Patsiaoura K, Katsiki E, Zafeiriadou E, et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. (2013) 62:121–6. doi: 10.1016/j.metabol.2012.06.007
14. Cindoruk M, Cirak MY, Unal S, Karakan T, Erkan G, Engin D, et al. Identification of Helicobacter species by 16S rDNA PCR and sequence analysis in human liver samples from patients with various etiologies of benign liver diseases. Eur J Gastroenterol Hepatol. (2008) 20:33–6. doi: 10.1097/MEG.0b013e3282efa4f2
15. Buzas GM. Helicobacter pylori and non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol. (2020) 66:267–79. doi: 10.23736/S1121-421X.20.02671-9
16. Abenavoli L, Milic N, Masarone M, Persico M. Association between non-alcoholic fatty liver disease, insulin resistance and Helicobacter pylori. Med Hypotheses. (2013) 81:913–5. doi: 10.1016/j.mehy.2013.08.011
17. Polyzos SA, Kountouras J. Helicobacter pylori infection and nonalcoholic fatty liver disease: Time for large clinical trials evaluating eradication therapy. Helicobacter. (2019) 24:e12588. doi: 10.1111/hel.12588
18. Okushin K, Takahashi Y, Yamamichi N, Shimamoto T, Enooku K, Fujinaga H, et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol. (2015) 15:25. doi: 10.1186/s12876-015-0247-9
19. Baeg MK, Yoon SK, Ko SH, Noh YS, Lee IS, Choi MG. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J Gastroenterol. (2016) 22:2592–600. doi: 10.3748/wjg.v22.i8.2592
20. Chen CX, Mao YS, Foster P, Zhu ZW, Du J, Guo CY. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. (2017) 42:295–301. doi: 10.1139/apnm-2016-0499
21. Kim TJ, Sinn DH, Min YW, Son HJ, Kim JJ, Chang Y, et al. A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J Gastroenterol. (2017) 52:1201–10. doi: 10.1007/s00535-017-1337-y
22. Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elhelaly R, Elzehery R, et al. Helicobacter pylori and non-alcoholic fatty liver disease: a new enigma? Helicobacter. (2018) 23:e12537. doi: 10.1111/hel.12537
23. Yu YY, Cai JT, Song ZY, Tong YL, Wang JH. The associations among Helicobacter pylori infection, white blood cell count and nonalcoholic fatty liver disease in a large Chinese population. Medicine (Baltimore). (2018) 97:e13271. doi: 10.1097/MD.0000000000013271
24. Goo MJ, Ki MR, Lee HR, Yang HJ, Yuan DW, Hong IH, et al. Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab Invest. (2009) 89:1291–303. doi: 10.1038/labinvest.2009.90
25. Ki MR, Goo MJ, Park JK, Hong IH, Ji AR, Han SY, et al. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-beta1-induced inflammatory signaling. Lab Invest. (2010) 90:1507–16. doi: 10.1038/labinvest.2010.109
26. Polyzos SA, Nikolopoulos P, Stogianni A, Romiopoulos I, Katsinelos P, Kountouras J. Effect of Helicobacter pylori eradication on hepatic steatosis, NAFLD fibrosis score and HSENSI in patients with nonalcoholic steatohepatitis: a MR imaging-based pilot open-label study. Arq Gastroenterol. (2014) 51:261–8. doi: 10.1590/s0004-28032014000300017
Keywords: Helicobacter pylori, non-alcoholic fatty liver disease, transient elastography, liver stiffness measurement, fat attenuation parameter
Citation: Liu Y, Li D, Liu Y and Shuai P (2021) Association Between Helicobacter Pylori Infection and Non-alcoholic Fatty Liver Disease, Hepatic Adipose Deposition and Stiffness in Southwest China. Front. Med. 8:764472. doi: 10.3389/fmed.2021.764472
Received: 08 October 2021; Accepted: 02 December 2021;
Published: 24 December 2021.
Edited by:
Mario Masarone, University of Salerno, ItalyReviewed by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyCopyright © 2021 Liu, Li, Liu and Shuai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Shuai, cGluZ3NoMjAxNEBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.