
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 13 October 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.757485
This article is part of the Research Topic Recent Updates in Advanced Gastrointestinal Endoscopy View all 25 articles
Treatment of mucosa-associated lymphoid tissue (MALT) lymphoma has recently received considerable attention. Here, we report a case of large esophageal MALT lymphoma that was successfully en bloc resected using endoscopic submucosal dissection (ESD). A 77-year-old woman was admitted to our hospital with progressive dysphagia for more than 2 months. Upper gastrointestinal endoscopy revealed a large rounded submucosal mass covered by normal mucosa, located at the lower esophagus. Endoscopic ultrasonography (EUS) showed a well-demarcated hypoechoic mass chiefly located in the esophageal wall, but the layers of the esophageal wall were not clear. ESD was performed for diagnostic and treatment purposes. No complications occurred during or after ESD. The resected specimen measured 4.3 cm × 2.8 cm × 1.5 cm. The histologic findings were diagnostic of esophageal MALT lymphoma. Infiltration of neoplastic cells in the lateral margins of the resected specimen was not observed. However, vertical margins showed an R1 situation and mild damage to the muscularis propria. After 3 months, her dysphagia disappeared. Additional radiation therapy was then administered. After 5 months, the patient was still under surveillance and free of recurrent disease. Resection with ESD of such a large mass of MALT in the esophageal region has rarely been reported before in the literature.
Esophageal lymphoma is usually secondary to the metastasis of lymph nodes from the cervical and mediastinal region or local invasion from the stomach (1), and thus primary esophageal lymphoma (PEL) is extremely rare, which accounts for <1% of cases of all primary gastrointestinal lymphoma (2). The clinical manifestations of PEL are non-specific, which may vary based on symptoms such as dyspepsia, dysphagia, nausea, vomiting, abdominal distention, weight loss, fever, and even epigastric pain to massive hemorrhage (3). The pathological subtypes of PEL are mainly represented by diffuse large B cell lymphoma (DLBCL) and mucosa-associated lymphoid tissue (MALT) lymphoma, and other B, T, or NK cell lymphoma and Hodgkin lymphoma in a few cases (4, 5). MALT lymphoma has the highest incidence in patients aged between 50 and 60 years (6), but it was observed that the incidence increased significantly in patients older than 40 years (7). To date, a few cases of esophageal MALT lymphoma have been reported in the literature. Due to the rarity, no standard treatment of primary esophageal MALT lymphoma has been established and its prognosis is unclear.
Here, we report a case of primary large MALT lymphoma of the esophagus that was successfully en bloc resected by endoscopic submucosal dissection (ESD) and diagnosed.
A 77-year-old woman was attended to our hospital for evaluation of an esophageal submucosal tumor (SMT) and complained of progressive dysphagia for more than 2 months. She had first noted intermittent difficulty in swallowing solids 2 months before the visit. Her symptoms worsened progressively over the past 1 month with difficulty in swallowing both solids and liquids. She had no significant weight loss during this time period. Her past medical history included a right buccal mass that had undergone resection 1 year ago. She had no history of any immunosuppressive disease, alcohol abuse, or smoking. The findings of physical examinations showed no abnormalities, and the superficial lymph nodes, liver, and spleen were not palpable. Laboratory tests revealed normal levels of blood routine, liver function, kidney function, and blood electrolytes. No hepatitis B surface antigen and hepatitis C virus antibodies were detected. No elevation of tumor markers or autoimmune antibodies was observed. Upper gastrointestinal endoscopy revealed a large rounded esophageal submucosal mass covered by normal mucosa, located at the lower esophagus, 30–34 cm from the central incisors (Figure 1A). Endoscopic ultrasonography (EUS) showed a well-demarcated hypoechoic mass chiefly located in the esophageal wall (Figure 1B), clearly separated from the surrounding adventitia. Findings on chest contrast-enhanced computed tomography (CT) revealed a well-defined homogeneous mass in the lower esophageal region, with size 18 mm × 28 mm (Figures 2A,B). Despite her age, in consultation with the patient, we chose and performed an ESD by injection-and-cut technique to completely remove the large esophageal lesion to allow for accurate histological diagnosis.
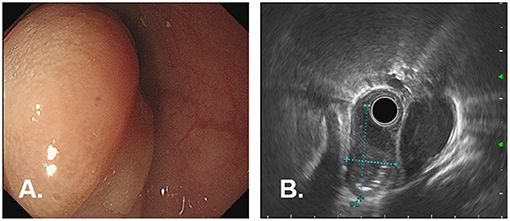
Figure 1. Endoscopic findings in a 77-year-old woman with dysphagia. (A) Upper gastrointestinal endoscopy showed a large, rounded mass with smooth normal overlying mucosa, which is seen extending longitudinally along the lower esophagus, 30–34 cm from the incisor teeth. (B) EUS shows a well-demarcated, hypoechoic mass in the esophagus wall, clearly margined from the surrounding adventitia.
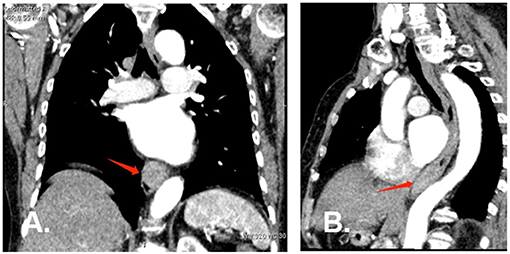
Figure 2. Chest contrast-enhanced computed tomography at diagnosis. (A) (Coronal plane) and (B) (Sagittal plane) CT scan revealed a well-defined homogeneous soft tissue mass at the lower esophagus (red arrow).
ESD was performed for diagnostic and treatment purposes. In this case, we used ESD-derived technique of submucosal tunneling endoscopic resection (STER). The submucosal injection was performed from the oral side at a distance of 3–5 cm from the tumor. Fluids were injected beneath the mucosa by a submucosal injection needle through the endoscopic channel to create a cushion. The fluid was a normal saline solution combined with 1:10,000 epinephrine and 1% methylene blue. A 2-cm longitudinal mucosal incision for a tunnel entry was made using a Dual knife. The submucosal layer was dissected using Dual knife and IT nano knife. Carbon dioxide was used for insufflation. After accomplishing the dissection, the lesion was removed using a basket and processed for histological evaluation. The dual knife was used for treating the possible vessels during the inspection. The mucosal incision site was closed with endoscopic clips (Figures 3A–D). The resected specimen measured 4.3 cm × 2.8 cm × 1.5 cm (Figures 3E,F).
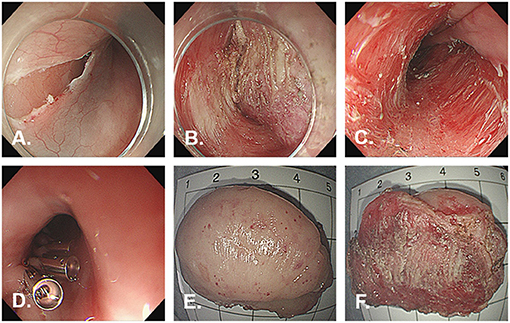
Figure 3. Images during endoscopic submucosal dissection. Endoscopic submucosal tunnel dissection is performed without any complication. (A) Initial incision of the mucosa after injection. (B) Exposure of the tumor. (C) The wound after ESD. (D) Complete closure of the mucosal incision site with endoclips. (E) On the external surface of the resected specimen. (F) On the cut surface of the resected specimen.
ESD was completed without any complications. A broad-spectrum antibiotic and proton pump inhibitor were administered intravenously for the next 3 days after the procedure. The patient was fasting and receiving fluid therapy for 3 days. She was discharged 5 days after the surgery, and an oral proton pump inhibitor was prescribed for the next 4 weeks. The histopathological findings of the resected specimen showed infiltration of small- to medium-sized lymphoid cells with slightly irregular dark nuclei and abundant cytoplasm (Figure 4A). Neoplastic cells infiltrated the lamina propria to the submucosal layers. Infiltration of neoplastic cells in the lateral margins of the resected specimen was not observed. However, vertical margins showed an R1 situation and mild damage to the muscularis propria. Immunohistochemical studies revealed that the lymphoid cells were positive for CD20 (Figure 4B), CD19 (Figure 4C), PAX5 (Figure 4D), as well as BCL2 (Figure 4E), and negative for CD3 (Figure 4F), CD5 (Figure 4G), CD10 (Figure 4H), and cyclin D1 (Figure 4I). The percentage of tumor cells positive for Ki-67-staining was <5%, indicating few mitotic cells. The diagnosis of esophageal MALT lymphoma was confirmed based on these pathological features. The patient was not tested for Helicobacter pylori during hospitalization. Therefore, she was not treated for Helicobacter pylori eradication. During the follow-up visit, the patient complained that her 13 C-urea breath test result was negative for Helicobacter pylori in the past.
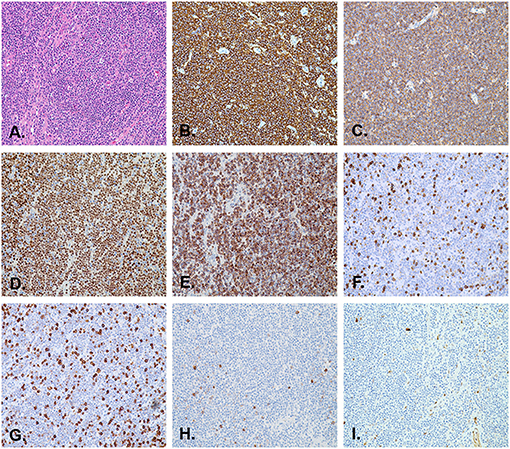
Figure 4. Pathological images of the resected specimen. (A) The histological section shows lymphoid hyperplasia in the lamina propria and submucosa (H&E stain, orig. mag. ×200). Immunohistochemistry revealed that the lymphoma cells are positive for CD20 (B), CD19 (C), PAX5 (D), and BCL2 (E), and negative for CD3 (F), CD5 (G), CD10 (H), and cyclin D1 (I) (orig. mag. ×200).
After 3 months, her dysphagia disappeared and a follow-up endoscopy showed no recurrence or complication at the ESD site, except for the presence of a scar. During the follow-up visit, the patient received additional radiation therapy according to the oncologist's suggestion. After 5 months, the patient was still under surveillance and free of recurrent disease.
Lymphoma arising in the esophagus is uncommon, accounting for <1% of patients with primary gastrointestinal lymphoma. Moriya et al. collected cases of PEL in stage I using the Lugano system staging and found that only 12 of the 37 cases (32.4%) were MALT lymphoma (8, 9). No finding from imaging was specific for the diagnosis of MALT lymphoma. Under barium swallow examination, PEL showed irregular filing defects due to segmental ulceration or narrowing and submucosal nodules which are similar to adenocarcinoma, esophageal varices, and achalasia (10, 11). CT findings show a thickened esophageal wall with a narrowed lumen that was not a target sign. PEL should be absent from cervical or mediastinal lymphadenopathy, so CT examination could exclude the involvement of lymph nodes in the cervical or mediastinal region (12). Endoscopic findings of PEL were variable and included submucosal nodular, polypoid growth, ulceration, and stenosis. EUS could detect structural changes of the digestive tract, which makes it valuable in assessing the depth of invasion, the extraluminal extent of the disease, or the extension in the lymph nodes. However, its findings were also non-specific, varying from anechoic, hypoechoic, or even hyperechoic masses (3, 13, 14).
MALT lymphoma appears in association with chronic inflammation induced by persistent infection and autoimmune diseases such as Helicobacter pylori (HP) infection and Hashimoto's thyroiditis, respectively (15, 16). Gastric MALT lymphomas without t (11; 18) translocation are well-known to be associated with HP infection, although there are very few cases of localized esophageal MALT lymphoma in HP-infected patients. Hence, HP infection was not a frequent marker for esophageal MALT lymphoma (17). Other factors such as the mechanical stimuli of food, hot water, chemicals in meals, other infections, and reflux esophagitis could also be involved in the development of primary esophageal MALT lymphoma. The underlying mechanisms of esophageal MALT lymphoma remained to be investigated.
The most frequent symptoms were dysphagia due to narrowing of the esophagus, epigastric pain, and weight loss. All of them were non-specific. Currently, because of the rarity of esophageal MALT lymphoma, a standard treatment for esophageal MALT lymphoma has not yet been established and recommended. Though radiotherapy is a treatment of choice for patients with large lesions in many other sites (18, 19), in esophageal MALT lymphoma, 64% of the cases were first treated with surgical resection or endoscopic resection (20, 21). In general, MALT lymphoma is not sensitive to chemotherapy, so chemotherapy is not recommended as the first-line treatment. In MALT lymphoma, the use of chemotherapy has been reserved for patients with disseminated disease or local treatment failure (22). ESD was confirmed as a useful therapeutic procedure for early gastric and esophageal cancers. The procedure also results in an improved differential diagnosis of malignant lymphoma occurring in digestive organs. To our knowledge, there have only been a few reports on ESD of early MALT lymphoma in the English literature (23–26). In the case reported here, ESD of the esophagus en bloc removed the large lesions and additional radiation was administered. If the MALT lymphoma is limited to the submucosa, ESD should be one of the most adequate and effective treatments. The indication of ESD for MALT lymphoma is limited to stage I, which means a tumor is located in the mucosa or submucosa without any lymphadenopathy. Compared to endoscopic mucosal resection (EMR), ESD has been shown to be superior because it is able to reach an en bloc resection and perform accurate histological examination (27). A pathological evaluation is extremely important in the diagnosis of lymphomas. The histological phenotype of a typical MALT lymphoma is positive for CD19, CD20, CD22, CD79a, and BCL2, and negative for CD3, CD5, CD10, CD23, and cyclin D1 (28–30).
In conclusion, although rare, primary esophageal MALT lymphoma should be considered in the differential diagnosis of esophageal SMT-like lesions. In addition, in cases in which the primary esophageal MALT lymphoma is confined to the deep mucosa and/or submucosa on EUS and the other sites are free of the disease, the lesion can be curatively removed by endoscopic resection. We report a case of successful ESD with primary esophageal MALT lymphoma. ESD may be a suitable and reasonable option as an attractive and less invasive local treatment for primary esophageal MALT lymphoma. The clinical profile of primary esophageal MALT lymphoma remains unclear, so it is important to accumulate more information on this rare entity.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YX and ML were involved in the study design and concept. YW was involved in data acquisition. JH revised the manuscript for critical intellectual content. All authors have read and approved the final manuscript and were involved in the review and editing of the manuscript.
This work was supported by the National Natural Science Foundation of China (NSFC 81700515).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors gratefully acknowledge all colleagues of the Department of Gastroenterology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, for helping with this study.
1. Ghai S, Pattison J, O'Malley ME, Khalili K, Stephens M. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics. (2007) 27:1371–88. doi: 10.1148/rg.275065151
2. Quiroga-Centeno AC, Bautista-Parada IR, Tapias LF, Gómez-Ochoa SA. Primary esophageal non-Hodgkin's lymphoma: demographics, clinical characteristics, histopathologic types, and survival in 179 patients from the SEER program and systematic review of the literature. Esophagus. (2021) 18:734–42. doi: 10.1007/s10388-021-00842-0
3. Zhu Q, Xu B, Xu K, Li J, Jin XL. Primary non-Hodgkin's lymphoma in the esophagus. J Dig Dis. (2008) 9:241–4. doi: 10.1111/j.1751-2980.2008.00354.x
4. Howell JM, Auer-Grzesiak I, Zhang J, Andrews CN, Stewart D, Urbanski SJ. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol. (2012) 26:452–6. doi: 10.1155/2012/480160
5. Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. (2003) 97:2462–73. doi: 10.1002/cncr.11415
6. Fischbach W, Kestel W, Kirchner T, Mossne J, Wilms K. Malignant lymphomas of the upper gastrointestinal tract. Results of a prospective study in 103 patients. Cancer. (1992) 70:1075–80. doi: 10.1002/1097-0142(19920901)70:5<1075::aid-cncr2820700511>3.0.co;2-1
7. Ge Z, Liu Z, Hu X. Anatomic distribution, clinical features, and survival data of 87 cases primary gastrointestinal lymphoma. World J Surg Oncol. (2016) 14:85. doi: 10.1186/s12957-016-0821-9
8. Moriya K, Tamura H, Nakamura K, Hosone M, Inokuchi K. A primary esophageal MALT lymphoma patient with Helicobacter pylori infection achieved complete remission after H. pylori eradication without anti-lymphoma treatment. Leuk Res Rep. (2016) 7:2–5. doi: 10.1016/j.lrr.2016.12.001
9. Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. (1994) 5:397–400. doi: 10.1093/oxfordjournals.annonc.a058869
10. Carnovale RL, Goldstein HM, Zornoza J, Dodd GD. Radiologic manifestations of esophageal lymphoma. AJR Am J Roentgenol. (1977) 128:751–4. doi: 10.2214/ajr.128.5.751
11. Coppens E, El Nakadi I, Nagy N, Zalcman M. Primary Hodgkin's lymphoma of the esophagus. AJR Am J Roentgenol. (2003) 180:1335–7. doi: 10.2214/ajr.180.5.1801335
12. Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. (2011) 17:697–707. doi: 10.3748/wjg.v17.i6.697
13. Ghimire P, Wu GY, Zhu L. Primary esophageal lymphoma in immunocompetent patients: two case reports and literature review. World J Radiol. (2010) 2:334–8. doi: 10.4329/wjr.v2.i8.334
14. Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract. J Dig Dis. (2015) 16:169–76. doi: 10.1111/1751-2980.12234
15. Alpen B, Neubauer A, Dierlamm J, Marynen P, Thiede C, Bayerdörfer E, et al. Translocation t(11;18) absent in early gastric marginal zone B-cell lymphoma of MALT type responding to eradication of Helicobacter pylori infection. Blood. (2000) 95:4014–5. doi: 10.1182/blood.V95.12.4014
16. Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. (2001) 357:39–40. doi: 10.1016/S0140-6736(00)03571-6
17. Weston AP, Cherian R, Horvat RT, Lawrinenko V, Dixon A, McGregor D, et al. Mucosa-associated lymphoid tissue (MALT) in Barrett's esophagus: prospective evaluation and association with gastric MALT, MALT lymphoma, and Helicobacter pylori. Am J Gastroenterol. (1997) 92:800–4.
18. Sugimoto M, Kajimura M, Shirai N, Furuta T, Kanaoka S, Ikuma M, et al. Outcome of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Intern Med. (2006) 45:405–9. doi: 10.2169/internalmedicine.45.1473
19. Tomita N, Kodaira T, Tachibana H, Nakamura T, Mizoguchi N, Takada A. Favorable outcomes of radiotherapy for early-stage mucosa-associated lymphoid tissue lymphoma. Radiother Oncol. (2009) 90:231–5. doi: 10.1016/j.radonc.2008.12.004
20. Hosaka S, Nakamura N, Akamatsu T, Fujisawa T, Ogiwara Y, Kiyosawa K, et al. A case of primary low grade mucosa associated lymphoid tissue (MALT) lymphoma of the oesophagus. Gut. (2002) 51:281–4. doi: 10.1136/gut.51.2.281
21. Yano S, Usui N, Dobashi N, Yahagi Y, Takahara S, Sugiyama K, et al. A case of primary esophageal mucosa-associated lymphoid tissue lymphoma with a numerical abnormality of 18q21 detected by fluorescence in situ hybridization. Ann Hematol. (2009) 88:703–4. doi: 10.1007/s00277-008-0653-y
22. Raderer M, Paul de Boer J. Role of chemotherapy in gastric MALT lymphoma, diffuse large B-cell lymphoma and other lymphomas. Best Pract Res Clin Gastroenterol. (2010) 24:19–26. doi: 10.1016/j.bpg.2009.11.001
23. Kudo K, Ota M, Narumiya K, Shirai Y, Ohki T, Yamamoto M. Primary esophageal mucosa-associated lymphoid tissue lymphoma treated by endoscopic submucosal dissection. Dig Endosc. (2014) 26:478–81. doi: 10.1111/den.12138
24. Kobayashi S, Iwamuro M, Nishida K, Tanaka T, Kawano S, Kawahara Y, et al. Primary localized esophageal mucosa-associated lymphoid tissue lymphoma treated by endoscopic submucosal dissection. Intern Med. (2018) 57:2347–52. doi: 10.2169/internalmedicine.0487-17
25. Baek DH, Kim GH, Song GA, Ryu KD, Cho EJ, Shin MJ, et al. Primary esophageal mucosa-associated lymphoid tissue lymphoma treated with endoscopic resection. Gastrointest Endosc. (2012) 75:1282–3. doi: 10.1016/j.gie.2011.06.003
26. Ling T, Min H, Zou X. A rare esophageal neoplasm. Gastroenterology. (2014) 147:e8–9. doi: 10.1053/j.gastro.2014.05.038
27. Ahmed Y, Othman M. EMR/ESD: techniques, complications, and evidence. Curr Gastroenterol Rep. (2020) 22:39. doi: 10.1007/s11894-020-00777-z
28. Piña-Oviedo S, Weissferdt A, Kalhor N, Moran CA. Primary pulmonary lymphomas. Adv Anat Pathol. (2015) 22:355–75. doi: 10.1097/PAP.0000000000000090
29. Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. (2002) 20:750–62. doi: 10.1183/09031936.02.00404102
Keywords: esophagus, mucosa-associated lymphoid tissue lymphoma, endoscopic submucosal dissection, pathology, treatment
Citation: Xia Y, Wang Y, Han J and Liu M (2021) En Bloc Resection of Primary Large Esophageal Mucosa-Associated Lymphoid Tissue Lymphoma by Endoscopic Submucosal Dissection: A Case Report. Front. Med. 8:757485. doi: 10.3389/fmed.2021.757485
Received: 12 August 2021; Accepted: 09 September 2021;
Published: 13 October 2021.
Edited by:
Abhilash Perisetti, University of Arkansas for Medical Sciences, United StatesReviewed by:
Abu Baker Sheikh, University of New Mexico, United StatesCopyright © 2021 Xia, Wang, Han and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujia Xia, eGlheWFyZW5AMTI2LmNvbQ==; Mei Liu, ZmxpdW1laUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.