- Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Vaccination is one of the cornerstones in the efforts towards ending the coronavirus disease 2019 (COVID-19) pandemic. However, several adverse effects of COVID-19 vaccination have been identified. Pityriasis rosea (PR)-like eruption is a rare cutaneous complication of immunization. To the best of our knowledge, there have been no reports of PR-like eruptions following inoculation with Oxford/AstraZeneca ChAdOx1 nCoV-19 (AZD1222) vaccine. Here, we described a case of PR-like eruption that developed 14 days after Oxford/AstraZeneca vaccination in a 52-year-old Thai woman with glioblastoma. Treatment with topical 0.1% triamcinolone acetonide twice per day showed partial response after seven days. Despite this rare complication, our report highlights that the presence of PR-like eruption is not a contraindication for subsequent vaccinations.
Introduction
Pityriasis rosea (PR)-like eruption is a cutaneous complication associated with several medications and vaccinations. Due to the rising incidence of coronavirus disease 2019 (COVID-19) worldwide, mass vaccination has been the cornerstone in preventing viral spread and decreasing morbidity and mortality. However, adverse effects to COVID-19 vaccines, particularly cutaneous reactions, have been reported. Here, we describe a case of PR-like eruption following ChAdOx1 nCoV-19 (AZD1222) vaccination in a Thai patient with glioblastoma.
Case Description
A 52-year-old Thai woman with a one-year history of recurrent glioblastoma was admitted to our hospital for a planned targeted therapy. Following the national COVID-19 vaccination guidelines, she received a shot of ChAdOx1 nCoV-19 (AZD1222) vaccine before the therapy. However, after 14 days, she developed multiple discrete blanching erythematous oval patches and plaques on the lower abdomen, back, and bilateral upper thighs (Figures 1A,B). Physical examination revealed that lesions were distributed along cleavage lines, similar to PR, without herald patch or prominent scales (Figure 1C). Moreover, central dusky-red erythematous was observed on some plaques (Figure 2A). Few brownish patches also appeared, suggestive of post-inflammatory hyperpigmentation. Unfortunately, due to her deteriorating neurologic status, her associated symptoms were not evaluated properly. Nevertheless, she did not present with fever or respiratory tract symptoms. In addition to COVID-19 vaccination, she was currently taking medications (i.e., levetiracetam and thyroxin) for glioblastoma, and the two drugs had been prescribed for approximately nine months.
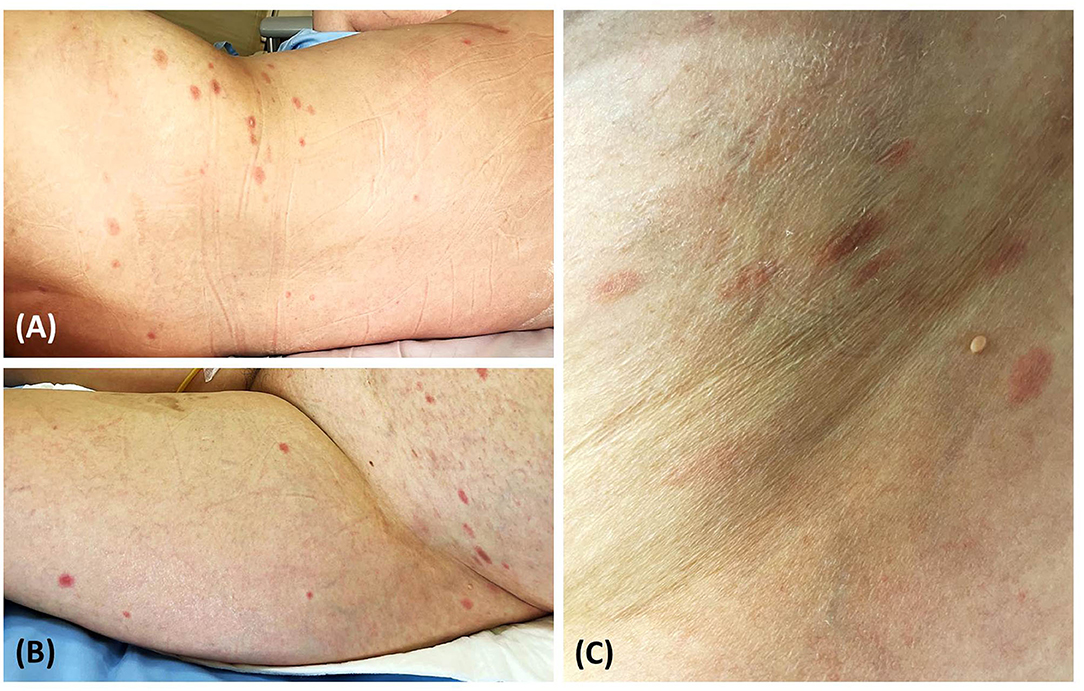
Figure 1. Pityriasis rosea-like eruption presenting as multiple discrete erythematous oval patches and plaques on the back (A) lower abdomen, and bilateral upper thighs (B); lesions distributed along cleavage lines without prominent scales (C).
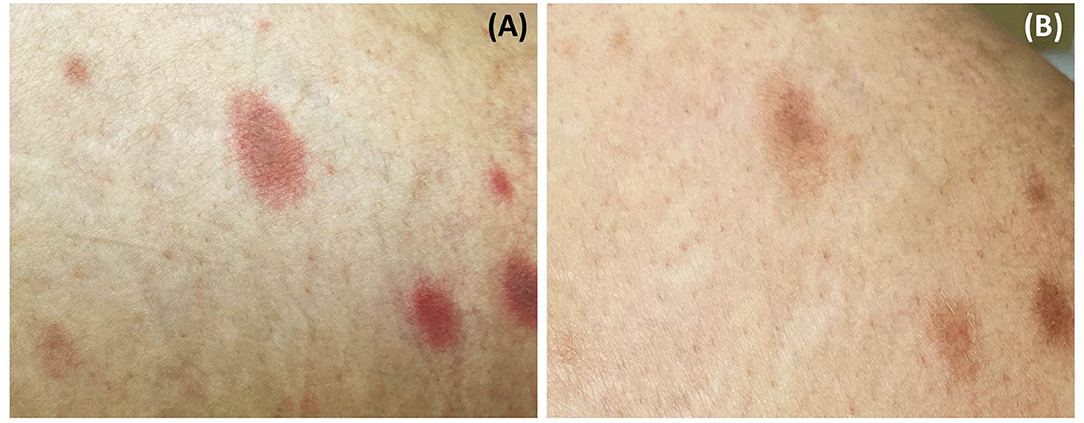
Figure 2. Central dusky-red erythematous on some plaques (A); almost all lesions fading with associated post-inflammatory hyperpigmentation after treatment (B).
Laboratory investigation results (i.e., complete blood count, blood chemistry, C-reactive protein level, erythrocyte sedimentation rate, serological test for syphilis, plasma polymerase chain reaction (PCR) for human herpesvirus (HHV)-6 and HHV-7, and reverse transcription-PCR for COVID-19) were unremarkable. Dermoscopy showed dotted and linear vessels forming a network on a yellowish background, with some whitish peripheral scales (Figure 3A). Histopathologic examination revealed epidermal focal spongiosis, interface dermatitis with necrotic keratinocytes, perivascular lymphocytic infiltration, and few eosinophilic infiltrations in the superficial dermis (Figures 3B,C). These findings were consistent with a diagnosis of PR-like eruption. Due to the temporal relationship between vaccination and onset of rash, COVID-19 vaccination was the most likely etiology. Treatment with topical 0.1% triamcinolone acetonide twice daily showed partial response after seven days. Moreover, almost all lesions faded with associated post-inflammatory hyperpigmentation (Figure 2B).
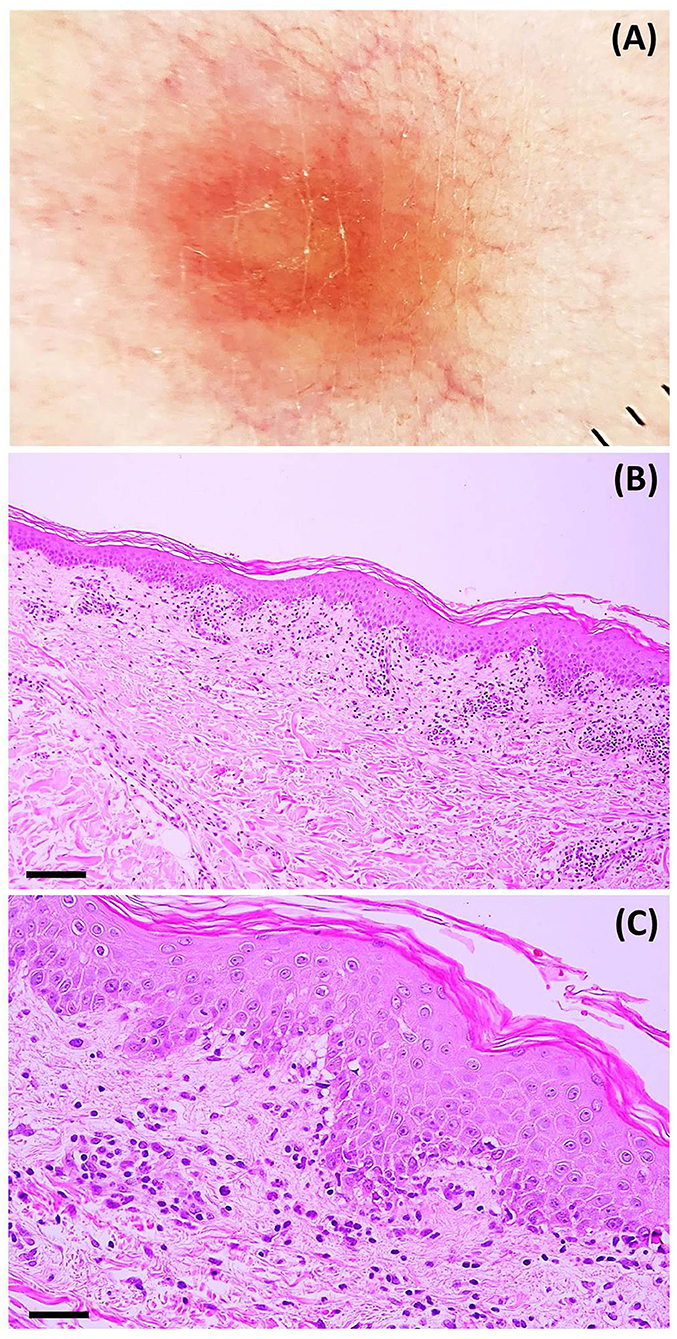
Figure 3. (A) Dermoscopic features: dotted and linear vessels forming a network on a yellowish background with some whitish peripheral scales (original magnification x20). Histopathologic features: (B) microscopic image at a low power field showing psoriasiform epidermal hyperplasia, perivascular infiltration, and interface dermatitis (hematoxylin-eosin; scale bar represents 100 μm); (C) microscopic image at a high power field showing focal spongiotic dermatitis, interface dermatitis, and perivascular infiltration of lymphocytes and eosinophils (hematoxylin-eosin; scale bar represents 40 μm).
Discussion
PR is a common self-limited papulosquamous eruption typically presenting with a large solitary pink-colored patch or plaque (i.e., herald patch), followed by secondary eruptions of smaller erythematous patches or plaques with associated peripheral collarette scaling distributed along the cleavage lines of the trunk, forming a Christmas tree pattern (5). However, in cases of atypical PR, it may present with erythema multiforme-like lesions and purpura (5).
Although the exact pathogenesis of PR remains unknown, the endogenous systemic reactivation of HHV (i.e., type 6 and 7) in the skin has been implicated as a precipitating factor. Among the common cutaneous manifestations of COVID-19 (e.g., morbilliform eruptions, chilblains, and varicella-like vesicles), PR and PR-like eruptions have rarely been described (6, 7). The mechanisms behind these COVID-19-related symptoms remain unclear. Some studies have identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike proteins on endothelial cells and lymphocytes from PR-like lesions, consistent with direct viral invasion (3, 4). Moreover, COVID-19 may solely induce human herpesvirus reactivation through the immunomodulatory ability of SARS-CoV-2 to reactivate latent viruses (8, 9).
Drug-induced PR-like eruption accounts for 2% of all cutaneous adverse drug reactions (10). Common causes of drug-induced PR-like eruptions include angiotensin-converting enzyme inhibitors, non-steroidal anti-inflammatory drugs, and gold. It often presents with diffuse and confluent severely pruritic dusky-red erythematous lesions in the absence of herald patch (11). Involvement of the oral mucosa has also been reported in as many as 50% of all cases (11). Although histopathologic characteristics of PR are also observed in PR-like lesions, the presence of dyskeratotic keratinocytes, interface dermatitis, and eosinophilic infiltration in the dermis may be more prominent in PR-like eruptions (11). Moreover, while dermoscopy may facilitate the diagnosis of PR, its utility for atypical PR or PR-like lesions is still limited.
Vaccinations have been identified to rarely cause several cutaneous reactions. The most commonly inoculated vaccines include those against smallpox, followed by tuberculosis, poliomyelitis, influenza, and human papillomavirus virus (12, 13). Among patients who received COVID-19 vaccines, PR-like eruptions have been reported following the first (n = 1) or second dose (n = 2) of BNT162b2 mRNA-based vaccines (3, 4). In some cases, central hyperpigmentation was identified. Nevertheless, the incidence of skin reactions after mRNA vaccinations was low. In a registry-based study, PR-like eruption was observed in only 1–2% of patients (14).
To the best of our knowledge, only one case of ChAdOx1 nCoV-19 vaccine-induced PR-like eruption has been reported (2). The patient developed papulovesicular lesions in the crease lines of the trunk and extremities, four days after inoculation with a recombinant coronavirus vaccine (Covishield™, India). Among patients who received inactivated vaccines, another case of PR-like eruption was reported four days after the first dose of CoronaVac® (SinoVac) (1). Here, we report the first case of PR-like eruption following ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccination. Table 1 summarizes all reported cases of PR-like eruption following COVID-19 vaccination. Although the exact pathophysiologic mechanisms of PR after vaccination remain unclear, immune responses to COVID-19 vaccines and disruption of T-cell mediated control of latent infections were implicated (12, 15–18). Other plausible theories include molecular mimicry with viral epitopes and type IV delayed hypersensitivity reaction (12).
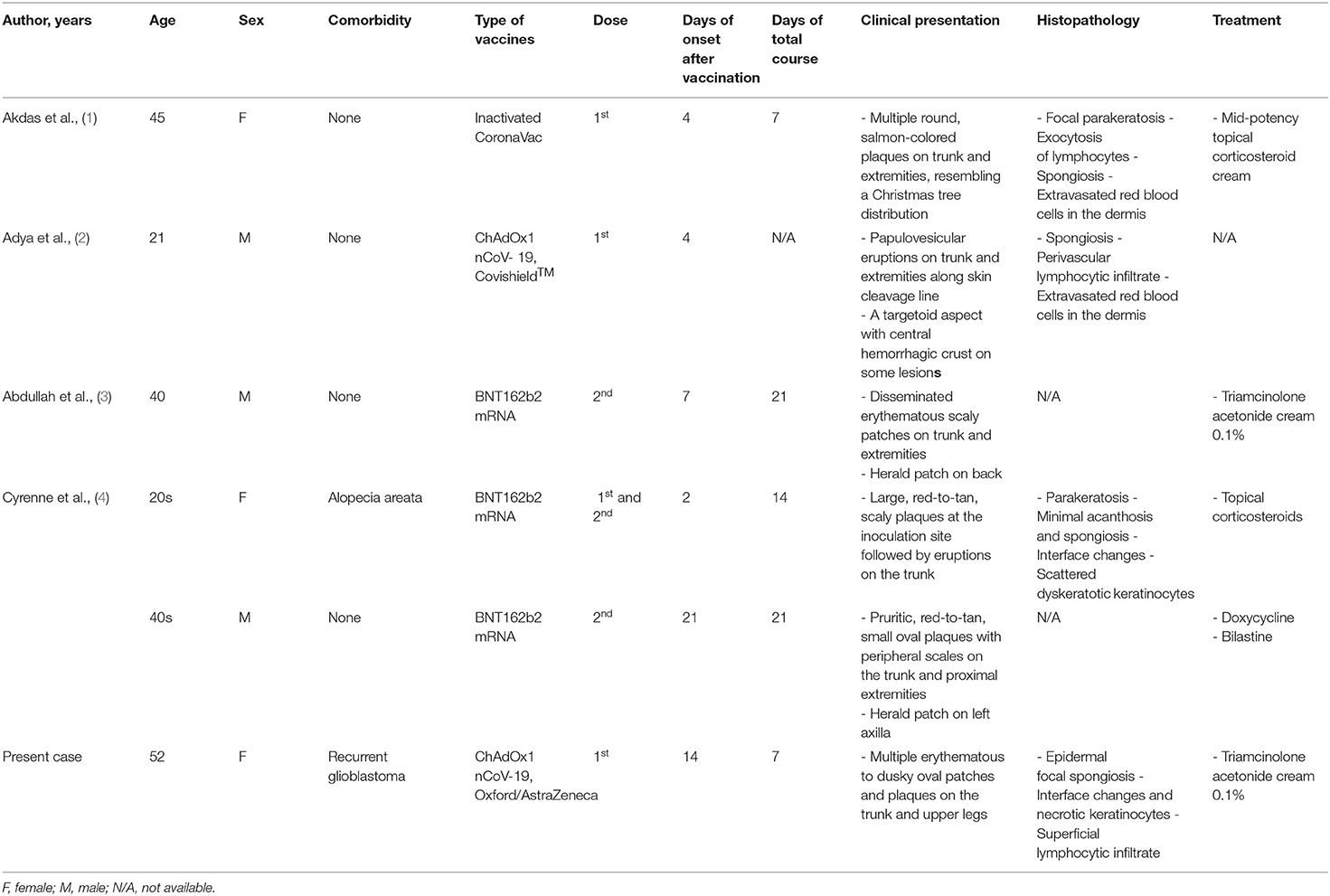
Table 1. Clinical manifestations and histopathologic findings of reported patients with pityriasis-rosea like eruptions following COVID-19 vaccination.
In conclusion, we have described the first case of PR-like eruption following ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccination. Fortunately, PR-like eruption is responsive to topical corticosteroids without any systemic adverse effects. Despite this rare complication, our report highlights that the presence of PR-like eruption is not a contraindication for subsequent vaccinations. With COVID-19 vaccines being authorized for emergency use, monitoring for potential adverse effects (e.g., cutaneous reactions) is necessary.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KL drafted the original manuscript. KL and KP collated resources. PS conceptualized the case report and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Akdas E, Ilter N, Ogut B, Erdem O. Pityriasis rosea following CoronaVac COVID-19 vaccination: a case report. J Eur Acad Dermatol Venereol. (2021) 35:e491–3. doi: 10.1111/jdv.17316
2. Adya KA, Inamadar AC, Albadri W. Post Covid-19 vaccination papulovesicular pityriasis rosea-like eruption in a young male. Dermatol Ther. (2021) p. e15040. doi: 10.1111/dth.15040
3. Abdullah L, Hasbani D, Kurban M, Abbas O. Pityriasis rosea after mRNA COVID-19 vaccination. Int J Dermatol. (2021) 60:1150–1. doi: 10.1111/ijd.15700
4. Cyrenne BM, Al-Mohammedi F, DeKoven JG, Alhusayen R. Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. (2021) 35:e546–8. doi: 10.1111/jdv.17342
5. Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. (2017) 5:203–11. doi: 10.12998/wjcc.v5.i6.203
6. Suchonwanit P, Leerunyakul K, Kositkuljorn C. Diagnostic and prognostic values of cutaneous manifestations in COVID-19. Dermatol Ther. (2020) 33:e13650. doi: 10.1111/dth.13650
7. Suchonwanit P, Leerunyakul K, Kositkuljorn C. Cutaneous manifestations in COVID-19: Lessons learned from current evidence. J Am Acad Dermatol. (2020) 83:e57–60. doi: 10.1016/j.jaad.2020.04.094
8. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus-6,−7, and Epstein-Barr virus reactivation in pityriasis rosea during COVID-19. J Med Virol. (2021) 93:1850–1. doi: 10.1002/jmv.26549
9. Ciccarese G, Parodi A, Drago F. SARS-CoV-2 as possible inducer of viral reactivations. Dermatol Ther. (2020) 33:e13878. doi: 10.1111/dth.13878
10. Atzori L, Pinna AL, Ferreli C, Aste N. Pityriasis rosea-like adverse reaction: review of the literature and experience of an Italian drug-surveillance center. Dermatol Online J. (2006) 12:1. doi: 10.5070/D380D8301D
11. Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: How to distinguish them? JAAD Case Rep. (2018) 4:800–1. doi: 10.1016/j.jdcr.2018.04.002
12. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. (2016) 30:544–5. doi: 10.1111/jdv.12942
13. Suchonwanit P, Triamchaisri S, Wittayakornrerk S, Rattanakaemakorn P. Leprosy Reaction in Thai Population: A 20-Year Retrospective Study. Dermatol Res Pract. (2015) 2015:253154. doi: 10.1155/2015/253154
14. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. (2021) 85:46–55. doi: 10.1016/j.jaad.2021.03.092
15. Suchonwanit P, Rojhirunsakool S, Khunkhet S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med Sci. (2019) 34:1857–64. doi: 10.1007/s10103-019-02783-8
16. Sriphojanart T, Khunkhet S, Suchonwanit P. A retrospective comparative study of the efficacy and safety of two regimens of diphenylcyclopropenone in the treatment of recalcitrant alopecia areata. Dermatol Reports. (2017) 9:7399. doi: 10.4081/dr.2017.7399
17. Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. (2016) 55:e338–43. doi: 10.1111/ijd.13061
18. Chanprapaph K, Pomsoong C, Tankunakorn J, Eden C, Suchonwanit P, Rutnin S. Comparative analyses of clinical features, histopathology, and CD123 immunohistochemistry of oral lupus erythematosus, lichen planus, and other lichenoid lesions. Dermatology. (2021). doi: 10.1159/000517971. [Epub ahead of print].
Keywords: adverse effect, coronavirus, cutaneous eruptions, immunization, pityriasis rosea, SARS-CoV-2, vaccine, case report
Citation: Leerunyakul K, Pakornphadungsit K and Suchonwanit P (2021) Case Report: Pityriasis Rosea-Like Eruption Following COVID-19 Vaccination. Front. Med. 8:752443. doi: 10.3389/fmed.2021.752443
Received: 03 August 2021; Accepted: 17 August 2021;
Published: 07 September 2021.
Edited by:
Angelo Valerio Marzano, University of Milan, ItalyReviewed by:
Giulia Ciccarese, San Martino Hospital (IRCCS), ItalyHiroki Hashimoto, Kyusyu University, Japan
Copyright © 2021 Leerunyakul, Pakornphadungsit and Suchonwanit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Poonkiat Suchonwanit, cG9vbmtpYXRAaG90bWFpbC5jb20=
†ORCID: Kanchana Leerunyakul orcid.org/0000-0002-0669-5611
Kallapan Pakornphadungsit orcid.org/0000-0003-4783-0282
Poonkiat Suchonwanit orcid.org/0000-0001-9723-0563
 Kanchana Leerunyakul
Kanchana Leerunyakul Kallapan Pakornphadungsit
Kallapan Pakornphadungsit Poonkiat Suchonwanit
Poonkiat Suchonwanit