
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 01 November 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.750378
This article is part of the Research Topic Insights in Coronavirus Disease (COVID-19) - Surveillance, Prevention and Treatment View all 84 articles
Background: Persistent coronavirus disease 2019 (COVID-19) symptoms are increasingly well-reported in cohort studies and case series. Given the spread of the pandemic, number of individuals suffering from persistent symptoms, termed ‘long COVID', are significant. However, type and prevalence of symptoms are not well reported using systematic literature reviews.
Objectives: In this scoping review of the literature, we aggregated type and prevalence of symptoms in people with long COVID.
Eligibility Criteria: Original investigations concerning the name and prevalence of symptoms were considered in participants ≥4-weeks post-infection.
Sources of Evidence: Four electronic databases [Medline, Web of Science, Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL)] were searched.
Methods: A scoping review was conducted using the Arksey and O'Malley framework. Review selection and characterisation was performed by three independent reviewers using pretested forms.
Results: Authors reviewed 2,711 titles and abstracts for inclusion with 152 selected for full-text review. 102 articles were subsequently removed as this did not meet inclusion criteria. Thus, fifty studies were analysed, 34 of which were described as cohort studies or prospective cohort studies, 14 were described as cross-sectional studies, one was described as a case control study, and one was described as a retrospective observational study. In total, >100 symptoms were identified and there was considerable heterogeneity in symptom prevalence and setting of study. Ten studies reported cardiovascular symptoms, four examined pulmonary symptoms, 25 reported respiratory symptoms, 24 reported pain-related symptoms, 21 reported fatigue, 16 reported general infection symptoms, 10 reported symptoms of psychological disorders, nine reported cognitive impairment, 31 reported a sensory impairment, seven reported a dermatological complaint, 11 reported a functional impairment, and 18 reported a symptom which did not fit into any of the above categories.
Conclusion: Most studies report symptoms analogous to those apparent in acute COVID-19 infection (i.e., sensory impairment and respiratory symptoms). Yet, our data suggest a larger spectrum of symptoms, evidenced by >100 reported symptoms. Symptom prevalence varied significantly and was not explained by data collection approaches, study design or other methodological approaches, and may be related to unknown cohort-specific factors.
An unprecedented surge in research following the onset of the severe acute respiratory syndrome coronavirus (SARS-CoV)-2 [also termed Coronavirus-19 (COVID-19)] pandemic means that, despite being a relatively new condition, much is now known about acute COVID-19 presentation and management (1–9). However, as the pandemic developed, it became clear that a significant proportion of patients experienced symptoms which persisted beyond the initial viral infection. Named initially by patients themselves (10), the term long COVID has become the most commonly used phrase to describe the condition and broadly describes individuals who have recovered from acute COVID-19, but experience symptoms which are persistent or very slow to resolve (11). These individuals manage with severe and debilitating symptoms, which are often cyclical in nature with periods of remission, followed by periods of extreme symptom exacerbation (12). Moreover, because long COVID symptoms develop after the viral infection, there have been several calls to redefine recovery from COVID-19 infection as requiring more than the absence of active infection (13). A further complication is that not only are long COVID symptoms disparate from acute COVID-19 symptoms, their severity is unrelated to initial acute infection severity (14).
Long COVID symptoms are not well described, partly because this requires longitudinal tracking of individuals, and the emergence of such evidence will naturally be delayed compared to those of acute symptoms. Nevertheless, some relatively common symptoms have emerged, with effects of long COVID reported to include cardiovascular (15), pulmonary (16), and respiratory symptoms (17, 18), pain of several anatomical locations (17, 19–23), fatigue (24–26), general infection symptoms [e.g., nausea (19), diarrhoea (27), fever (28), etc.], psychological disorders (29), cognitive impairment (30), sensory impairment (31), dermatological complaints (32), and functional impairment (33). Indeed, one of the remarkable aspects of the condition is the wide variety of symptoms associated with it. Furthermore, the prevalence with which different physiological systems are involved appears to vary considerably. For example, prevalence of fatigue in people with long COVID ranges between 53% in Italy (24) and 98% in the UK (34). This divergence may be in part due to study design. For example, if an investigation is conducted in a smell and taste clinic, soon after acute COVID-19 recovery, it is likely a large proportion of participants will present with dysnosmia or dysgeusia [e.g., (35); 100% of participants]. Conversely, if an investigation includes all those recovered from acute COVID-19, months after acute COVID-19 recovery, prevalence of sensory impairment will be significantly less [e.g., (17); 11% of participants]. However, this has not been extensively examined in systematic reviews of the literature to date and therefore warrants further investigation. The two systematic reviews that exist to our knowledge (36, 37) report considerable divergence in results despite similar objectives. Indeed, Iqbal et al. (36) identify multiple flaws in data capture and interpretation, and thus urge caution in application of the meta-analytical findings.
A comprehensive review of long COVID symptoms is important for clinicians to ensure they can support individuals with appropriate care and prescription. As such, it seemed pragmatic to conduct a scoping review in this area to map existing literature in terms of the volume, nature, and characteristics of the primary research (38). We used a scoping review rather than systematic review and meta-analysis because 1) our aim was to characterise symptoms of long COVID as reported in the available literature, rather than pose a specific and focused research question (39), and 2) the wide variations in study designs, inclusion criteria, and sampling meant effective pooling of data was unlikely to be feasible (40). A comprehensive review of long COVID symptoms is an essential tool to guide clinical decision making. However, a standard systematic review requires a strong understanding of the area to which specific research questions can be addressed. Given reports of broad heterogeneity in symptoms, severity, and prevalence, and that clear diagnostic criteria for long COVID are not yet established, our understanding of the development and symptoms of long COVID is not sufficient to develop such a question. As such, a traditional systematic review and meta-analysis would have been premature. Consequently, we elected to undertake a scoping review as the current state of the literature was relatively unknown in terms of methodologies and data reporting. This approach retains the systematic approach to literature searching but aims to map out a new and rapidly developing area where a consensus of findings may be unlikely (39). Using the framework of Arksey and O'Malley, a scoping review aims to use a broad set of search terms and include a wide range of study designs and methods [in contrast to a systematic review (38)]. This approach, however, has the benefit of clarifying key concepts, surveying current data collection approaches, and identifying critical knowledge gaps.
We aimed to provide an overview of existing literature concerning long COVID symptoms. Our three specific objectives of this scoping review were to 1) conduct a systematic search of the published literature concerning long COVID symptoms and their prevalence, 2) map characteristics and methodologies used, and 3) provide recommendations for the advancement of the investigative area.
The review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) guidelines (41) and the five-stage framework outlined in Arksey and O'Malley (38). A review protocol was not published.
Studies that met the following criteria were included: (1) involvement of human participants; (2) not a review; (3) an investigation which considered participants ≥4-weeks after acute COVID-19 infection (COVID-19 rapid guideline: managing the long-term effects of COVID-19; NICE); (4) employed a study design which was not a case study or case series; (5) published in English; (6) including outcome measures related to (i) symptoms, and (ii) symptom prevalence.
The search strategy consisted of a combination of free-text and MeSH terms relating to persistent symptoms following COVID infection which were developed through examination of published original literature and review articles. Example search terms for PubMed included: (COVID or COVID-19 OR Sars-Cov-2) AND (long COVID OR persistent symptoms OR post-acute OR post-viral).
Four electronic databases [Medline, Web of Science, Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL)] were searched to identify original research articles published from the earliest available date up until 5th February 2021. Additional records were identified through reading included studies.
Data were extracted by three reviewers (LH, JI, and NS) independently and compared in an unblinded and standardised manner. Once each database search was completed and manuscripts sourced, studies were downloaded into a single reference list with duplicates removed. Titles and abstracts were then screened for eligibility and full texts were only retrieved for studies with symptom prevalence incorporated. Full texts were then assessed using the complete eligibility criteria with all authors confirming inclusion and exclusion. Following this assessment, the same reviewers read the studies and assessed the following: design method, participant characteristics, setting, study duration, and symptoms. Descriptions were extracted with as much detail provided by the authors. Any uncertainty by reviewers was discussed in consensus meetings and resolved by agreement. Data extracted from each study included sample size, group descriptions, study design, and outcome data. The primary outcome variables were defined as symptom type and symptom prevalence.
After the initial database search, 2,852 records were identified (Figure 1). Once duplicates were removed, 2,711 titles and abstracts remained, and titles and abstracts were screened for inclusion resulting in 152 full-text articles being sourced and screened. Of these, 102 were excluded and 50 remained.
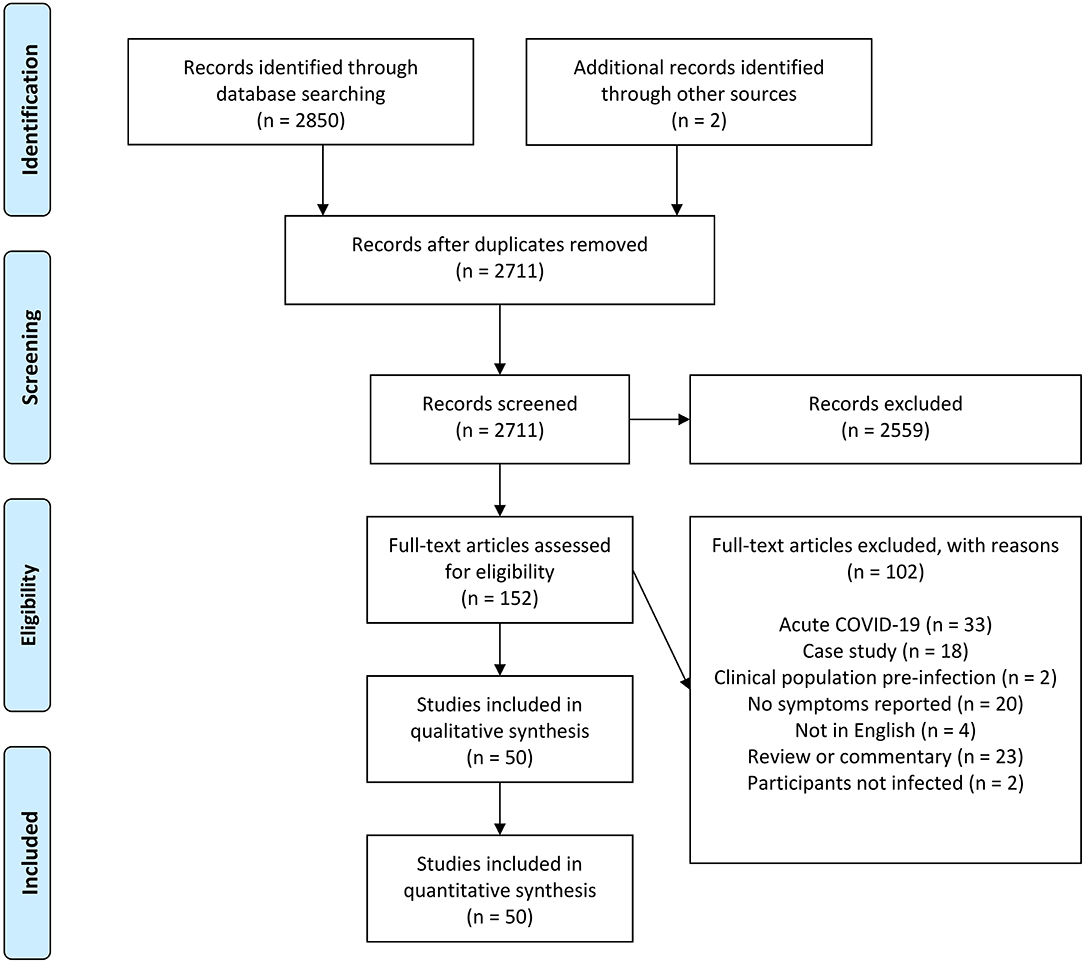
Figure 1. Schematic flow diagram describing exclusions of potential studies and final number of studies.
Of the 50 studies included, 34 were described as cohort studies or prospective cohort studies, 14 were described as cross-sectional studies, one was described as a case control study, and one was described as a retrospective observational study (Table 1). Where a study had multiple symptoms described, they were extracted separately and grouped by symptom, rather than study (Table 2).

Table 2. Summary of long COVID symptoms, grouped by category, with prevalence reported in each study and studies listed in order of prevalence for each symptom.
In total, 108 distinct symptoms were described by authors of the original articles, despite us grouping taste dysfunction, smell dysnfunction, and breathing problems together into three categories. There were 10 studies which reported cardiovascular symptoms, four which examined pulmonary symptoms, 25 which reported respiratory symptoms, 24 which reported pain-related symptoms, 21 which reported on fatigue of some description, 16 which reported general infection symptoms, 10 which reported symptoms of psychological disorders, 9 which reported cognitive impairment, 31 which reported a sensory impairment, seven which reported a dermatological complaint, 11 which reported a functional impairment, and 18 which reported a symptom which did not fit into any of the above categories.
Dyspnoea/breathlessness/shortness of breath/breathing problems (all one category) was the most reported symptom (27 cohorts), with smell dysfunction (26 cohort) second, fatigue/tiredness second (24 cohorts) third. Symptom prevalence varied significantly between studies, often from <10 to >70% (e.g., dyspnoea/breathlessness/shortness of breath/breathing problems, cough, sore throat, chest pain, headache, joint pain/arthralgia, pain or discomfort, fatigue, fever, neurocognitive impairment, smell dysfunction, and taste dysfunction).
Of the 50 studies, 37 were from Europe, four from North America, six from Asia, one from South America, one from Africa, and one where the location was unclear. Of the 37 studies from Europe, ten were conducted in Italy, five in France, five in Spain, five in the UK, two in the Netherlands, two in the Netherlands and Belgium, two in Germany, two in Greece, one in Austria, one in Denmark, one in Norway, and one in Ireland.
Of the 50 studies, 27 concerned hospitalised individuals only, 13 were in both hospitalised and non-hospitalised combined, and three were in only non-hospitalised participants. The remaining studies were unclear as to whether participants were included or excluded based on whether they were hospitalised. Of the 27 studies concerning exclusively hospitalised participants, five exclusively studied participants from the ICU only, three were conducted in participants from the general ward only, and 19 that were explicitly in both ICU or general ward patients or were hospitalised but unclear whether to the ICU or general ward. For clarity, two studies had two cohorts (19, 32), and have been considered as individual data sets.
This scoping review examined the range of outcomes from studies pertaining to long COVID symptoms, aligned to our primary aim. Firstly, >100 symptoms have been reported by original investigations, which emphasises the diverse nature of long COVID. Secondly, the volume of articles published from 2020 onwards speaks to this rapidly emerging area of research. This review catalogues existing symptom literature, with a view to aiding physicians and healthcare practitioners better understand the range and prevalence of symptoms of long COVID. Moreover, we believe this information can facilitate discussion of research opportunities and issues that need to be addressed in future studies.
Results of this review support recent observations that long COVID can result in a wide variety of symptoms. From the studies included in this review, we identified more than 100 symptoms. A recent report by Davis et al. (73) similarly detailed over 200 symptoms in an international cohort of long COVID patients. The difference between their data and ours being largely explained by our grouping of similar classifications of symptoms (e.g., we grouped dyspnoea, shortness of breath, breathlessness as a single category). Nevertheless, this work supports the growing view that long COVID is typified by a disparate array of symptoms, across multiple physiological systems, and may often result in individuals experiencing their own idiosyncratic manifestation of the condition.
Unsurprisingly symptoms associated with acute COVID-19 infection appear most frequently in the literature, include sensory alterations, respiratory symptoms, chest pain, headaches, and fever. However, because of their association with acute infection (72, 74), it is difficult to determine the degree to which they occur in long COVID. Indeed, it is reasonable to assume that most studies designed their surveys to reflect acute symptoms. Thus, even though these categories are most commonly associated with long COVID, this may be due, in part at least, to them being the symptoms about which researchers most frequently enquired. Conversely, although not as commonly reported as acute symptoms, this review identified other common symptoms of long COVID, which are less closely aligned to acute COVID-19 infection. These include cognitive impairments, fatigue, neuralgia and myalgic pain, sleep difficulties, mobility impairments, and psychological symptoms (e.g., anxiety and depression). These findings support previous research reports (11, 56, 75), and case studies (76, 77) from which the defining characteristics of long COVID have emerged. It also supports prior work suggesting long COVID is a distinct condition rather than slowly resolving acute COVID-19 and associated symptoms (78).
It was noted prevalence of symptoms displayed considerable divergence between investigations. A plausible a priori hypothesis would have been that heterogeneity in symptoms may be due to differences in study protocols or data collection methods (e.g., such as whether inclusion criteria required a prior confirmed COVID-19 test). However, there was limited evidence to support this view. For a variety of symptoms including dyspnoea, cough, sore throat, chest pain, headache, fatigue, and diarrhoea there was no clear pattern that explained observed heterogeneity. Studies reporting a high prevalence included online surveys of individuals self-reporting as having persistent symptoms (19), studies using in person evaluation of only those with a positive COVID-19 test (28) and studies using online surveys of both suspected and confirmed COVID-19 cases (21). Similarly, those reporting low prevalence also included self-reported COVID-19 infection (44) and those with positive PCR tests. Neither were there clear differences in duration of follow-up with similar follow up durations utilised in investigations reporting high [e.g., 74 days (21) −4 months (28)] and low [e.g., 60 days (44) −6 months (31)] prevalence. Taken together, these data suggests that, in these symptoms at least, long COVID is an inherently variable condition. While some symptoms are commonly considered to be associated with the condition (such as fatigue), they are by no means ubiquitous among patients. Practitioners should be aware of the idiosyncratic symptoms and experiences of people with the condition, which in turn will likely require personalised rehabilitation strategies. As an exemplar to emphasise this point, diarrhoea prevalence is a prime example of homogeneity in study characteristics yet heterogeneity in results. The greatest prevalence was reported by Dennis et al. [(28); 59%], and the lowest by Bellan et al. [(29); 1%]. These studies have similar samples sizes (n = 201 vs. n = 238), similar follow-up durations (3 vs. 4 months), similar study design (cohort), both studies considered only confirmed cases of COVID-19, and both studies considered hospitalised participants [Bellan et al (29) considered exclusively hospitalised patients whereas Dennis et al. (28) considered both hospitalised and non-hospitalised] individuals. Both studies were robust in research design, with few difference in methodology, yet divergence in prevalence of diarrhoea was reported.
In addition, it is also worth noting there was no discernible pattern concerning participants who had a confirmed COVID-19 infection vs. those with suspected COVID-19 (but who may not have been tested at the time of infection). This finding is supported by studies which have specifically investigated confirmed vs. suspected cases [e.g., Meys et al. (21)]. Consequently, it may not be necessary in future studies to have a positive COVID-19 test as an inclusion criterion, since the symptom range (and variation) appears to be similar in both confirmed and suspected cases. This is particularly useful finding for researchers and patient groups given that, particularly early in the pandemic, testing was unlikely to have taken place, despite obvious acute symptoms. A caveat to this suggestion is that although this was applicable to those infected with COVID-19 in 2020/2021, this may not be true for 2021/2022 when other viruses (e.g., influenza) may be circulating to a greater extent in the population the addition of a positive test as an inclusion criteiron may be necessary to exclude other potential causes of post-viral symptoms.
Whilst considerable variance between studies was evident, variation within each study in terms of its prevalence rank in Table 2 was small. By this, we mean prevalence rates may be related to some unknown, cohort-specific factor as whole study cohorts were relatively consistent when studies were ranked by their reported symptom prevalence. For example, for the 15 symptoms they have reported, Dennis et al. (28) had the greatest prevalence in eight categories and were in the top three for the remainder. Similarly, Goertz et al. (19) reported on two cohorts (hospitalised and non-hospitalised), and frequently report some of the highest incidence rates for the symptoms they assessed. Conversely, Bellan et al. (29) reported 14 symptoms and for 10 of those they consistently report one of, or the, lowest prevalence rates, (and for two of the remainder they are the only reporting study, so comparisons are unfeasible). It is difficult to speculate from the available data what specific factors are explanatory in this context. Some potential factors include differences in geographic location, treatment algorithms, cohort profile (e.g., existing co-morbidities). However, further longitudinal studies will be required to provide a more comprehensive assessment of risk factors for long COVID.
In relation to our second objective, studies included were mostly cohort studies or cross sectional studies, which are both observational studies (79). We chose to report study design as reported by the authors of the original investigation but often these studies utilised the same research design in that several individuals who had recovered from acute COVID-19 were contacted and asked for their symptom at that time point. Thus, we would suggest that in many cases, authors who defined their study as ‘cross sectional' had actually conducted retrospective cohort studies (79).
Follow-up periods ranged from 4 weeks to approximately 6 months. As mentioned previously, it would have been reasonable to speculate a priori that this influenced symptom prevalence as some symptoms may have been evident at 4 weeks but resolved by 6 months. However, follow-up period had little effect on prevalence differences between studies. Conversely, within each cohort, following participants for a greater time course may have influence within study prevalence rates. This is an inherent problem with a single follow-up point, which several of the cohort studies in this review utilised. It is possible that some research groups involved in the articles included in this review will have research projects ongoing, continually detailing symptoms which would permit dissemination of information concerning time course of symptom resolution.
Studies were conducted worldwide (Europe; n = 37, North America; n = 4, Asia; n = 6, South America; n = 1, Africa; n = 1, location unclear; n = 1). Whilst the geographical location of investigations conducted may be of surprise to some because COVID-19 originated in China and therefore the healthcare system of China had greatest potential for follow-up duration, Europe has to date experienced the most absolute number of confirmed cases. This was likely the fact the Chinese government implemented more drastic lockdown measures than did European governments. This likely attenuated virus transmission and is evidenced by China having ~95,000 confirmed cases at the time of writing, whilst the UK has had >7.3 million confirmed cases.
Sample sizes ranged from 12 to 1,939, with 16 studies having n <100. This supports our rationale to scope the literate rather than to meta-analyse the field, as we feel reporting prevalence as a percentage of 19 individuals is not epidemiologically valid [e.g., Woo et al. (30)]. As mentioned previously, it would have been reasonable to speculate a priori that this influenced symptom prevalence but as mentioned above, this was not the case.
In relation to our third objective, we believe the investigative area concerning long COVID could be improved by greater methodological detail. As evidenced from Table 1, we were often unable to extract details concerning methods utilised which may have influenced results, and thus interpretations. For example, given the known effect of chronological age on acute COVID-19 severity (1), we believe this information should be present in methods of articles included in this review, although this was not always the case. On the topic of age, there is now some emerging evidence that children may experience similar long-term effects to adults after COVID-19 infection (80–83). Whilst we did not specifically exclude studies on the basis of age, it is evident from Table 1 that few studies were conducted in children. Thus, long COVID in children may be an area for further exploration.
To improve the investigative area in the future, serial longitudinal follow-ups within each cohort would allow for information around time course of symptoms. We believe this would assist physicians better understand the prevalence of symptoms at each relevant time point (e.g., whether sensory dysregulation is typically present at 1 month, 2 months, or 3 months post-acute COVID-19 recovery). However, this may be labour-intensive so remote symptom tracking using mobile technology may prove advantageous in this context. This would alleviate resource commitments associated with data collection but may result in greater time and expense concerning data management and analysis. Finally, and most importantly, precision of reporting follow-up timing, prevalence of comorbidities, and setting (i.e., outpatients' clinic, smell and taste clinic) would all enhance the existing literature base.
In conclusion, this review catalogued the range and prevalence of symptoms of long COVID. We report the most reported symptoms fell into categories of sensory, respiratory, pain, and fatigue respectively. Prevalence of each symptom varied significantly, but unlikely because of study heterogeneity, and appeared to be related to unknown cohort-specific factors. By this, we mean that study design, participant age, study setting, participant sex, and follow-up duration did not appear to explain differences in symptom prevalence, but instead prevalence differed from one study to another, despite methodological similarities in some instances. It is expected that as the investigative area advances and more is known about the long COVID condition, a regression towards the mean will occur and a better knowledge of symptom prevalence will arise.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LH, JI, and NS: conceptualisation, methodology, formal analysis and investigation, writing—original draft preparation, writing—review and editing, visualisation, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the Chief Scientist Office (grant no COV/LTE/20/08).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Authors wish to acknowledge the support of respective employers in preparation of this review.
1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. (2020) 324:782–93. doi: 10.1001/jama.2020.12839
2. Coronavirus (COVID-19) events as they happen. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed June 10, 2021).
3. De Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS ONE. (2020) 15:e0239570. doi: 10.1371/journal.pone.0239570
4. Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. (2020) 12:135. doi: 10.3390/v12020135
5. Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. (2021) 1–11. doi: 10.1007/s00259-021-05215-4
6. Overview. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19 Guidance NICE. Available online at at: https://www.nice.org.uk/guidance/ng188 (accessed September 14, 2021).
7. V'kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. (2020) 19:155–70. doi: 10.1038/s41579-020-00468-6
8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
9. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
10. Why we need to keep using the patient made term “Long Covid ”. The BMJ. (2020). Available online at: https://blogs.bmj.com/bmj/2020/10/01/why-we-need-to-keep-using-the-patient-made-term-long-covid/ (accessed September 20, 2021).
11. Mendelson M, Nel J, Blumberg L, Madhi SA, Dryden M, Stevens W, et al. Long-COVID: An evolving problem with an extensive impact. Samj South Afr Med J. (2021) 111:10–2. doi: 10.7196/SAMJ.2020.v111i11.15433
12. Kingstone T, Taylor AK, O'Donnell CA, Atherton H, Blane DN, Chew-Graham CA. Finding the “right” GP: a qualitative study of the experiences of people with long-COVID. BJGP Open. (2020) 4:bjgpopen20X101143. doi: 10.3399/bjgpopen20X101143
13. Alwan NA. A negative COVID-19 test does not mean recovery. Nature. (2020) 584:170. doi: 10.1038/d41586-020-02335-z
14. Tenforde MW. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:993–8. doi: 10.15585/mmwr.mm6930e1
15. Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. (2021) 27:89–95. doi: 10.1016/j.cmi.2020.09.023
16. Machado FVC, Meys R, Delbressine JM, Vaes AW, Goërtz YMJ, van Herck M, et al. Construct validity of the Post-COVID-19 functional status scale in adult subjects with COVID-19. Health Qual Life Outcomes. (2021) 19:40. doi: 10.1186/s12955-021-01691-2
17. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
18. Mannan A, Mehedi HMH, Chy NUHA, Qayum MO, Akter F, Rob MA, et al. A multi-centre, cross-sectional study on coronavirus disease 2019 in Bangladesh: clinical epidemiology and short-term outcomes in recovered individuals. New Microbes New Infect. (2021) 40:100838. doi: 10.1016/j.nmni.2021.100838
19. Goertz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? Erj Open Research. (2020) 6. doi: 10.1183/23120541.00542-2020
20. Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, Nyirenda T, Friedman T, Gupta A, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE. (2020) 15:e0243882. doi: 10.1371/journal.pone.0243882
21. Meys R, Delbressine JM, Goërtz YMJ, Vaes AW, Machado FVC, Van Herck M, et al. Generic and respiratory-specific quality of life in non-hospitalized patients with COVID-19. J Clin Med. (2020) 9:3993. doi: 10.3390/jcm9123993
22. Tomasoni D, Bai F, Castoldi R, Barbanotti D, Falcinella C, Mulè G, et al. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J Med Virol. (2021) 93:1175–9. doi: 10.1002/jmv.26459
23. Trinkmann F, Müller M, Reif A, Kahn N, Kreuter M, Trudzinski F, et al. Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection. Eur Respir J. (2021) 57:2003002. doi: 10.1183/13993003.03002-2020
24. Carfì A, Bernabei R, Landi F Gemelli Against COVID-9 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. (2020) 324:603. doi: 10.1001/jama.2020.12603
25. Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth. (2020) 126:e110–3. doi: 10.1016/j.bja.2020.12.007
26. Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. (2020) 15:e0240784. doi: 10.1371/journal.pone.0240784
27. Boscolo-Rizzo P, Borsetto D, Fabbris C, Spinato G, Frezza D, Menegaldo A, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. (2020) 146:729–32. doi: 10.1001/jamaoto.2020.1379
28. Dennis A, Wamil M, Kapur S, Alberts J, Badley AD, Decker GA, et al. Multi-organ impairment in low-risk individuals with long COVID. medRxiv. (2020) 2020.10.14.20212555. doi: 10.1101/2020.10.14.20212555
29. Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. (2021) 4:e2036142. doi: 10.1001/jamanetworkopen.2020.36142
30. Woo MS, Malsy J, Pöttgen J, Seddiq Zai S, Ufer F, Hadjilaou A, et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. (2020) 2:fcaa205. doi: 10.1093/braincomms/fcaa205
31. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. (2020) 76:405–7. doi: 10.1136/thoraxjnl-2020-216377
32. Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. (2020) 81:E4–6. doi: 10.1016/j.jinf.2020.08.029
33. van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. (2020) 73:e1089–98. doi: 10.1093/cid/ciaa1750
34. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. (2020) 2020.10.19.20214494. doi: 10.1101/2020.10.19.20214494
35. Kandemirli SG, Altundag A, Yildirim D, Tekcan Sanli DE, Saatci O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad Radiol. (2021) 28:28–35. doi: 10.1016/j.acra.2020.10.006
36. Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. (2021) 36:100899. doi: 10.1016/j.eclinm.2021.100899
37. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. (2021) 11:16144. doi: 10.1038/s41598-021-95565-8
38. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
39. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
40. Mays N, Roberts E, Popay J. Synthesising research evidence. In: Fulop N, Allen P, Clarke A, Black N, editors. Studying the Organisation and Delivery of Health Services: Research Methods. London: Routledge (2001). p. 188–220.
41. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
42. Cai X, Hu X, Ekumi IO, Wang J, An Y, Li Z, et al. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am J Geriatr Psychiatry. (2020) 28:1030–9. doi: 10.1016/j.jagp.2020.07.003
43. Caronna E, Ballvé A, ó A, Gallardo VJ, Ariton DM, Lallana S, et al. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. (2020) 40:1410–21. doi: 10.1177/0333102420965157
44. Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. (2020) 27:258–63. doi: 10.1016/j.cmi.2020.09.052
45. Chiesa-Estomba CM, Lechien JR, Radulesco T, Michel J, Sowerby LJ, Hopkins C, et al. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol. (2020) 27:2318–21. doi: 10.1111/ene.14440
46. Curci C, Negrini F, Ferrillo M, Bergonzi R, Bonacci E, Camozzi DM, et al. Functional outcome after inpatient rehabilitation in post-intensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur J Phys Rehabil Med. (2021) 57:443–50. doi: 10.23736/S1973-9087.20.06660-5
47. Fjaeldstad AW. Prolonged complaints of chemosensory loss after COVID-19. Dan Med J. (2020) 67:A05200340.
48. Frija-Masson J, Debray M-P, Gilbert M, Lescure F-X, Travert F, Borie R, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. (2020) 56:2001754. doi: 10.1183/13993003.01754-2020
49. Galván-Tejada CE, Herrera-García CF, Godina-González S, Villagrana-Bañuelos KE, Amaro JDL, Herrera-García K, et al. Persistence of covid-19 symptoms after recovery in mexican population. Int J Environ Res Public Health. (2020) 17:1–12. doi: 10.3390/ijerph17249367
50. Hall J, Myall K, Lam JL, Mason T, Mukherjee B, West A, et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax. (2021) 76:408–11. doi: 10.1136/thoraxjnl-2020-215861
51. Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc Imaging. (2020) 13:2330–9. doi: 10.1016/j.jcmg.2020.05.004
52. Iannuzzi L, Salzo AE, Angarano G, Palmieri VO, Portincasa P, Saracino A, et al. Gaining back what is lost: recovering the sense of smell in mild to moderate patients after COVID-19. Chem Senses. (2020) 45:875–81. doi: 10.1093/chemse/bjaa066
53. Janiri D, Kotzalidis GD, Giuseppin G, Molinaro M, Modica M, Montanari S, et al. Psychological distress after Covid-19 recovery: reciprocal effects with temperament and emotional dysregulation. an exploratory study of patients over 60 years of age assessed in a post-acute care service. Front Psychiatry. (2020) 11:590135. doi: 10.3389/fpsyt.2020.590135
54. Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. (2020) 75:e13746. doi: 10.1111/ijcp.13746
55. Konstantinidis I, Delides A, Tsakiropoulou E, Maragoudakis P, Sapounas S, Tsiodras S. Short-term follow-up of self-isolated COVID-19 patients with smell and taste dysfunction in greece: two phenotypes of recovery. ORL J Otorhinolaryngol Relat Spec. (2020) 82:295–303. doi: 10.1159/000511436
56. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. (2020) 76:396–98. doi: 10.1136/thoraxjnl-2020-215818
57. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. (2020) 89:594–600. doi: 10.1016/j.bbi.2020.07.037
58. Méndez R, Balanzá-Martínez V, Luperdi SC, Estrada I, Latorre A, González-Jiménez P, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. (2021) 290:621–31. doi: 10.1111/joim.13262
59. Moreno-Pérez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a mediterranean cohort study. J Infect. (2021) 82:378–83. doi: 10.1016/j.jinf.2021.01.004
60. Munro KJ, Uus K, Almufarrij I, Chaudhuri N, Yioe V. Persistent self-reported changes in hearing and tinnitus in post-hospitalisation COVID-19 cases. Int J Audiol. (2020) 59:889–90. doi: 10.1080/14992027.2020.1798519
61. Niklassen AS, Draf J, Huart C, Hintschich C, Bocksberger S, Trecca EMC, et al. COVID-19: recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope. (2021) 131:1095–100. doi: 10.1002/lary.29383
62. Ortelli P, Ferrazzoli D, Sebastianelli L, Engl M, Romanello R, Nardone R, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci. (2021) 420:117271. doi: 10.1016/j.jns.2020.117271
63. Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME Á, Steig B, et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa1792. [Epub ahead of print].
64. Poncet-Megemont L, Paris P, Tronchere A, Salazard J-P, Pereira B, Dallel R, et al. High prevalence of headaches during Covid-19 infection: a retrospective cohort study. Headache. (2020) 60:2578–82. doi: 10.1111/head.13923
65. Printza A, Katotomichelakis M, Metallidis S, Panagopoulos P, Sarafidou A, Petrakis V, et al. The clinical course of smell and taste loss in COVID-19 hospitalized patients. Hippokratia. (2020) 24:66–71.
66. Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. (2021) 31:100683. doi: 10.1016/j.eclinm.2020.100683
67. Shah AS, Wong AW, Hague CJ, Murphy DT, Johnston JC, Ryerson CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. (2020) 76:402–4. doi: 10.1136/thoraxjnl-2020-216308
68. Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur Respir J. (2020) 57:2003481. doi: 10.1183/13993003.03481-2020
69. Taboada M, Cariñena A, Moreno E, Rodríguez N, Domínguez MJ, Casal A, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect. (2020) 82:e31–3. doi: 10.1016/j.jinf.2020.12.022
70. Yan CH, Prajapati DP, Ritter ML, DeConde AS. Persistent Smell Loss Following Undetectable SARS-CoV-2. Otolaryngol Head Neck Surg. (2020) 163:923–5. doi: 10.1177/0194599820934769
71. Zhao Y, Shang Y, Song W, Li Q, Xie H, Xu Q, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. (2020) 25:100463. doi: 10.1016/j.eclinm.2020.100463
72. Curci C, Pisano F, Bonacci E, Camozzi DM, Ceravolo C, Bergonzi R, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehabil Med. (2020) 56:633–41. doi: 10.23736/S1973-9087.20.06339-X
73. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021). doi: 10.1016/j.eclinm.2021.101019. [Epub ahead of print].
74. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
75. Vink M, Vink-Niese A. Could cognitive behavioural therapy be an effective treatment for long COVID and post COVID-19 fatigue syndrome? Lessons from the qure study for Q-Fever fatigue syndrome. Health Care. (2020) 8:552. doi: 10.3390/healthcare8040552
76. Garner P. Paul garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustion. BMJ. (2020). Available online at: https://blogs.bmj.com/bmj/2020/05/05/paul-garner-people-who-have-a-more-protracted-illness-need-help-to-understand-and-cope-with-the-constantly-shifting-bizarre-symptoms/
77. Lokugamage AU, Taylor S, Rayner C. Patients' experiences of “longcovid” are missing from the NHS narrative. BMJ Opinion. (2020). Available online at: https://blogs.bmj.com/bmj/2020/07/10/patients-experiences-of-longcovid-are-missing-from-the-nhs-narrative/
78. Baig AM. (2020). Chronic COVID Syndrome: Need for an appropriate medical terminology for Long-COVID and COVID Long-Haulers. J Med Virol. doi: 10.1002/jmv.26624
79. Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. (2003) 20:54–60. doi: 10.1136/emj.20.1.54
80. Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. (2021) 110:2208–11. doi: 10.1111/apa.15870
81. Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. (2021) 110:914–21. doi: 10.1111/apa.15673
82. Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. (2021) 2101341. doi: 10.1183/13993003.01341-2021
Keywords: coronavirus–COVID-19, COVID-19, long COVID, SARS-CoV-2, persistent, symptoms, post acute covid syndrome (PACS)
Citation: Hayes LD, Ingram J and Sculthorpe NF (2021) More Than 100 Persistent Symptoms of SARS-CoV-2 (Long COVID): A Scoping Review. Front. Med. 8:750378. doi: 10.3389/fmed.2021.750378
Received: 30 July 2021; Accepted: 05 October 2021;
Published: 01 November 2021.
Edited by:
Marc Jean Struelens, Université Libre de Bruxelles, BelgiumReviewed by:
Oana Sandulescu, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2021 Hayes, Ingram and Sculthorpe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lawrence D. Hayes, bGF3cmVuY2UuaGF5ZXNAdXdzLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.