- 1Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China
- 2Department of Ophthalmology, Tianjin Medical University General Hospital, Tianjin, China
Background: With the advent of aging society of China, fundus diseases related to pathological neovascularization, including age-related macular degeneration (AMD), diabetic macular edema (DME), and pathological myopia (PM), have become an increasingly serious medical and health problems. As effective drugs of the treatment, conbercept and ranibizumab have been commonly used and covered by the national basic medical insurance in China. However, the pharmacoeconomic evaluation of conbercept vs. ranibizumab for DME and PM remains lacking. This study would assess the cost-effectiveness of conbercept and ranibizumab for the treatment of AMD, DME, and PM from the perspective of Chinese payers.
Methods: A Markov chain model was constructed based on the visual conditions of the patient indicated by the number of letters in best corrected visual acuity (BCVA). We conducted models based on real-world scenario to calculate the cost per the quality-adjusted life-year (QALY) gained. A 1-year cycle length and a 10-year simulation treatment were applied and the number of injections of conbercept and ranibizumab was assumed to the average number within 10 years. Transition probabilities, costs, utility data, and other parameters were obtained from literature searches. A 3.5% discounting rate was applied for both the costs and utilities.
Results: The incremental cost-effectiveness ratios (ICERs) were more favorable for conbercept than ranibizumab in treatment of AMD, DME, and PM, with associated ICER of 66,669 renminbi (RMB), −258,813 RMB, and −373,185 RMB per QALY gained. Compared with ranibizumab, the incremental effectiveness of conbercept in treatment of AMD, DME, and PM was −0.665 QALYs, 0.215 QALYs, and 0.029 QALYs, respectively. The sensitivity analysis showed the same findings, although the ICER is sensitive to the costs of this program.
Conclusion: Under the current Chinese healthcare setting, conbercept is suitable and cost-effective in treatment of AMD, DME, and PM compared with ranibizumab.
Introduction
Age-related macular degeneration (AMD), a chronic macular disease that affects the central retina, is the third leading cause of irreversible blindness and the first cause of visual impairment in developed countries (1–3). The total number of patients with AMD was estimated to be 196 million in 2020 and might rise to 288 million by 2040 (4). Global burden of disease in 2010 showed that vision-related quality-adjusted life-years (QALYs) caused by AMD increased at an exponential rate of 160% (5). Diabetic retinopathy is the leading blinding eye disease for working-aged people and diabetic macular edema (DME) secondary to diabetic retinopathy is the direct cause of visual impairment (6). Epidemiological surveys in China show that the incidence of DME in diabetic patients is 5% (7, 8). Pathological myopia (PM) is one of the three most common causes of blindness in the world. As many as 3% of the population of the world suffer from PM, especially in Asian countries, and the global prevalence is generally increasing (9). Thus, the visual impairment caused by fundus diseases related to pathological neovascularization, including AMD, DME, and PM, has brought a heavy economic burden to the society.
In China, with the advent of an aging society, AMD, DME, and PM have become an increasingly serious medical and health problems, for which intravitreal injection of antivascular endothelial growth factor (VEGF) drugs is an effective method. Until now, the main anti-VEGF drugs in clinical use are ranibizumab, aflibercept, bevacizumab, and conbercept, which have been proven to be superior to conventional treatments (10).
Ranibizumab, the first drug proven to improve eyesight, has been confirmed that it has good effects through randomized controlled trials (RCTs) (11–13). Conbercept (Lumitin, Chengdu Kanghong Biotech Corporation Ltd., Chengdu, Sichuan, China), a genetically engineered fusion protein, is an anti-VEGF drug independently developed by China in the recent years. It can effectively combine with VEGF in blood vessels and tissues to treat a variety of eye diseases related to pathological angiogenesis (14). A recent systematic review demonstrated that conbercept had comparable safety and efficacy profiles rather than ranibizumab (15). Ranibizumab and conbercept have been commonly used and covered by the national basic medical insurance in China, so it is necessary to conduct an economic evaluation of these two drugs, which socioeconomic burden would be fueled soon for their high cost and widespread used. An economic evaluation has concluded previously that conbercept is more cost-effective than aflibercept and ranibizumab for the treatment of AMD in China, but only based on the 2-year medication situation in clinical scenarios (16). Even worse, there is still a lack of studies on the pharmacoeconomic evaluation between conbercept and ranibizumab treatment of DME and PM. Therefore, it is urgent to evaluate the cost-effectiveness between conbercept and ranibizumab in treatment of AMD, DME, and PM from the perspective of Chinese payers in order to optimally allocate and utilize the limited medical resources.
Methods
A Markov chain model, the simplest Markov model, was used to estimate the cost-effectiveness of conbercept and ranibizumab in treatment of AMD, DME, and PM. The principle is to simulate the development of the disease according to the transition probability of its natural process, divided into different states (Markov state) in a certain period of time (Markov cycle). Combined with the health utility value of the disease and the consumption of public health resources, the outcome and cost of disease progression were estimated through multiple iterations.
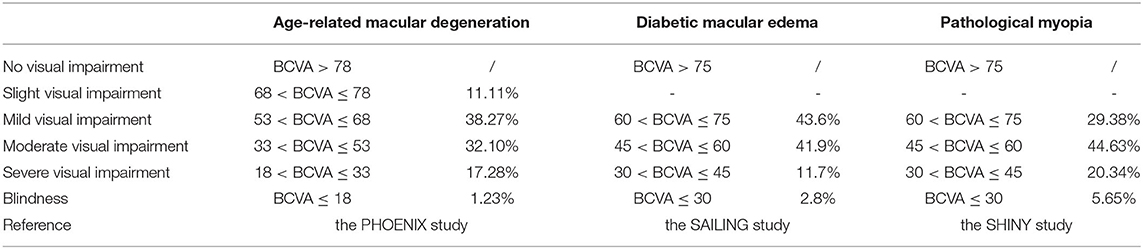
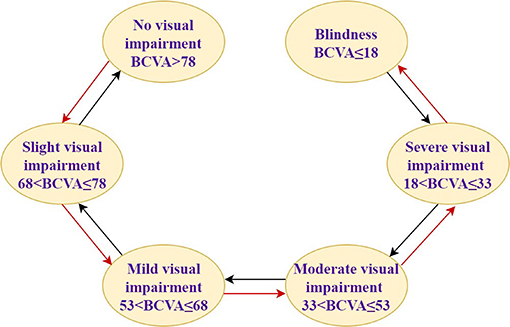
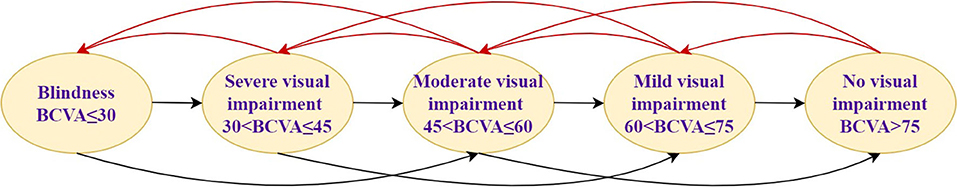
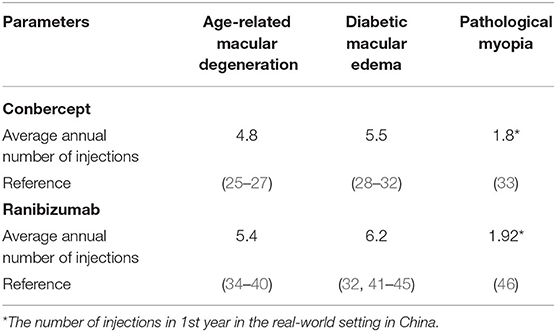
In this study, assuming that the simulated population is patients with AMD, DME, and PM, the baseline characteristics are consistent with that in PHOENIX (identifier NCT01436864), SAILING (identifier NCT02194634), and SHINY (identifier NCT01809223) studies, respectively (Appendix Part A). Health states were represented by different vision conditions of the patients, which were indicated by the number of letters in best corrected visual acuity (BCVA). There are six health states of AMD and five health states of the other two eye diseases. The specific health status classification and baseline visual acuity distribution of the patient are shown in Table 1. The baseline distribution of visual acuity among the patients with AMD, DME, and PM was derived from unpublished data in PHOENIX, SAILING, and SHINY studies, respectively. The average annual number of injections was used to simulate the injection situation within 10 years, assuming that remained the same. The number of injections for conbercept and ranibizumab was set according to the existing literature and research. The difference between the costs of the two drugs has remained approximately the same within 10 years due to focus on the incremental cost-effectiveness ratio (ICER) in this study, which is the ratio of incremental cost to incremental effectiveness. The ICER is used to evaluate the relative economics of two or more alternative treatment options. The Markov transition processes of the eye diseases were shown in Figures 1–3, respectively, and the arrows indicate whole possible transitions between the states 1 year later.
After a cycle, participants in one stage could stay in that stage or move onto the next stage with a certain transition probability. Suppose that after 1 year of the treatment, there were three types of therapeutic effects of AMD and DME: BCVA increased by 15 letters or more, BCVA remained unchanged, BCVA decreased by 10 letters or more, five types of therapeutic effects of PM: BCVA increased or decreased by 30 letters, BCVA increased or decreased by 15 letters, and BCVA remained unchanged. The model structure is shown in Figures 1–3.
Input Parameters
Transition Probabilities
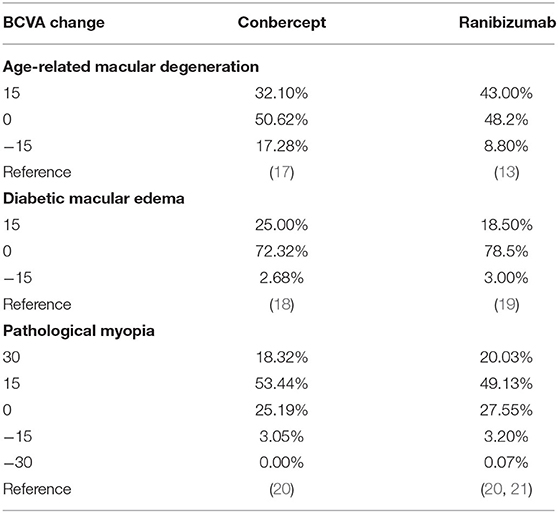
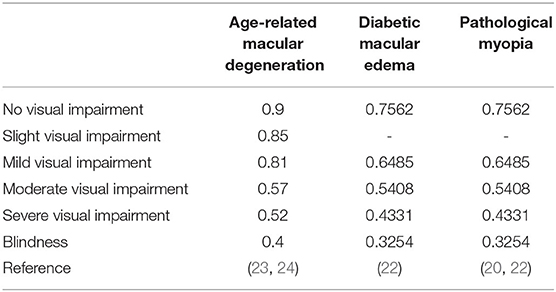
The conditional probability of a given stage moving to another stage after a certain time is expressed by transition probabilities. The metastasis probability table of AMD, DME, and PM is shown in Table 2, which represented the conditional probability of transition from a given stage to another stage after 1 year. The transition probability of this study was obtained from the related clinical trial literature to simulate the real-world treatment situation.
Utilities
The generic health status and health-related quality of life were expressed by health utility weights. According to how the individuals perceive his or her health status, health utility weights are valued between 0 and 1. Utility of 1 was considered as “perfect health,” while utility of 0 was considered as “death.” AMD, DME, and PM can be monitored by changes in visual acuity (VA) and VA results are presented using the logarithm of the minimum angle of resolution (logMAR) scale. A previous study performed the univariate ordinary least squares regression analyses to investigate the strength of the relationship between VA and the utility value obtained from time trade-off of the patients and found that the utility value has a linear relationship with VA, which manifested as the utility value equals 0.828–0.359 × logMAR (22). We used this formula to estimate the health utility value of DME and PM under different letter states due to the difficulty of obtaining them. Utility values of AMD obtained from the literature are included in Table 3.
Costs
In this study, costs for patient management were considered as direct medical costs (including drug costs, inspection costs, surgical costs, nursing costs, and treatment costs) for efficacy end point events. The prices of single injections of conbercept and ranibizumab were 5,550 RMB and 5,700 RMB, respectively, according to the National Reimbursement Drug List (NRDL) (2019). A 1-year drug treatment cycle was adopted in our analysis. The injections of conbercept and ranibizumab were used as average annual number within 10 years to investigate the influence of the variance on cost estimates. Both the cost and the health utility value used a discount rate of 3.5% (Table 4).
Sensitivity Analysis
Because the decisions informed by the model are affected by parameter uncertainty, it is necessary to carry out extensive sensitivity analyses including the fluctuation of drug prices and different numbers of injections to test the robustness of the results. First, we have compared the cost-effectiveness of treating AMD, DME, and PM when the prices of conbercept and ranibizumab fluctuate by 5 and 10%. Moreover, we have compared the cost-effectiveness of conbercept and ranibizumab at latest negotiated prices that are 4,160 RMB and 3,950 RMB, respectively, since January 1, 2020 to December 31, 2021, according to the NRDL (2020). Second, we have carried out one-way and two-way sensitivity analysis on the number of injections of conbercept and ranibizumab based on the number of drug injections in real world in the existing literatures. Third, we assumed that the cost obeyed the gamma distribution in probabilistic sensitivity analysis using Monte Carlo simulation (47). The cost-effectiveness acceptability curves of conbercept and ranibizumab in treatment of AMD, DME, and PM are shown in Appendix Part B. Finally, the number of injections in the real world is often smaller than that in RCT, so the cost-effectiveness analysis of different injections in RCT scenario is also part of the sensitivity analysis. The number of injections in RCT scenario was derived from previous studies (Table 5).
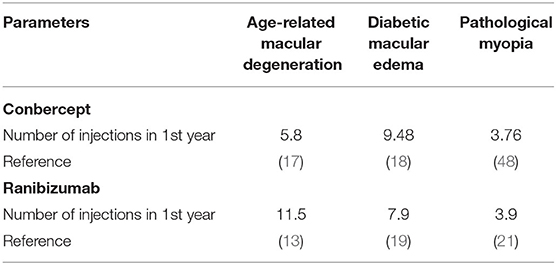
Table 5. Sensitivity analyses about number of injections of conbercept and ranibizumab in randomized controlled trial (RCT) scenario.
Willingness to Pay
In developing countries, 3-fold the per capita gross domestic product (GDP) is usually taken as the maximum WTP (33). In 2019, GDP per capita of China was 70,892 RMB. The maximum WTP for a QALY gained in this program of about 212,676 RMB was estimated based on the above approach.
Results
Cost-Effectiveness Comparison of Conbercept and Ranibizumab in Real-World Scenario
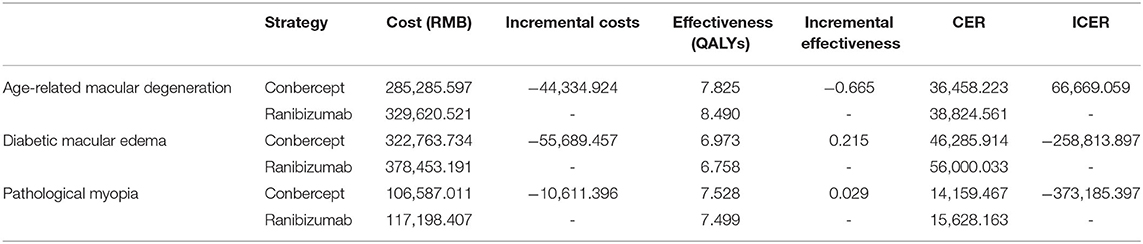
The costs of conbercept and ranibizumab for AMD, DME, and PM within 10 years in real-world scenario are shown in Table 6.
For ranibizumab, the costs were 329,620 RMB, 378,453 RMB, and 117,198 RMB in treatment of AMD, DME, and PM within 10 years, respectively, while the costs of conbercept were 285,285 RMB, 322,763 RMB, and 106,587 RMB, respectively. Compared with ranibizumab, the incremental costs of conbercept were −44,334 RMB, −55,689 RMB, and −10,611 RMB, respectively, and the ICER were 66,669 RMB, −258,813 RMB, and −373,185 RMB per QALY gained, less than the threshold of triple GDP per capita. Conbercept had significant cost-effectiveness in treatment of AMD, DME, and PM because ranibizumab was dominated by conbercept, meaning that it was more costly and less effective.
Cost-Effectiveness Comparison of Conbercept and Ranibizumab in RCT Scenario
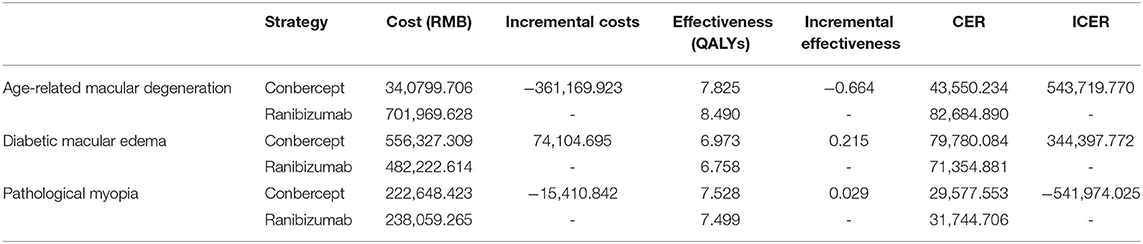
The treatment costs of conbercept and ranibizumab for AMD, DME, and PM within 10 years in RCT scenario are shown in Table 7.
The costs of ranibizumab were 70,1969 RMB, 482,222 RMB, and 238,059 RMB for AMD, DME, and PM within 10 years, respectively, while the costs of conbercept were 340,799 RMB, 556,327 RMB, and 222,648 RMB, respectively. Compared with ranibizumab, the incremental costs of conbercept were−361,169 RMB, 74,104 RMB, and−15,410 RMB, respectively. The ICER was−54,1974 RMB per QALY gained for PM, with much less than the threshold of triple GDP per capita and ranibizumab cost twice as much as conbercept for AMD with a similar QALY, which means that compared with ranibizumab, conbercept had cost-effectiveness in treatment of AMD and PM in RCT scenario.
Extensive Sensitivity Analyses
The sensitivity analyses have indicated that at latest negotiated prices, conbercept still has a significant cost-effectiveness in treatment of AMD, DME, and PM compared with ranibizumab. When the price remains and the number of drug injections changes, conbercept has a certain cost-effectiveness in treatment of AMD with similar number of injections (Appendix Part B).
Discussion
To the best of our knowledge, this is the first study to analyze and compare the cost-effectiveness of conbercept and ranibizumab for the treatment of AMD, DME, and PM based on the real-world scenarios. The results of the economic evaluation study suggest that compared with ranibizumab, conbercept has lower cost and more effective in treatment of DME and PM, which indicated that conbercept had significant cost-effectiveness. In treatment of AMD, the total cost and total utility value of conbercept were lower than that of ranibizumab within 10 years, while the ICER was lower than the threshold of three times per capita GDP. The results indicated that conbercept had a certain cost-effectiveness as well relative to ranibizumab in treatment of AMD.
Previous cost-effectiveness study based on the status change of BCVA, using a similar Markov structure, has explored the cost-effectiveness of conbercept and ranibizumab for wet AMD and found that conbercept was a more cost-effective option for the treatment of AMD in a Chinese healthcare setting (16). A meeting abstract from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) also mentioned that compared with ranibizumab, conbercept is a superior cost-saving alternative for the treatment of Chinese AMD (49). Additionally, conbercept also has certain advantages in clinical application. Unlike ranibizumab, a recombinant humanized anti-VEGF monoclonal antibody Fab fragment, which can only neutralize all the active components of VEGF-A, conbercept is a recombinant fusion protein of the extracellular segments of VEGF receptors 1 and 2 and the human immunoglobulin Fc segment, which can target VEGF-A, VEGF-B, and placental growth factor (15, 50). Therefore, among the four anti-VEGF drugs such as ranibizumab, aflibercept, bevacizumab, and conbercept, conbercept is a 100% humanized protein with the highest affinity and the most binding VEGF targets, which avoids the immune risk of murine protein. The high affinity also means a longer active period of the drug, thereby extending the treatment interval, reducing the burden of the patient, and reflecting the therapeutic advantage in AMD, DME, and PM.
Age-related macular degeneration, induced by a variety of factors and closely related to age, can occur in middle age and its incidence shows an exponential increase after the age of 70 years (51). At the same time, it has been estimated that 25% of Asians will be aged over 60 years by 2050 (52). Therefore, with aging global populations, it was anticipated that AMD would continue to be major causes of vision impairment (4). High myopia is particularly prevalent in East Asian populations and the prevalence of high myopia in the Chinese population in Singapore is 9.1%, China is 3.3%, Taiwan is 2.4%, and Japan is 8.2% (53, 54). PM can develop from high myopia, brings severe visual impairment, and its prevalence is relatively high (about 9–21%) in adults in Asian populations (55). DME is a diabetic microvascular complication that occurs in the retina (56) and diabetic retinopathy (DR) is a common diabetic complication and the main cause of moderate-to-severe visual impairment in diabetic patients (57). About one-third of diabetic patients develop DR and about one-third of patients with DR develop DME (58). Until now, diabetes mellitus (DM) remains a major health burden, with ~80% occurring in low- and middle-income countries (59, 60). The number of DM in China is expected to increase to 142.7 million by 2035 (61). Similarly, it was predicted that the diabetic population in Malaysia would continue to rise (62).
In summary, in low- and middle-income countries that have many similarities and comparability with China in terms of national conditions, such as Malaysia and Singapore, the disease burden of AMD, DME, and PM is also heavy. Although the European Society of Retina Specialists recommends anti-VEGF therapy as a first-line treatment, it is unclear which anti-VEGF should be used first (63). Economic evaluation and analysis should be carried out to obtain greater benefits at the minimum cost and then optimize the allocation and utilization of limited medical resources. This study was based on real-world data for long-term evaluation, which is objective, scientific, and authentic, and can reflect the medication and health of the patient under real conditions to the maximum extent. Therefore, the conclusion throws significant light on the treatment of AMD, DME, and PM in low- and middle-income countries such as Malaysia.
Our research findings showed that in order to maximize health within the fixed general healthcare budget, in the case of the equivalence of conbercept and ranibizumab, the preferential price of conbercept enables it to achieve good therapeutic effect and saves the medical cost during treatment, which has certain advantages. Therefore, clinicians could consider using conbercept instead of ranibizumab to treat AMD, DME, and PM.
This analysis has some limitations that need to be considered along with the results. Firstly, this study mainly used parameters extracted from foreign reports and articles, which might cause a certain degree of uncertainty, so we carried out extensive sensitivity analyses to prove the credibility and robustness of our research results. Secondly, there were more or less differences in population characteristics under different research backgrounds, which may cause the results to have a certain orientation and lack the ability to generalize. Moreover, indirect costs such as the loss of productivity and adverse effects of conbercept and ranibizumab were not included in this study, which may cause a heavy socioeconomic burden for the family and society of the patient. According to clinical trials, the incidence of adverse effects of the two treatment options is very low and mild; the cost-effectiveness of conbercept vs. ranibizumab would become more credible, if this study included the indirect costs. Finally, we simply compared the cost-effectiveness of conbercept and ranibizumab. In fact, compared with other potential anti-VEGF therapies (aflibercept and bevacizumab), ranibizumab has not been shown to be cost-effective in treatment of AMD, DME, and PM (64–66). In light of fact that bevacizumab has been admitted to medical insurance in China in 2017, it is necessary to conduct the cost-effectiveness of conbercept vs. aflibercept or bevacizumab in follow-up studies.
Conclusion
In conclusion, from the third-party payer perspective, conbercept was more cost-effective than ranibizumab in treatment of AMD, DME, and PM. Therefore, due to its favorable economic outcomes, conbercept was the most suitable option in the current Chinese healthcare setting, although conbercept and ranibizumab were both licensed for the treatment of the three eye diseases in China.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ZC and WZ collected data. TZ, HW, and QC designed the entire plan of this study and wrote the manuscript. XM and YL did data analysis and interpretation. ZC, WZ, and HY discussed and supervised this study. All the authors wrote the manuscript and approval the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Numbers 82020108007 and 81830026) and the Beijing-Tianjin-Hebei Special Project (Grant Numbers 19JCZDJC64300(Z) and 20JCZXJC00180).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We give sincere thanks to the researchers in PHOENIX (Identifier NCT01436864), SAILING (Identifier NCT02194634), and SHINY (Identifier NCT01809223) studies for their data support and all the authors for their contributions to the writing manuscript and data analysis of this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.750132/full#supplementary-material
References
1. Klein R, Klein BE, Cruickshanks KJ. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog Retin Eye Res. (1999) 18:371–89. doi: 10.1016/S1350-9462(98)00025-1
2. Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. (2004) 82:844–51. doi: 10.1076/opep.11.2.67.28158
3. Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. (2008) 115:116–26. doi: 10.1016/j.ophtha.2007.03.008
4. Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. (2014) 2:e106–16. doi: 10.1016/S2214-109X(13)70145-1
5. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). (2012) 380:2163–96. doi: 10.1016/S0140-6736(12)61729-2
6. Varma R, Bressler NM, Doan QV, Gleeson M, Danese M, Bower JK, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. (2014) 132:1334–40. doi: 10.1001/jamaophthalmol.2014.2854
7. Cheung GC, Yoon YH, Chen LJ, Chen SJ, George TM, Lai TY, et al. Diabetic macular oedema: evidence-based treatment recommendations for Asian countries. Clin Exp Ophthalmol. (2018) 46:75–86. doi: 10.1111/ceo.12999
8. Chua J, Lim CXY, Wong TY, Sabanayagam C. Diabetic retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila). (2018) 7:3–16. doi: 10.22608/APO.2017511
9. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. (2014) 157:9–25.e12. doi: 10.1016/j.ajo.2013.08.010
10. Alexander M, Halmos B. VEGF inhibitors in EGFR-mutated lung cancer: a never-ending story? Ann Transl Med. (2018) 6:446. doi: 10.21037/atm.2018.11.20
11. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. (2006) 355:1432–44. doi: 10.1056/NEJMoa062655
12. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. (2006) 355:1419–31. doi: 10.1056/NEJMoa054481
13. Zhao J, Li X, Tang S, Xu G, Xu X, Zhang F, et al. EXTEND II: an open-label phase III multicentre study to evaluate efficacy and safety of ranibizumab in Chinese patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. BioDrugs. (2014) 28:527–36. doi: 10.1007/s40259-014-0106-1
14. Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. (2014) 121:1740–7. doi: 10.1016/j.ophtha.2014.03.026
15. Zhang J, Liang Y, Xie J, Li D, Hu Q, Li X, et al. Conbercept for patients with age-related macular degeneration: a systematic review. BMC Ophthalmol. (2018) 18:142. doi: 10.1186/s12886-018-0807-1
16. Chen R, Wu B. Cost-effectiveness of intravitreal conbercept versus other treatments for wet age-related macular degeneration. Ann Transl Med. (2020) 8:939. doi: 10.21037/atm-20-1334
17. Liu K, Song Y, Xu G, Ye J, Wu Z, Liu X, et al. Conbercept for treatment of neovascular age-related macular degeneration: results of the randomized phase 3 PHOENIX study. Am J Ophthalmol. (2019) 197:156–67. doi: 10.1016/j.ajo.2018.08.026
18. Liu K, Wang H, He W, Ye J, Song Y, Wang Y, et al. Intravitreal conbercept for diabetic macular oedema: 2-year results from a randomised controlled trial and open-label extension study. Br J Ophthalmol. (2021). doi: 10.1136/bjophthalmol-2020-318690. [Epub ahead of print].
19. Li X, Dai H, Li X, Han M, Li J, Suhner A, et al. Efficacy and safety of ranibizumab 05 mg in Chinese patients with visual impairment due to diabetic macular edema: results from the 12-month REFINE study. Graefes Arch Clin Exp Ophthalmol. (2019) 257:529–41. doi: 10.1007/s00417-018-04213-x
20. Xuan J. Pharmacoeconomics Study of Lumitin® (Combercept) in the Treatment of Pathological Myopia with Choroidal Neovascularization (CNV). Guangzhou, Sun Yat-sen University (2019).
21. Chen Y, Sharma T, Li X, Song Y, Chang Q, Lin R, et al. Ranibizumab versus verteporfin photodynamic therapy in asian patients with myopic choroidal neovascularization: brilliance, a 12-month, randomized, double-masked study. Retina. (2019) 39:1985–94. doi: 10.1097/IAE.0000000000002292
22. Czoski-Murray C, Carlton J, Brazier J, Young T, Papo NL, Kang HK. Valuing condition-specific health states using simulation contact lenses. Value Health. (2009) 12:793–9. doi: 10.1111/j.1524-4733.2009.00527.x
23. Choi S, Park SM, Jee D. Utility values for age-related macular degeneration patients in Korea. PLoS ONE. (2018) 13:e0201399. doi: 10.1371/journal.pone.0201399
24. Aixia M. Pharmacoeconomic evaluation of conbercept and rezumumab in the treatment of age-related macular degeneration. China J Pharm Econ. (2018) 13:27–31. doi: 10.12010/j.issn.1673-5846.2018.09.005
25. Cui J, Sun D, Lu H, Dai R, Xing L, Dong H, et al. Comparison of effectiveness and safety between conbercept and ranibizumab for treatment of neovascular age-related macular degeneration. A retrospective case-controlled non-inferiority multiple center study. Eye (Lond). (2018) 32:391–9. doi: 10.1038/eye.2017.187
26. Gao L, Tao Y, Liu M, Li L, Zhang P, Wang H, et al. Different conbercept injection strategies for the treatment of exudative age-related macular degeneration: A retrospective cohort study. Medicine (Baltimore). (2020) 99:e19007. doi: 10.1097/MD.0000000000019007
27. Wu BH, Wang B, Wu HQ, Chang Q, Lu HQ. Intravitreal conbercept injection for neovascular age-related macular degeneration. Int J Ophthalmol. (2019) 12:252–7. doi: 10.18240/ijo.2019.02.11
28. Cheng Y, Yuan L, Zhao MW, Qian T. Real-world outcomes of two-year Conbercept therapy for diabetic macular edema. Int J Ophthalmol. (2021) 14:416–22. doi: 10.18240/ijo.2021.03.14
29. Li F, Zhang L, Wang Y, Xu W, Jiao W, Ma A, et al. One-year outcome of conbercept therapy for diabetic macular edema. Curr Eye Res. (2018) 43:218–23. doi: 10.1080/02713683.2017.1379542
30. Xu Y, Qu Y, Suo Y, Gao J, Chen X, Liu K, et al. Correlation of retinal layer changes with vision gain in diabetic macular edema during conbercept treatment. BMC Ophthalmol. (2019) 19:123. doi: 10.1186/s12886-019-1131-0
31. Xu Y, Rong A, Bi Y, Xu W. Intravitreal conbercept injection with and without grid laser photocoagulation in the treatment of diffuse diabetic macular edema in real-life clinical practice. J Ophthalmol. (2016) 2016:2143082. doi: 10.1155/2016/2143082
32. Xu Y, Rong A, Xu W, Niu Y, Wang Z. Comparison of 12-month therapeutic effect of conbercept and ranibizumab for diabetic macular edema: a real-life clinical practice study. BMC Ophthalmol. (2017) 17:158. doi: 10.1186/s12886-017-0554-8
33. Chen J, Zhang L, Li Z. Observation of the curative effect of anti-VEG drug Conbercept in the treatment of choroidal neovascularization in pathological myopia. J Chin Ophthalmol Otorhinolaryngol. (2017) 7:228–31. doi: 10.3969/j.issn.1674-9006.2017.04.013
34. Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Queré S, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. (2013) 33:474–81. doi: 10.1097/IAE.0b013e31827b6324
35. Frennesson CI, Nilsson SE. A three-year follow-up of ranibizumab treatment of exudative AMD: impact on the outcome of carrying forward the last acuity observation in drop-outs. Acta Ophthalmol. (2014) 92:216–20. doi: 10.1111/aos.12091
36. Hjelmqvist L, Lindberg C, Kanulf P, Dahlgren H, Johansson I, Siewert A. One-year outcomes using ranibizumab for neovascular age-related macular degeneration: results of a prospective and retrospective observational multicentre study. J Ophthalmol. (2011) 2011:405724. doi: 10.1155/2011/405724
37. Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. (2015) 99:220–6. doi: 10.1136/bjophthalmol-2014-305327
38. Lotery A, Griner R, Ferreira A, Milnes F, Dugel P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye (Lond). (2017) 31:1697–706. doi: 10.1038/eye.2017.143
39. Muniraju R, Ramu J, Sivaprasad S. Three-year visual outcome and injection frequency of intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Ophthalmologica. (2013) 230:27–33. doi: 10.1159/000350238
40. Rakic JM, Leys A, Brié H, Denhaerynck K, Pacheco C, Vancayzeele S, et al. Real-world variability in ranibizumab treatment and associated clinical, quality of life, and safety outcomes over 24 months in patients with neovascular age-related macular degeneration: the HELIOS study. Clin Ophthalmol. (2013) 7:1849–58. doi: 10.2147/OPTH.S49385
41. Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. (2016) 172:51–7. doi: 10.1016/j.ajo.2016.09.002
42. Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. (2017) 101:75–80. doi: 10.1136/bjophthalmol-2016-309313
43. Ziemssen F, Wachtlin J, Kuehlewein L, Gamulescu MA, Bertelmann T, Feucht N, et al. Intravitreal ranibizumab therapy for diabetic macular edema in routine practice: two-year real-life data from a non-interventional, multicenter study in Germany. Diabetes Ther. (2018) 9:2271–89. doi: 10.1007/s13300-018-0513-2
44. Tsai MJ, Hsieh YT, Peng YJ. Real-life experience of ranibizumab for diabetic macular edema in Taiwan. Int Ophthalmol. (2019) 39:1511–22. doi: 10.1007/s10792-018-0970-7
45. Mitchell P, Sheidow TG, Farah ME, Mahmood S, Minnella AM, Eter N, et al. Effectiveness and safety of ranibizumab 05 mg in treatment-naïve patients with diabetic macular edema: results from the real-world global LUMINOUS study. PLoS ONE. (2020) 15:e0233595. doi: 10.1371/journal.pone.0233595
46. Sun Z, Wan G, Qian C, Liang S, Wang J. Efficacy of intravitreal ranibizumab for the treatment of choroidal neovascularization secondary to pathological myopia. Recent Adv Ophthalmol. (2018) 38:925–9. doi: 10.13389/j.cnki.rao.2018.0219
47. Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. (2000) 17:479–500. doi: 10.2165/00019053-200017050-00006
48. Yan M, Huang Z, Lian HY, Song YP, Chen X. Conbercept for treatment of choroidal neovascularization secondary to pathologic myopia. Acta Ophthalmol. (2019) 97:e813–e4. doi: 10.1111/aos.13632
49. Zhao M, Feng W, Zhang L, Ke X, Zhang W, Xuan J. Cost-effectiveness analysis of conbercept versus ranibizumab for the treatment of age-related macular degeneration in China. Value Health. (2015) 18:A421-A. doi: 10.1016/j.jval.2015.09.558
50. Horowitz SA, Damasceno NP, Damasceno EF. Treatment of Radiation Retinopathy with Intravitreal Injection of Ranibizumab (Lucentis(®)). Int Med Case Rep J. (2020) 13:27–32. doi: 10.2147/IMCRJ.S191654
51. van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. Epidemiology of age-related maculopathy: a review. Eur J Epidemiol. (2003) 18:845–54. doi: 10.1023/A:1025643303914
52. Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. (2010) 117:921–7. doi: 10.1016/j.ophtha.2009.10.007
53. Yang MC, Chen YP, Tan EC, Leteneux C, Chang E, Chu CH, et al. Epidemiology, treatment pattern and health care utilization of myopic choroidal neovascularization: a population based study. Jpn J Ophthalmol. (2017) 61:159–68. doi: 10.1007/s10384-016-0496-3
54. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 123:1036–42. doi: 10.1016/j.ophtha.2016.01.006
55. Mitry D, Zambarakji H. Recent trends in the management of maculopathy secondary to pathological myopia. Graefes Arch Clin Exp Ophthalmol. (2012) 250:3–13. doi: 10.1007/s00417-011-1889-0
56. Lotery AJ. Long-term therapies for diabetic macular edema. Eur Ophthalmic Rev. (2012) 6:236–41. doi: 10.17925/EOR.2012.06.04.236
57. Wilkinson CP, Ferris FL III, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. (2003) 110:1677–82. doi: 10.1016/S0161-6420(03)00475-5
58. Lindner M, Arefnia B, Ivastinovic D, Sourij H, Lindner E, Wimmer G. Association of periodontitis and diabetic macular edema in various stages of diabetic retinopathy. Clin Oral Investig. (2021). doi: 10.1007/s00784-021-04028-x [Epub ahead of print].
59. Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. (2004) 27:2568–9; author reply 9. doi: 10.2337/diacare.27.10.2568
60. Flood D, Hane J, Dunn M, Brown SJ, Wagenaar BH, Rogers EA, et al. Health system interventions for adults with type 2 diabetes in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. (2020) 17:e1003434. doi: 10.1371/journal.pmed.1003434
61. Wang B, Zhang M, Wang S, Wang C, Wang J, Li L, et al. Dynamic status of metabolically healthy overweight/obesity and metabolically unhealthy and normal weight and the risk of type 2 diabetes mellitus: a cohort study of a rural adult Chinese population. Obes Res Clin Pract. (2018) 12:61–71. doi: 10.1016/j.orcp.2017.10.005
62. Ibrahim N, Ming Moy F, Awalludin IA, Mohd Ali Z, Ismail IS. Effects of a community-based healthy lifestyle intervention program (Co-HELP) among Adults with prediabetes in a developing country: a quasi-experimental study. PLoS ONE. (2016) 11:e0167123. doi: 10.1371/journal.pone.0167123
63. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European society of retina specialists (EURETINA). Ophthalmologica. (2017) 237:185–222. doi: 10.1159/000458539
64. Yu JS, Carlton R, Agashivala N, Hassan T, Wykoff CC. Brolucizumab vs. aflibercept and ranibizumab for neovascular age-related macular degeneration: a cost-effectiveness analysis. J Manag Care Spec Pharm. (2021) 27:743–52. doi: 10.18553/jmcp.2021.27.6.743
65. El-Dahiyat F, Eljilany I. Cost-minimization analysis of ranibizumab versus aflibercept for treating saudi patients with visual impairment owing to age-related macular degeneration or diabetic macular edema. Value Health Reg Issues. (2020) 22:23–6. doi: 10.1016/j.vhri.2019.09.007
66. Hykin P, Prevost AT, Sivaprasad S, Vasconcelos JC, Murphy C, Kelly J, et al. Intravitreal ranibizumab versus aflibercept versus bevacizumab for macular oedema due to central retinal vein occlusion: the LEAVO non-inferiority three-arm RCT. Health Technol Assess. (2021) 25:1–196. doi: 10.3310/hta25380
Keywords: age-related macular degeneration (AMD), diabetic macular edema (DME), pathological myopia (PM), ranibizumab, conbercept, cost-effectiveness, Markov model
Citation: Cui Z, Zhou W, Chang Q, Zhang T, Wang H, Meng X, Liu Y and Yan H (2021) Cost-Effectiveness of Conbercept vs. Ranibizumab for Age-Related Macular Degeneration, Diabetic Macular Edema, and Pathological Myopia: Population-Based Cohort Study and Markov Model. Front. Med. 8:750132. doi: 10.3389/fmed.2021.750132
Received: 30 July 2021; Accepted: 02 November 2021;
Published: 02 December 2021.
Edited by:
Yongwei Guo, Zhejiang University, ChinaReviewed by:
Yanling Wang, Capital Medical University, ChinaYe Liu, Zhejiang University, China
Kaiwen Ni, Zhejiang University, China
Copyright © 2021 Cui, Zhou, Chang, Zhang, Wang, Meng, Liu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yan, enl5eWFuaHVhQHRtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Zhuang Cui
Zhuang Cui Wei Zhou2†
Wei Zhou2† Qinxue Chang
Qinxue Chang Hua Yan
Hua Yan