- 1Department of Urology, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha City, China
- 2Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha City, China
Objective: To assess the safety and efficacy of low-dose everolimus maintenance therapy for tuberous sclerosis complex-related renal angiomyolipoma (TSC-RAML) patients that had previously undergone standard-dose treatment for a minimum of 6 months.
Materials and Methods: In total, 24 patients with a definitive TSC diagnosis were enrolled from April 2018 – April 2019 at Xiangya Hospital, Central South University. All patients underwent low-dose everolimus maintenance therapy following standard-dose everolimus induction therapy for a minimum of 6 months. Patients additionally underwent TSC1/TSC2 genetic testing, And they were followed-up at 3, 6, 12, 18, and 24 months. The Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) criteria were used to monitor patient RAML responses, while adverse events (AEs) were assessed as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0). P < 0.05 was the significance level for all analyses, which were performed using SPSS 19.0.
Results: TSC1/TSC2 gene mutations were present in all 24 patients, all of whom achieved a significant reduction in TSC-RAML volume within the initial 6-month induction therapy period, and exhibited volume stabilization during the low-dose maintenance therapy treatment period without any instances of TSC-RAML regrowth. Adverse events (AEs) were significantly less severe and less frequent over the course of maintenance therapy relative to standard therapy.
Conclusions: Low-dose everolimus maintenance therapy represents an effective approach to achieving TSC-RAML control following a minimum of 6 months of full-dose induction therapy, and may be associated with decreases in everolimus-related AE frequency and severity.
Introduction
Tuberous sclerosis complex (TSC) is an autosomaldominant syndrome that impacts between 1 in 6,000 and 1 in 10,000 individuals, resulting in characteristic neurodevelopmental features and the development of multiple tumors in organs including the skin, heart, lungs, brain, and kidneys (1). Upwards of 80% of TSC patients are affected by renal angiomyolipoma (RAML) (2), which is characterized by multiple bilateral lesions in the smooth muscles, adipose tissue, and vasculature (3). As these tumors typically grow over time, TSC-RAML can result in arterial hypertension and imposes a risk of life-threatening hemorrhage, which is the leading cause of TSC-associated mortality among adults with this condition (4).
Most TSC patients present with mutations in the TSC1 or TSC2 genes, which encode proteins that form the TSC1-TSC2 complex that serves to antagonize the signaling pathway downstream of mammalian target of rapamycin (mTOR) by promoting the activation of the small GTPase Rheb and thereby inhibiting cellular growth and proliferation. Pathogenic TSC1/TSC2 variants result in constitutive mTOR pathway hyperactivation, thereby contributing to the growth of benign tumors or hamartomas in multiple systems (5).
Everolimus is an mTOR inhibitor that has shown promise for the treatment of complications associated with TSC including RAML, seizures, facial angiofibromas, and subependymal giant cell astrocytomas (SEGAs) (6–9). Indeed, everolimus treatment can result in an initial rapid decrease in TSC-RAML volume, followed by a secondary phase during which these tumors slowly shrink or stabilize (7). The International Tuberous Sclerosis Complex Consensus Conference held in 2012 recommended the first-line use of mTOR inhibitors for the treatment of RAML ≥ 3 cm in diameter, even when not associated with any clinical symptoms (10).
Prior work suggests that TSC-RAML regrowth may occur following the cessation of mTOR inhibitor therapy, and the ideal duration for this therapeutic strategy remains to be defined optimal duration of mTOR inhibitor treatment has yet to be determined (11, 12). With respect to safety, the short-term adverse effects associated with everolimus are typically acceptable, although in a few instances more severe events have been reported (13). Long-term treatment-related safety outcomes, however, remain to be established. Wheless and Klim (14) proposed a dose reduction algorithm designed to minimize the negative impact of mTOR inhibitor treatment for patients with SEGAs that are shrinking or stable in size. We were thus interested in whether the everolimus dose could similarly be reduced to control the frequency and severity of adverse events (AEs) in patients with controlled TSC-RAML. This study was therefore designed to examine the safety and efficacy of low-dose everolimus maintenance therapy in TSC-RAML patients that had previously undergone treatment with a standard everolimus dose for a minimum of 6 months.
Methods
Study Group
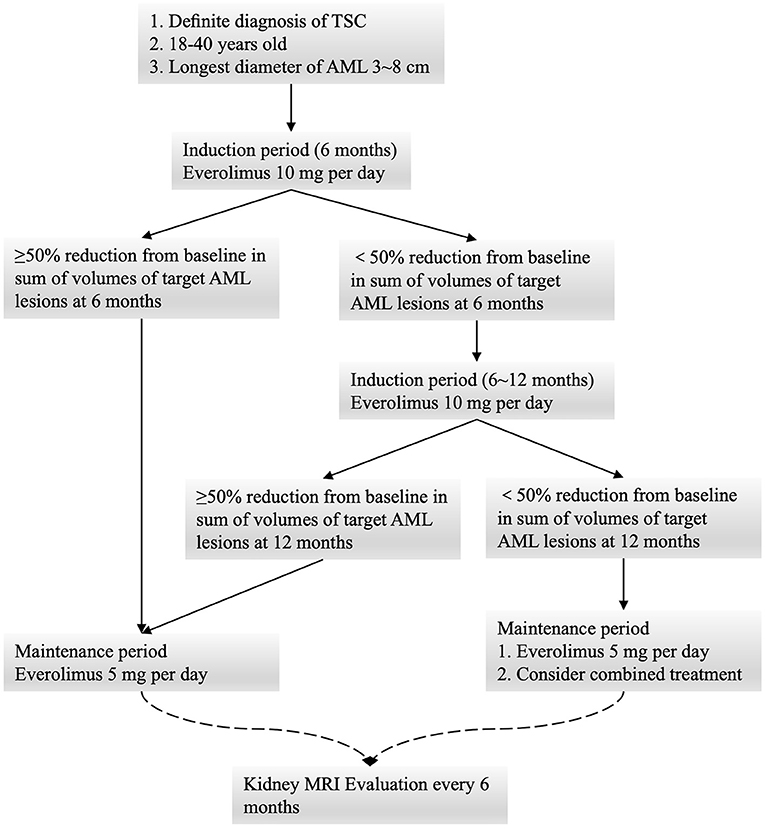
This was a single-center, open-label, single-arm, prospective interventional study performed between April 2018 and April 2019 at Xiangya Hospital, Central South University. The Human Ethics Committee of Xiangya Hospital, Central South University approved this study prior to patient enrollment, and all protocols were performed in accordance with the Declaration of Helsinki (15). Patients provided written informed consent prior to voluntary study participation. Patients eligible for inclusion were: 1) individuals with a definitive TSC diagnosis as defined by meeting 2 major criteria or 1 major criterion and ≥ 2 minor criteria recommended by the 2012 International Tuberous Sclerosis Complex Consensus Conference (10); 2) individuals ≥ 18 years old; 3) individuals with a minimum of one RAML ≥ 30 mm in diameter.
All patients underwent oral everolimus induction therapy (10 mg/day) for 6 months, after which they underwent radiographic follow-up and a safety evaluation. All patients that achieved a ≥ 50% decrease in the total volume of the target AML (relative to baseline) were assigned to the low-dose oral everolimus maintenance group (5 mg/day), while patients not meeting these criteria underwent induction therapy for an additional 6 months. Follow-up was then repeated at 12 months, at which time patients were assigned to undergo low-dose maintenance therapy regardless of the observed reduction in AML size. Combination treatment options were considered for individuals exhibiting a poor response to everolimus. Patient follow-up was performed at 3, 6, 12, 18, and 24 months (Figure 1).
Patient Evaluation and Follow-Up
Abdominal magnetic resonance imaging (MRI) was used to visualize RAML tumors at baseline, with up to four RAMLs with a maximum diameter ≥ 3.0 cm being identified as target lesions in each patient. The sum of the diameters of these target lesions was calculated. Over the course of follow-up, patients underwent routine urine, blood, physical, and radiographic analyses. The Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) criteria were used to monitor patient RAML responses, while adverse events (AEs) were assessed as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).
Genetic Analysis
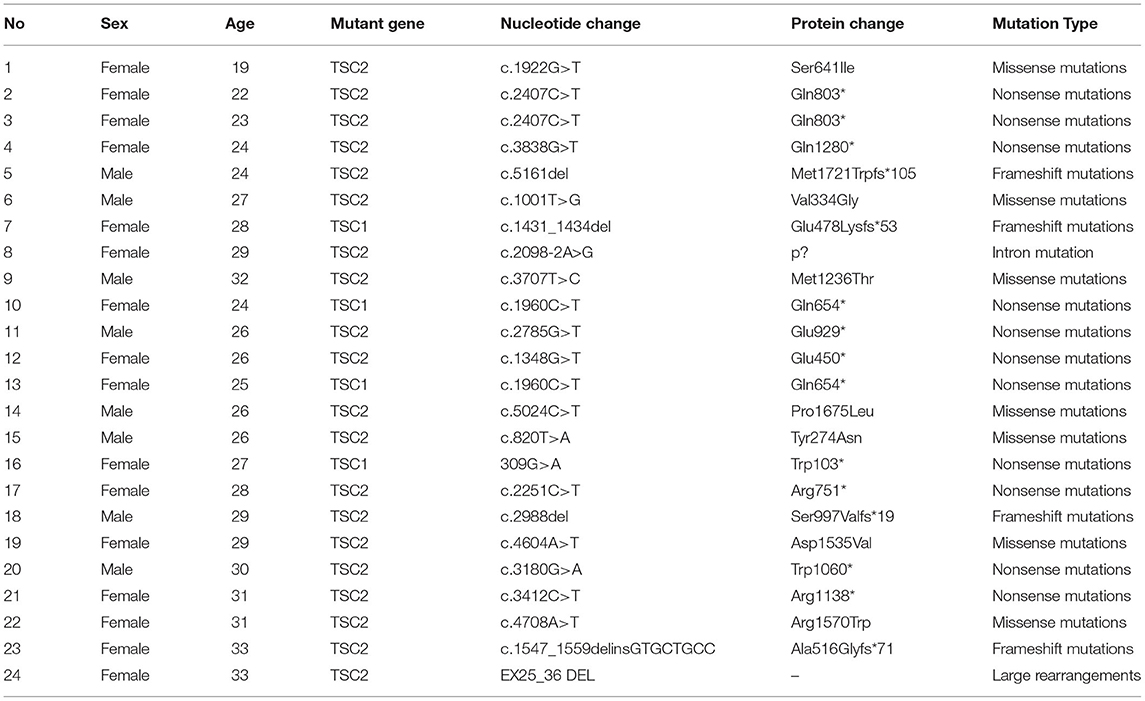
TSC1/TSC2 mutational status was assessed via a next-generation sequencing (NGS) approach at the NHC Key Laboratory of Cancer Proteomics (Hunan Province, China). Pathogenic mutations were confirmed through reference to the LOVD databases (www.lovd.nl/TSC1; www.lovd.nl/TSC2). The potential impact of newly identified mutations resulting in amino acid substitutions on protein function was assessed with the online SIFT and PolyPhen2 tools.
Statistical Analysis
Continuous variables are given as mean ± standard deviation (M ± SD), while categorical variables are given in the form of frequencies (n) and percentages (%). Categorical variables were compared using the chi-square test. SPSS 19.0 (SPSS, IL, USA) was used for all statistical testing, with P < 0.05 as the significance threshold.
Results
Patient Characteristics
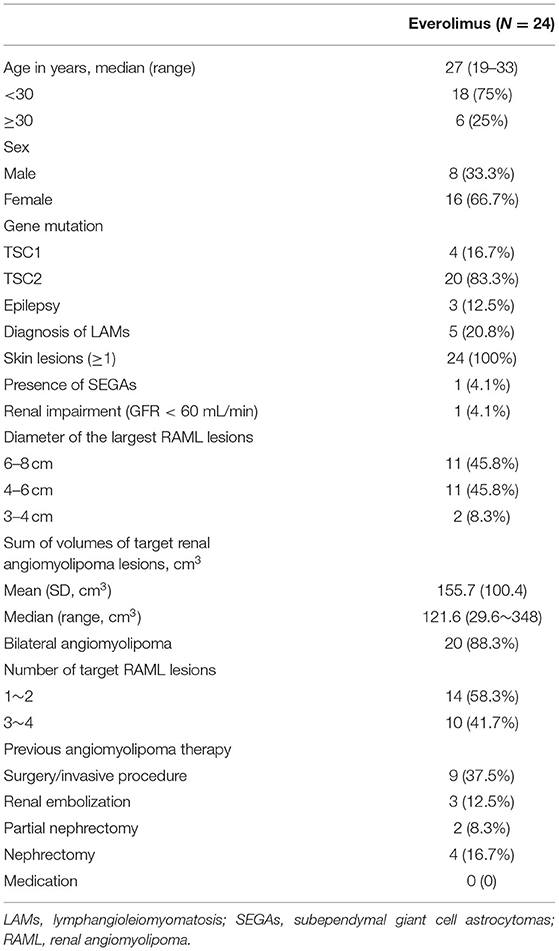
In total, 24 patients (8 male, 16 female) were enrolled in this study, with a median age of 27 years. Patient demographics are compiled in Table 1. Of these patients, 20 and 4 were found to harbor TSC2 and TSC1 mutations, respectively, via NGS (Table 2). Three of these patients had a history of epilepsy, two were treated with antiepileptic monotherapy (oxcarbazepine, lamotrigine), while the remaining one with antiepileptic combination therapy (oxcarbazepine + topiramate). Five of these patients were diagnosed with lymphangioleiomyomatosis (LAM), all suffered from skin lesions, and one presented with SEGAs. One patient had a renal impairment of GFR < 60 mL/min. With respect to the baseline RAML characteristics in these patients, 11 exhibited RAMLs with a maximum diameter ≥ 6 cm, 20 exhibited bilateral RAMLs, and 10 presented with 3-4 target RAML lesions.
Before enrollment in this study, 3, 2, and 4 of these patients had respectively undergone renal embolization, partial nephrectomy, and nephrectomy. No patients had undergone prior medication therapy. Over the follow-up period, two patients withdrew from the study at 24 months, leaving 22 patients for the assessment of RAML status. One gave up medication for economic reason, and the other withdrew from the study due to the unavailability of everolimus resulted from COVID-19 pandemic.
Treatment Efficacy
The patients' response of AML volume to everolimus during treatment is detailed in Table 3. The number of patients who achieved ≥ 50% reduction in RAML volume was 12 (50%) at 6 months and 13 (54%) at 12 months, respectively. The change in RAML volume for each patient over the study period is displayed in Figure 2, with the most significant decrease in tumor volume having been observed within the initial 6 months of standard-dose everolimus therapy. In total, 12 patients achieved ≥ 50% reduction in total target AML volume at 6 months, whereupon they initiated low-dose everolimus maintenance therapy. Just one of the remaining 12 patients achieved ≥ 50% reduction in target AML volume after an additional round of full-dose everolimus treatment. Target RAML volumes were well-controlled in all patients during the maintenance therapy period.
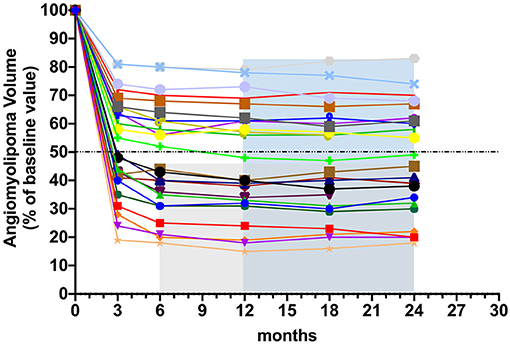
Figure 2. Changes in TSC-RAML volume from baseline during the induction therapy and the maintenance therapy. Each line represents TSC-RAML volume change in one patient.
The pulmonary function in 5 female patients with LAM during everolimus therapy are detailed in Table 4. At baseline, four patients showed moderate airflow obstruction (forced expiratory volume in 1 second (FEV1): 50–70% of the predicted value), while one patient showed severe airflow obstruction (FEV1 < 50% of the predicted value). During the medication period, an increase was observed in FEV1, forced vital capacity (FVC), total lung capacity, and diffusion capacity for carbon monoxide (DLCO) among all the five patients, while the residual volume decreased. The changes in FEV1, FVC, and residual volume for each patient are shown in Figure 3. It further displayed the improved pulmonary function in patients with LAM. These results indicated that both full-dose and low-dose everolimus treatment have a protective effect on pulmonary function in patients with TSC.
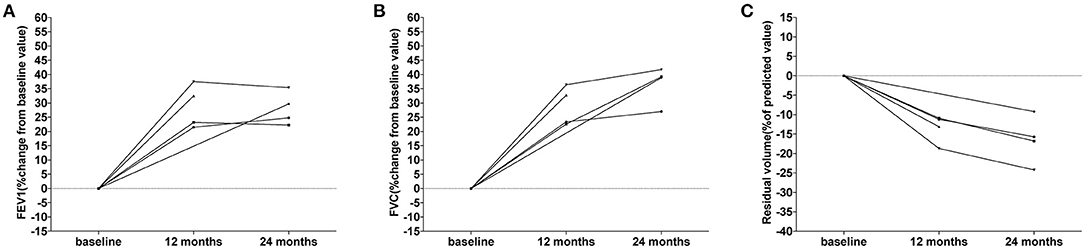
Figure 3. Changes of pulmonary function in the five patients with LAM. (A) The changes of FEV1 from baseline during treatment. Each line represents FEV1 change in one patient. (B) The changes of FVC from baseline during treatment. Each line represents FVC change in one patient. (C) The changes of residual volume from baseline during treatment. Each line represents residual volume change in one patient.
Adverse Events
All AEs recorded during the induction and maintenance phases of everolimus treatment are compiled in Table 5. While six grade 3–4 AEs occurred during full-dose induction therapy, none occurred during low-dose maintenance therapy. The most common AEs during full-dose induction therapy included oral mucositis (22/24), abdominal pain (10/24), hypertriglyceridemia (10/24), and headache (9/24), while the most common AEs during low-dose maintenance treatment were oral mucositis (10/24) and hypertriglyceridemia (9/24). AEs with significant incidence reductions during low-dose maintenance therapy included oral mucositis (P < 0.001), irregular menstruation (P = 0.04), upper respiratory infections (P = 0.02), and fever (P = 0.04). No unexpected AEs or mortality were reported, and no patients declined treatment or withdrew from the study due to AEs.
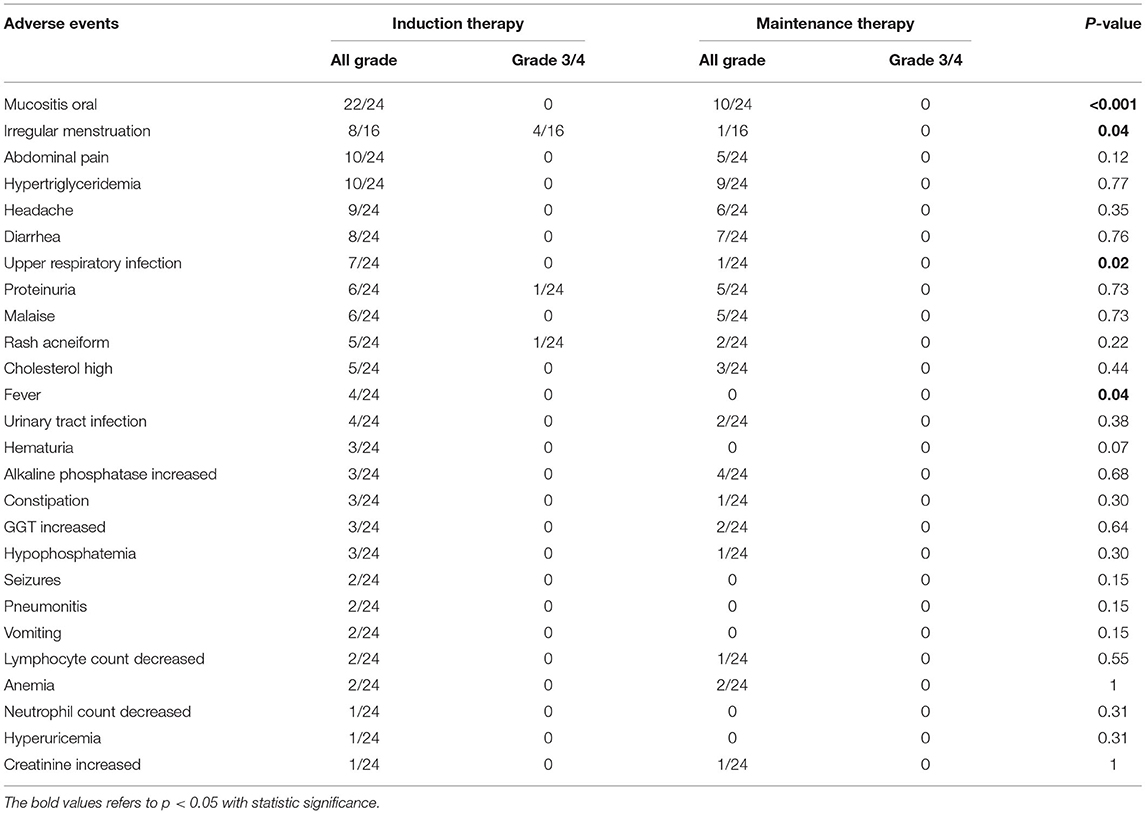
Table 5. Adverse events associated with everolimus in the study group during induction and maintenance therapy.
Discussion
This study is the first to our knowledge to have assessed the efficacy and safety of low-dose everolimus maintenance therapy for the treatment of TSC-RAML patients after a minimum 6-month full-dose induction therapy period.
Owing to the potential for rebound after withdrawal, sustained everolimus therapy is necessary to effectively control TSC-RAML. However, continuous everolimus treatment is associated with a number of issues. First, prolonged standard everolimus treatment is expensive and can impose a major economic burden on patients that decreases their compliance. Second, lifelong mTOR inhibitor treatment is often required for TSC patients, particularly for individuals < 40 years of age, emphasizing the need to explore more feasible or cost-effective solutions. Third, sustained standard everolimus treatment can result in potentially severe AEs. Lastly, the most prominent tumor growth reduction generally occurs within the initial 3–6 months of treatment in patients, whereafter tumor volumes tend to stabilize or decrease gradually (16). We therefore designed the present study to assess the ability of low-dose everolimus maintenance to control RAML volumes and to reduce AE incidence, given that such an approach has previously been reported to be successful in the treatment of TSC-related SEGAs (17).
Herein, the everolimus dose utilized for low-dose maintenance therapy was reduced to 5 mg/day from 10 mg/day. All patients achieved a significant reduction in TSC-RAML volume over the first 6 months of induction treatment, and maintained a stable TSC-RAML volume during the low-dose maintenance period without any evidence of target AML lesion growth or progression. This suggests that low-dose everolimus maintenance therapy is an effective therapeutic option for TSC-AML patients. Moreover, pulmonary functions, including FEV1, FVC, total lung capacity, DLCO, and residual volume, were improved in five patients with LAM during both full- and low-dose everlimus therapy, which further confirmed the efficacy of low-dose everlimus therapy.
Everolimus therapy is commonly associated with a range of AEs that can affect treatment efficacy and compliance in some patients. We found that these everolimus therapy-related AEs were significantly less frequent and less severe during the low-dose maintenance therapy compared with the standard treatment period, consistent with a previous study (17). Reducing the incidence of oral mucositis is critical to improving patient compliance. Most importantly, no grade 3-4 AEs were observed in the context of low-dose maintenance therapy, in contrast to the incidence of such complications during full-dose everolimus treatment. These results thus suggest that low-dose everolimus maintenance therapy is a feasible and well-tolerated option for patients with TSC-RAML.
While these results are promising, this study is nonetheless limited by the fact that it is a single-center analysis of a relatively small patient population. Even so, we hope that this study can provide a reference for the everolimus treatment of TSC-RAML patients, particularly those patients that exhibit poor tolerance for full-dose everolimus therapy. We plan to recruit more patients for treatment with this low-dose maintenance therapy regimen, and as such, our available efficacy and safety data will continue to expand in the future. Another noteworthy limitation of this study is that we were unable to assess drug concentrations in patient blood owing to technical limitations. However, we believe that consistent dosing will largely mitigate the potential bias associated with this limitation.
Conclusions
Low-dose everolimus maintenance therapy is an effective therapeutic approach to controlling TSC-RAML following full-dose induction therapy, and may reduce the frequency and severity of AEs associated with everolimus.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CL, W-RY, Y-LL, and YC participated in its design and coordination and drafted the manuscript. CL, W-RY, and Y-LL participated in the design of the study and performed the statistical analysis. CL, W-RY, M-FC, LQ, X-BZ, Y-LL, and YC conceived of the study, participated in its design, and coordination and helped to draft the manuscript. All authors have read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 81800590) and the Natural Science Foundation of Hunan Province (Grant No. 2020JJ5882).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.744050/full#supplementary-material
References
1. Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. (1991) 615:125–7. doi: 10.1111/j.1749-6632.1991.tb37754.x
2. Henske EP, Józwiak S, Kingswood JC, Sampson JR, Thiele EA. Tuberous sclerosis complex. Nat Rev Dis Primers. (2016) 2:16035. doi: 10.1038/nrdp.2016.35
3. Cai Y, Li H, Zhang Y. Assessment of tuberous sclerosis complex associated with renal lesions by targeted next-generation sequencing in Mainland China. Urology. (2017) 101:70.e1. doi: 10.1016/j.urology.2016.10.056
4. Kapoor A, Girard L, Lattouf JB, Pei Y, Rendon R, Card P, et al. Evolving Strategies in the Treatment of Tuberous Sclerosis Complex-associated Angiomyolipomas (TSC-AML). Urology. (2016) 89:19–26. doi: 10.1016/j.urology.2015.12.009
5. Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. (2003) 5:578–81. doi: 10.1038/ncb999
6. Mizuguchi M, Ikeda H, Kagitani-Shimono K, Yoshinaga H, Suzuki Y, Aoki M, et al. Everolimus for epilepsy and autism spectrum disorder in tuberous sclerosis complex: EXIST-3 substudy in Japan. Brain Dev. (2019) 41:1-10. doi: 10.1016/j.braindev.2018.07.003
7. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2013) 381:817–24. doi: 10.1016/S0140-6736(12)61767-X
8. Wataya-Kaneda M, Nakamura A, Tanaka M, Hayashi M, Matsumoto S, Yamamoto K, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex : a randomized clinical trial. JAMA Dermatol. (2017) 153:39–48. doi: 10.1001/jamadermatol.2016.3545
9. Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. (2013) 381:125–32. doi: 10.1016/S0140-6736(12)61134-9
10. Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. (2013) 49:243–54. doi: 10.1016/j.pediatrneurol.2013.08.001
11. Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. (2008) 358:140–51. doi: 10.1056/NEJMoa063564
12. Cai Y, Guo H, Wang W, Li H, Sun H, Shi B, et al. Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: a two years trial. Orphanet J Rare Dis. (2018) 13:43. doi: 10.1186/s13023-018-0781-y
13. Trelinska J, Dachowska I, Kotulska K, Fendler W, Jozwiak S, Mlynarski W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anticancer Drugs. (2015) 26:437–42. doi: 10.1097/CAD.0000000000000207
14. Wheless JW, Klimo P Jr. Subependymal giant cell astrocytomas in patients with tuberous sclerosis complex: considerations for surgical or pharmacotherapeutic intervention. J Child Neurol. (2014) 29:1562–71. doi: 10.1177/0883073813501870
15. Gandevia B, Tovell A. Declaration of Helsinki. Med J Aust. (1964) 2:320–1. doi: 10.5694/j.1326-5377.1964.tb115781.x
16. Bissler JJ, Budde K, Sauter M, Franz DN, Zonnenberg BA, Frost MD, et al. Effect of everolimus on renal function in patients with tuberous sclerosis complex: evidence from EXIST-1 and EXIST-2. Nephrol Dial Transplant. (2019) 34:1000–8. doi: 10.1093/ndt/gfy132
Keywords: tuberous sclerosis complex, renal angiomyolipoma, everolimus, low-dose maintenance therapy, safety
Citation: Luo C, Ye W-R, Zu X-B, Chen M-F, Qi L, Li Y-L and Cai Y (2021) Low-Dose Everolimus Maintenance Therapy for Renal Angiomyolipoma Associated With Tuberous Sclerosis Complex. Front. Med. 8:744050. doi: 10.3389/fmed.2021.744050
Received: 19 July 2021; Accepted: 05 November 2021;
Published: 24 November 2021.
Edited by:
Liangyou Gu, People's Liberation Army General Hospital, ChinaReviewed by:
Elzbieta Radzikowska, National Institute of Tuberculosis and Lung Diseases, PolandAlice Chen, National Cancer Institute (NCI), United States
Copyright © 2021 Luo, Ye, Zu, Chen, Qi, Li and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang-Le Li, bHlsZTIwMDNAY3N1LmVkdS5jbg==; Yi Cai, Y2FpLXlpQGNzdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Cong Luo
Cong Luo Wen-Rui Ye
Wen-Rui Ye Xiong-Bin Zu1
Xiong-Bin Zu1 Yi Cai
Yi Cai