- 1Division of Nephrology, Department of Medicine, Cathay General Hospital, School of Medicine, Fu-Jen Catholic University, Taipei, Taiwan
- 2National Defense Medical Center, Graduate Institute of Medical Sciences, Taipei, Taiwan
Immune checkpoints inhibitors (ICPIs), as either a frontline or adjuvant therapy, showed favorable outcomes among diverse malignancies. Immune-related adverse events (IRAEs) are increasingly encountered, but the kidneys are rarely affected. A 67-year-old man with stage IV squamous cell carcinoma of the lung presented with acute kidney injury and hypercalcemia secondary to bone metastasis. After an aggressive saline infusion and subcutaneous denosumab 60mg administration, his renal function and serum calcium level were recovered on day 4. Due to his intolerance to chemotherapy, immunotherapy with a monoclonal antibody targeting programmed cell death protein-1 (PD-1), pembrolizumab 2mg/kg, was used on day 4. On day 11, polyuria, non-albumin dominant proteinuria, and severe deficiencies of electrolytes (potassium 2.5 mmol/L, calcium 5.5 mg/dL, magnesium 1.3 mg/dL, and phosphate 1.5 mg/dL) along with concomitant renal wasting were developed acutely. Except for postponing the next pembrolizumab, prednisolone at 1 mg/kg/day was given on day 13. On day 27, his polyuria subsided and urine protein loss resolved. Serum levels of potassium, phosphate, calcium, and magnesium all returned within the reference range. This case highlighted that renal IRAEs, even though uncommon, could be severe and potentially life-threatening if left unrecognized and untreated. Early recognition of renal IRAEs and prompt withdrawal of ICPIs may result in lower renal morbidity.
Introduction
Immune checkpoints are composed of different pathways functioning to maintain self-tolerance and modify immune response. Recent progress in understanding that tumor cells could hijack these pathways to evade immune elimination urged the development of immune therapies targeting immune checkpoints (1). Immune checkpoints inhibitors (ICPIs) include monoclonal antibodies blocking either programmed cell death protein-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway or cytotoxic T-lymphocyte associated protein 4 (CTLA-4)/CD28 axis (2). Favorable outcomes have been increasingly reported in using ICPIs among diverse malignancies and approval indications have been expanding rapidly in either frontline or adjuvant settings. With the increasing use of ICPIs, the lurking threats of ICPIs related toxicity became widely recognized, especially immune-related adverse events (IRAEs) (3).
The spectrum of clinical manifestations in IRAEs is broad because a number of organs could be affected by immune intolerance including skin, gastrointestinal tract, lung, heart, endocrine organs, and nervous system being (4). Pembrolizumab is one of PD-1 inhibitors and, similar to other ICPIs, its IRAEs rarely affect the kidneys (5). However, pembrolizumab-associated proteinuria, acute kidney injury, and rarely renal tubular acidosis are increasingly reported recently (6, 7). To the best of our knowledge, pembrolizumab or other PD-1 inhibitors have not been published to cause a diffuse renal tubulopathy-related lethally acid-base and electrolytes disturbance. In this article, we present a case of developing a life-threatening electrolytes disturbance after receiving pembrolizumab therapy.
Case Description
A 67-year-old man presented with 1 week of fatigue, abdominal bloating, and constipation. He was found to have stage IV squamous cell carcinoma of the lung with bone metastasis 4 months ago, for which he received the first cycle of gemcitabine combined with cisplatin until 4 weeks ago. He denied a prior history of systemic diseases.
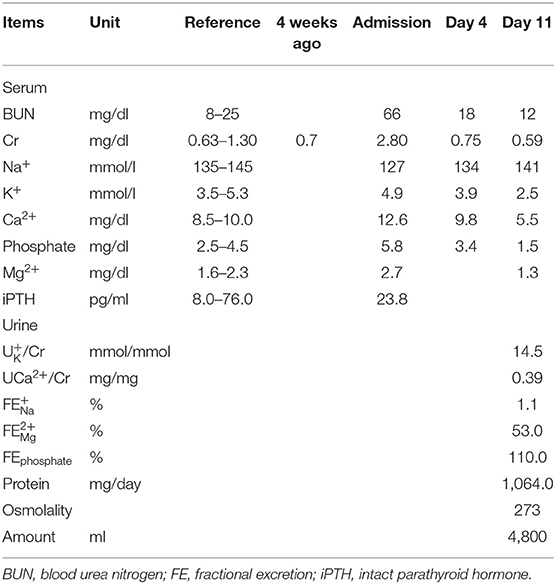
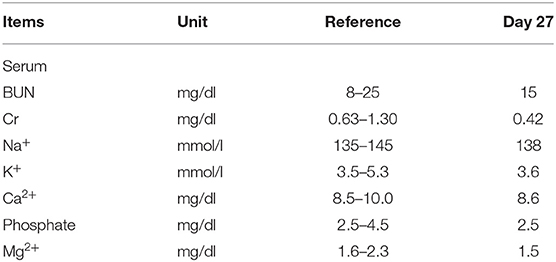
On admission, his blood pressure was 123/82 mmHg, pulse rate 102 beats/min, respiratory rate 18/min, and body temperature 37°C. Physical examination revealed poor skin turgor and mildly distended abdomen with tympanic percussion. The remainder of the physical examination was unremarkable. Laboratory tests showed an elevated serum creatinine (2.8 mg/dL; baseline 0.7 mg/dL 4 weeks ago), hypercalcemia (corrected Ca2+ 12.6 mg/dL), hyperphosphatemia (5.8 mg/dL) and a normal parathyroid hormone (PTH) level (23.7 pg/mL) (Table 1). Intravenous saline and subcutaneous denosumab 60 mg were used for treating hypercalcemia. On day four, his serum creatinine and Ca2+ was recovered. Due to chemotherapy intolerance, therapy was switched to immunotherapy with programmed cell death protein-1 (PD-1) receptor blocker, pembrolizumab 2mg/kg. On day 11, his urine output acutely increased to 4 to 5 L/day. He developed severe electrolytes disturbance, including hypokalemia (2.5 mmol/L), hypocalcemia (5.5 mg/dL), hypomagnesemia (1.3 mg/dL) and hypophosphatemia (1.5 mg/dL) along with renal wasting of these electrolytes (urine K+/Cr 14.5 mmol/mmol, urine Ca2+/Cr 0.39 mg/mg, fractional excretion (FE) of Mg2+ 53% and FE of phosphate 110%, respectively). He also had a new-onset of non-albumin predominant proteinuria (1,064 mg in 24-h urine collection) and persistent isothenuria in the absence of a deteriorated renal function (Table 1). Aggressive replacement of deficient electrolytes orally and intravenously was performed for 2 days but treatment was not successful. On day 13, oral prednisolone 1 mg/kg/day was immediately initiated along with discontinuation of pembrolizumab. On day 27, the serum levels of K+, phosphate, Ca2+, and Mg2+ all returned within the reference range and daily urine output decreased from 4–3 L to 1.0–1.5 L/day (Table 2).
Discussion
The presence of polyuria, isosthenuria, a new onset non-albumin predominant proteinuria, and severe disturbance of serum electrolytes including hypokalemia, hypocalcemia, hypophosphatemia, and hypomagnesemia with inappropriate renal wasting of K+, Ca2+, Mg2+, and phosphate suggests diffuse renal tubular injury. In the absence of other acquired tubulopathy causes such as systemic diseases, diuretics or the use of nephrotoxic agents, pembrolizumab-induced diffusely renal tubulopathy was favored.
ICPIs have revolutionized cancer treatments, either as first-line therapies or as adjuvant therapies. Tumors are able to evade immunosurveillance via diverse mechanisms including induction of immune tolerance through activating immune checkpoints (8). Pembrolizumab is one of the ICPIs targeting PD-1 on T lymphocytes to hinder the binding between PD-1 and its ligands (9). The prevalence of pembrolizumab-associated IRAEs, like other ICPIs, was reported higher than 50% but kidneys were rarely affected (7). However, with the increasing experience of pembrolizumab use, it has been proposed that the prevalence of pembrolizumab-associated renal toxicities may be underestimated (10). The most common renal toxicity of pembrolizumab is acute kidney injury (AKI) and the incidence has been reported ~1.7–6.7% with acute tubular injury or acute interstitial nephritis (AIN) being the most common pathological findings (5, 6, 11). In contrast, glomerular diseases are less frequent renal IRAEs, mainly podocytopathy. Distal renal tubule acidosis, although rare, was also identified in some case reports (12). However, diffusely renal tubule damages with severe electrolytes deficiency in the absence of AKI, as presented in our case study, have not been described before. In addition to assess renal function, our case highlights that the levels of serum electrolytes may warrant a close monitor after pembrolizumab therapy to prevent unrecognizing life-threatening electrolytes disturbance.
Denosumab, a monoclonal antibody directly against receptor activator of nuclear factor-κB ligand (RANKL), is widely used in osteoporosis or bone metastasis. Denosumab can cause hypocalcemia with a mechanism similar to hungry bone syndrome (13) as well as hypophosphatemia due to renal phosphate losing which is mediated by hypocalcemia-related secondary hyperparathyroidism post denosumab injection and is also a hungry bone-like mechanism (14). The hypophosphatemia with renal phosphate wasting in our case is indistinguishable from those caused by denosumab. However, denosumab has not been reported to cause hypokalemia, hypomagnesemia, proteinuria, or impaired urine concentration ability (15). Besides, urine calcium excretion will be decreased during denosumab-related hypocalcemia. Accordingly, both onset and recovery of each electrolyte's disturbances with renal wasting and impaired concentration ability occurred concomitantly, which suggested that pembrolizumab may be the main culprit.
Some risk factors of renal IRAEs have been identified including the use of proton pump inhibitors, impaired renal function, and combined use of ICPIs (16). The mechanism of pembrolizumab-associated kidney injury remains unclear. The ligand of PD-1 (PD-L1) expressed on renal epithelial cells has been reported to protect the kidneys against ischemia-reperfusion injury via inducing immune tolerance, regulating the activation of different T cell subgroups, and modifying cytokines and chemokines (11, 17). The use of pembrolizumab soon after AKI in this patient may interrupt PD-1/PD-L1 interaction-related immune tolerance, and cause autoimmune response and dysregulation of post-AKI inflammation; therefore, leading to tubulointerstitial damages (17). However, the wide variation in clinicopathological presentations of ICPI-related nephropathy suggests a more complex mechanism. Recently, the RANK-RANKL pathway was discovered to be crucial in many immune processes such as regulatory T lymphocytes generation (17). The synergistic anti-tumor effects of denosumab were described during the combination with ICPIs (18, 19). Therefore, denosumab needs to be accessed due to the possible increased risks of ICPIs related IRAEs since the synergistic effects may aggravate the autoimmune response and dysregulation of inflammation in the injured kidneys of our patient.
Discontinuation of PD-1 inhibitors is imperative when severe adverse kidney effects occur. Temporary use of immunosuppressant agents is common regarding the potentially pathogenic role of activation of autoreactive lymphocytes by PD-1 inhibitors (20). Except for glucocorticoid, several immunosuppressant agents were investigated including mycophenolate mofetil, cyclophosphamide, and rituximab. Infliximab against tumor necrosis factor-α (TNF-α) was found to be effective while patients had a poor response to other immunosuppressant agents, which is possibly because TNF-α is upregulated during ICPIs treatment (21). In general, a favorable renal prognosis has been reported in pembrolizumab-induced nephrotoxicities if diagnosis and management are prompt, best illustrated by the complete recovery of renal function in our patient. The rapid recovery of renal IRAEs within 14 days in our patient may suggest the role of immunosuppressant agents, supported by the reports with recovery to baseline kidney function within 1 month under the immunosuppressant therapy (22).
In conclusion, renal IRAEs, albeit uncommon, may be severe and potentially life-threatening if left unrecognized and untreated. Our case highlighted that early recognition along with the prompt withdrawal of culprit ICPIs helps to achieve a better outcome.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
P-JT collected data and made the tables. P-JT and M-TY drafted and revised the paper. Both authors approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li B, Chan HL, Chen P. Immune checkpoint inhibitors: basics and challenges. Curr Med Chem. (2019) 26:3009–25. doi: 10.2174/0929867324666170804143706
2. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1
3. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
4. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
5. Herrmann SM, Perazella MA. Immune Checkpoint Inhibitors and Immune-Related Adverse Renal Events. Kidney Int Rep. (2020) 5:1139–48. doi: 10.1016/j.ekir.2020.04.018
6. Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J. (2019) 12:81–8. doi: 10.1093/ckj/sfy100
7. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. (2016) 90:638–47. doi: 10.1016/j.kint.2016.04.008
8. Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. (2017) 47:765–79. doi: 10.1002/eji.201646875
9. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. (2018) 360:k793. doi: 10.1136/bmj.k793
10. Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. (2017) 45:160–9. doi: 10.1159/000455014
11. Perazella MA. Checkmate: kidney injury associated with targeted cancer immunotherapy. Kidney Int. (2016) 90:474–6. doi: 10.1016/j.kint.2016.05.024
12. El Bitar S, Weerasinghe C, El-Charabaty E, Odaimi M. Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep Oncol Med. (2018) 2018:8408015. doi: 10.1155/2018/8408015
13. Dave V, Chiang CY, Booth J, Mount PF. Hypocalcemia post denosumab in patients with chronic kidney disease stage 4-5. Am J Nephrol. (2015) 41:129–37. doi: 10.1159/000380960
14. Megapanou E, Florentin M, Milionis H, Elisaf M, Liamis G. Drug-induced hypophosphatemia: current insights. Drug Saf. (2020) 43:197–210. doi: 10.1007/s40264-019-00888-1
15. Suzuki T, Nakamura Y, Kato H. Changes of bone-related minerals during DENOSUMAB administration in post-menopausal osteoporotic patients. Nutrients. (2017) 9:871. doi: 10.3390/nu9080871
16. Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical Features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. (2020) 31:435–46. doi: 10.1681/ASN.2019070676
17. Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. (2018) 29:2039–52. doi: 10.1681/ASN.2018050488
18. Liede A, Hernandez RK, Wade SW, Bo R, Nussbaum NC, Ahern E, et al. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology. (2018) 7:e1480301. doi: 10.1080/2162402X.2018.1480301
19. Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signaling. Nature. (2011) 470:548–53. doi: 10.1038/nature09707
20. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
21. Murakami N, Borges TJ, Yamashita M, Riella LV. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kidney J. (2016) 9:411–7. doi: 10.1093/ckj/sfw024
Keywords: drug therapy, acute kidney injury, comorbidity, creatinine, electrolytes, pembrolizumab, immune checkpoint inhibitors, case report
Citation: Tseng P-J and Yan M-T (2021) Acute Diffuse Renal Tubulopathy in a Patient With Lung Cancer: A Case Report. Front. Med. 8:742489. doi: 10.3389/fmed.2021.742489
Received: 16 July 2021; Accepted: 03 September 2021;
Published: 04 October 2021.
Edited by:
Chia-Ter Chao, National Taiwan University Hospital Bei-Hu branch, TaiwanReviewed by:
Chrysanthi Sardeli, Aristotle University of Thessaloniki, GreeceIlya Glezerman, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2021 Tseng and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Tso Yan, cXFoYWliZWFyQGdtYWlsLmNvbQ==
 Po-Jung Tseng1
Po-Jung Tseng1 Ming-Tso Yan
Ming-Tso Yan