- 1Department of Pediatrics, Chang Gung Memorial Hospital, Keelung, Taiwan
- 2Chang Gung University College of Medicine, Taoyuan, Taiwan
- 3Molecular Infectious Disease Research Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 4Division of Pulmonology, Department of Pediatrics, Chang Gung Children's Hospital, Taoyuan, Taiwan
- 5Division of Allergy, Asthma, and Rheumatology, Department of Pediatrics, Chang Gung Children's Hospital, Taoyuan, Taiwan
- 6Department of Pediatrics, New Taipei Municipal TuCheng Hospital, Chang Gung Memorial Hospital and Chang Gung University, New Taipei, Taiwan
Background: Methicillin-resistant Staphylococcus aureus (MRSA) colonization in infants may pose a risk for subsequent infection in children. The study aimed to determine S. aureus colonization patterns in infancy, and strain relatedness between maternal and infant colonization.
Methods: A prospective cohort study was conducted for nasopharyngeal S. aureus detection in neonates at delivery; in children at 1, 6, 12, 24, 36, and 60 months of age; and from mothers immediately after the delivery of their baby and when their child is 1 month old. A questionnaire for infants and mothers was administered at each planned visit.
Results: In total, 521 and 135 infant–mother dyads underwent nasopharyngeal swab collection at 1 month and immediately after delivery, respectively. Among the 521 dyads at 1 month of age, concordant S. aureus colonization was found in 95 dyads, including MRSA in 48.4% (46/95). No concordant MRSA carriage was present among the 135 dyads at delivery. The genetic relatedness of concurrent MRSA-colonized dyads showed that more than two-thirds (32/46 [69.6%]) had identical genotypes, mainly ST 59/PVL-negative/SCCmec IV. Infants aged 1 month had the highest incidence of S. aureus, and the trend declined to a nadir at the age of 12 months. Carrier mothers who smoked cigarettes may increase the risk of infant Staphylococcus colonization (odds ratio, 2.12; 95% confidence interval, 1.23–3.66; p < 0.01).
Conclusions: Maternal–infant horizontal transmission may be the primary source of MRSA acquisition in early infancy. The avoidance of passive smoking could be recommended for the prevention of S. aureus carriage.
Introduction
Staphylococcus aureus is a major cause of infectious diseases ranging from soft-tissue infections to bacteraemia, which causes substantial morbidity or mortality in hospital settings and communities (1, 2). Methicillin-resistant S. aureus (MRSA) is usually considered a hospital pathogen, and its infection has traditionally been confined to individuals with health care–associated factors, including recent hospitalization, residence in long-term care units, indwelling catheters, or haemodialysis (3). In the past decades, the prevalence of infections caused by MRSA has increased among healthy people, particularly among children without established risk factors for MRSA acquisition (4), namely, community-associated MRSA (CA-MRSA).
In Taiwan, CA-MRSA infections have been increasingly reported in children since 2002, and the proportion of MRSA infections to the community-associated S. aureus infections in children without risk factors increased from 9.8% during 1997–2000 to 56% in 2004–2005 (5). Chen et al. (4) showed that MRSA has accounted for 50% of childhood community-associated S. aureus infections in Taiwan since 2005. In addition, the burden of S. aureus infection in infants is high. In the United States, hospitalized infants under 1 yr of age had the highest risk of Staphylococcus infection, highest annual incidence, and highest mortality among children under 17 yr of age (6). Although a high incidence of childhood CA-MRSA infection has occurred in the past decade, the cause of this increase is not well understood.
Colonization by S. aureus may play an important role in the development of Staphylococcus infection (7). Previous studies have shown that MRSA colonization poses a significantly greater risk to the development of subsequent infection than methicillin-sensitive S. aureus (MSSA) colonization (8). To understand the increasing rate of CA-MRSA infections in children, an investigation on the timing and source of MRSA acquisition in infants is required. Maternal MRSA carriage may also play a role in neonatal or infantile colonization (9); however, the phenotypic and molecular features of S. aureus and MRSA isolates between mothers and infants have rarely been assessed.
A birth cohort study was conducted, and infants were prospectively examined for colonized S. aureus at the planned time points. The dynamics of S. aureus carriage during early childhood, the possible factors involved in MRSA colonization, and the molecular relationships between maternal and childhood S. aureus carriage were investigated.
Methods
Study Population
An ongoing prospective study launched in 2012 that follows a birth cohort of infants, was initiated in Keelung Chang Gung Memorial Hospital to investigate bacterial colonization and factors related to the development of asthma and other allergic diseases. Keelung Chang Gung Memorial Hospital, which is a district hospital with a capacity of 1,100 beds in Keelung, Taiwan, is capable of handling academic research, clinical services, and medical education. Women admitted to the obstetrics clinic of this hospital during the third trimester of pregnancy were invited to participate in the study. The study project was approved by the institutional review board of Keelung Chang Gung Memorial Hospital, and written informed consent was obtained from each participant.
Study Design and Sample Collection
From March 2013 to December 2019, the mothers were requested to bring the enrolled infants to the pediatric clinic of Keelung Chang Gung Memorial Hospital for S. aureus detection from nasopharyngeal swabs at 1, 6, 12, 24, 36, and 60 months of age. One swab per child was collected per visit. A nasopharyngeal swab was collected from the mother when the swab was taken from the infant at the age of one month. To further investigate maternal and infantile carrier status at delivery, nasopharyngeal swabs were collected from the mothers and infants on the day of delivery starting from March 2015. Additionally, vaginal group B Streptococcus (GBS) screening was performed between gestational weeks 35 and 37 during routine maternal prenatal examination, and samples from the volunteer pregnant women were collected for S. aureus detection during the same period. Questionnaires were administered at each planned visit to obtain information such as demographic data, housing, living conditions, risk factors for Staphylococcus carriage, history of Staphylococcus infection and hospitalization, and other medical illnesses.
Identification and Characterization of S. aureus
Nasopharyngeal or vaginal swabs were collected using separate cotton-tipped swabs (Copan Swab Applicator, Copan Diagnostics Inc., Brescia, Italy) at the scheduled visits. After being placed in a transported medium, swabs were transported to the microbiology laboratory within two h after collection and were cultured for bacteria by using standard methods for identification (10). Swab samples were inoculated onto trypticase soy agar with 5% sheep blood plates by using the streak plate method. On the basis of the patterns of beta-haemolysis and the macroscopic appearance, colonies of suspected S. aureus were further incubated on 5% sheep blood agar plates at 37 °C overnight. After incubation, a coagulase test was performed using rabbit plasma to identify S. aureus. To detect MRSA in specimens, S. aureus was first identified by a coagulase test, and a cefoxitin test was conducted to distinguish MRSA from MSSA in accordance with the recommendations of CLSI document M100-S18 (11). All S. aureus isolates were stored for further molecular characterization.
Pulsed-field gel electrophoresis (PFGE) with SmaI digestion was performed according to previously described procedures (12), and the genotypes were designated in alphabetical order. PFGE patterns with fewer than four band differences from an existing genotype were defined as subtypes of this genotype (13). Two isolates were considered indistinguishable, highly related, or distinct if they had the same subtype (no band difference), the same genotype (less than four band differences), or different types (equal to or greater than four band differences). Isolates with representative PFGE patterns were selected, and multilocus sequence typing (MLST) was conducted (http://www.mlst.net) (14). The allelic profiles were assigned by comparing the sequence at each locus with the sequences of the known alleles in the S. aureus MLST database, and the resulting profiles were defined as sequence types (STs) accordingly.
The Staphylococcus cassette chromosome (SCCmec) typing of isolates was determined using multiplex PCR, as described previously (15). The control strains for SCCmec types I, II, III, and IVa were as follows: type I, NCTC10442; type II, N315; type III, 85/2082; and type IVa, JCSC4744. SCCmec typing for type VT was determined using a specific primer described elsewhere (13), and the TSGH-17 strain was used as a control. The presence of Panton–Valentine leucocidin (PVL) genes was determined by PCR, as described by Lina et al. (16).
Statistical Analysis
Data were analyzed using SPSS (version 22.0; SPSS Inc., Chicago, IL, USA). Student's t-test was used to analyse the numerical data. If the data were not normally distributed, the Mann–Whitney U test was used for analysis. The chi-square test or Fisher's exact test was used when appropriate to analyzed the categorical data. In univariate analysis, clinical variables were compared between carrier and non-carrier mothers if their infants were S. aureus carriers at the same time. Variables for which P < 0.1 were chosen for model selection in multivariate analysis. Statistical significance was set at P < 0.05.
Results
Study Population
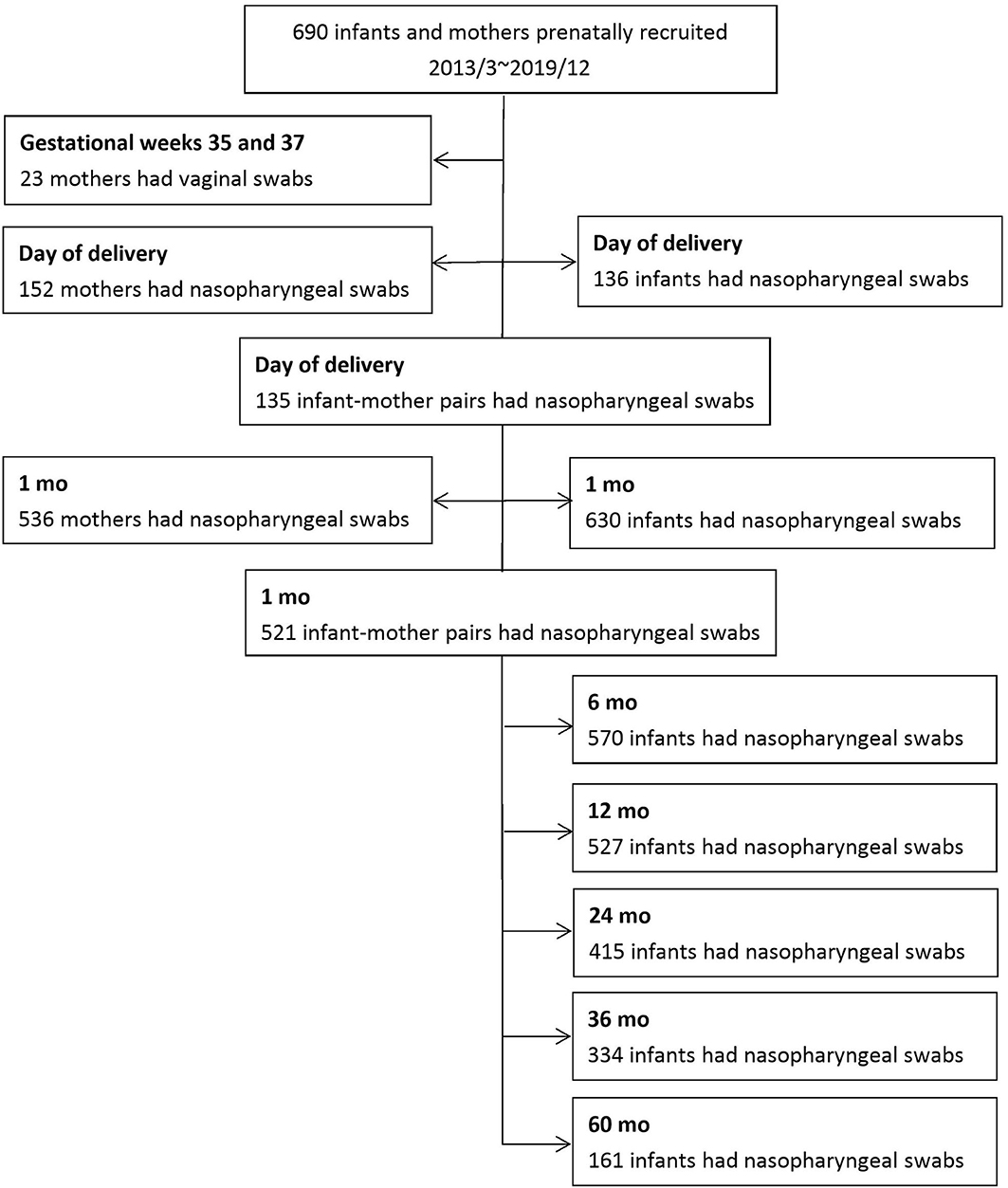
From March 2013 to December 2019, 690 infants and their mothers were prenatally recruited. Questionnaire-based surveys and nasopharyngeal swabs were received from 630 infants and 536 mothers one month past delivery. Since March 2015, nasopharyngeal swabs were collected from 136 infants and 152 mothers on the day of delivery. In total, 521 infant–mother pairs were enrolled one month past delivery, and 135 dyads were collected at delivery. Vaginal swabs from 23 volunteer pregnant women were obtained when routine GBS cultures were performed between gestational weeks 35 and 37. Figure 1 shows the detailed enrollment of this cohort.
Regarding the S. aureus carriage in children during the first 60 months of life, S. aureus colonization was observed in 3.7% (5/136) at delivery, 48.7% (307/630) at 1 month of age, 18.4% (105/570) at 6 months of age, 10.2% (54/527) at 12 months of age, 15.9% (66/415) at 24 months of age, 24.3% (81/334) at 36 months of age, and 29.8% (48/161) at 60 months of age. MRSA colonization was noted in 2.2% (3/136) at birth, 30.5% (192/630) at 1 month, 10.9% (62/570) at 6 months, 6.6% (35/527) at 12 months, 9.9% (41/415) at 24 months, 12.3% (41/334) at 36 months, and 23.0% (37/161) at 60 months. Infants aged 1 month had the highest incidence of S. aureus and MRSA carriage, and the trend declined to a nadir at the age of 12 months. However, the carriage rate gradually increased between 24 and 60 months of age. Figure 2 shows the trend of S. aureus and MRSA colonization at the planned sampling time points during the first 60 months of life.
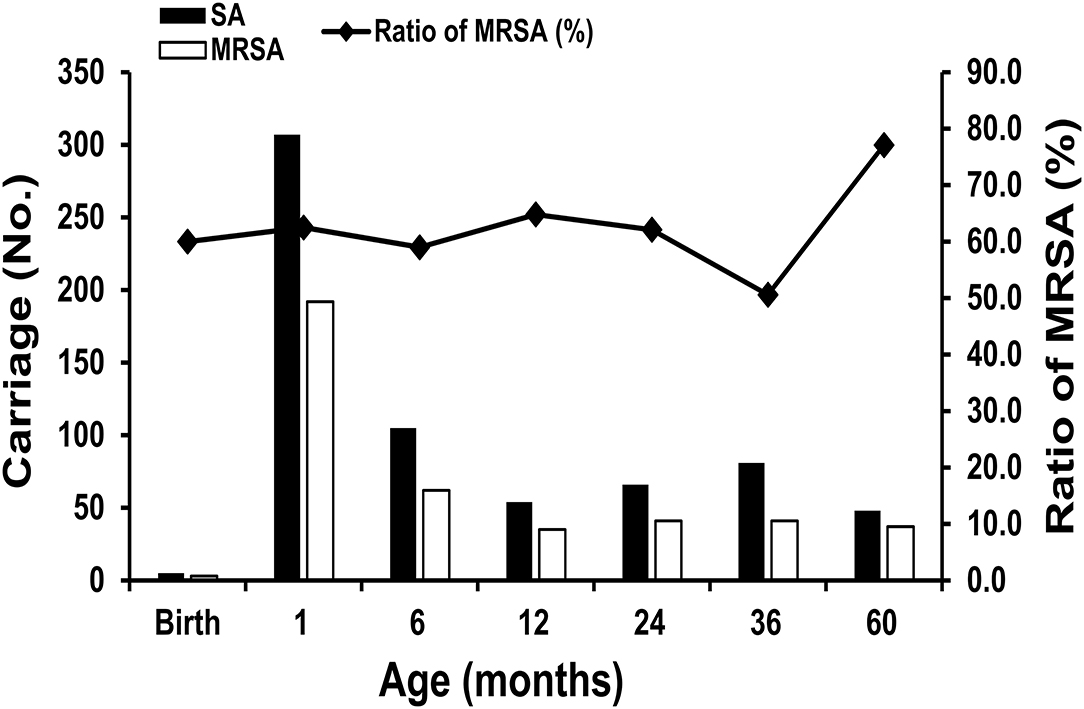
Figure 2. The trend of Staphylococcus aureus colonization, including methicillin-resistant Staphylococcus aureus (MRSA) at the planned sampling time points during the first 60 months of life.
Characterization of S. aureus Carriage and Genetic Relatedness Among Infant–Mother Pairs
Among the 521 dyads at the age of 1 month, S. aureus colonization was observed in 49.7% (259/521) of infants and 27.6% (144/521) of mothers. Concordant S. aureus colonization was found in 95 infant–mother dyads, including MRSA in 48.4% (46/95), MSSA in 33.7% (32/95), and mixed MRSA/MSSA in 17.9% (17/95). Additionally, 3.7% (5/135) colonized infants and 23.7% (32/135) mothers were noted among the 135 dyads at delivery. However, only one pair of concordant S. aureus carriage was found among these dyads, and the S. aureus isolates belonged to MSSA.
For the molecular investigation of concordant S. aureus–colonized isolates, 95 infant–mother pairs at 1 month of age were obtained to analyze strain relatedness. The results revealed that 56.8% (54/95) had identical genotypes, including 69.6% (32/46) concordant MRSA [mainly ST 59/PVL-negative/ SCCmec IV, 54.3% (25/46)], 56.3% (18/32) concordant MSSA, and 23.5% (4/17) mixed MRSA/MSSA colonized pairs belonging to genetically indistinguishable genotypes. Table 1 and Table S1 show the molecular characteristics of S. aureus isolates among infant–mother pairs.
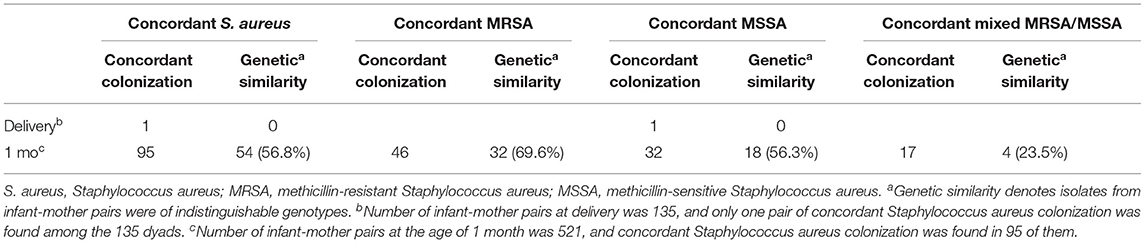
Table 1. Molecular relatedness of concordant colonization with Staphylococcus aureus in infant-mother pairs.
Among the 135 dyads at delivery, nasopharyngeal swabs were also administered for 124 dyads at 1 month of age. To longitudinally investigate the association between infant and mother carriers, the S. aureus characteristics of the 124 infant–mother pairs were further analyzed. Among these dyads, S. aureus colonization was observed in 1 infant (MSSA) and 31 mothers at delivery, but no concordant S. aureus carriage was present. In addition, 51.6% (64/124) of infants and 25.8% (32/124) of mothers were colonized at the age of 1 month, and concordant S. aureus colonization was found in 25 dyads, including MRSA in 52.0% (13/25), MSSA in 32.0% (8/25), and mixed MRSA/MSSA in 16.0% (4/25). With regard to the investigation of molecular genotype, most of the concordant MRSA-colonized dyads (69.2%, 9/13) were genetically indistinguishable genotypes, followed by 37.5% (3/8) with identical genotypes in MSSA dyads and 25.0% (1/4) in mixed MRSA/MSSA dyads.
Twenty-three mothers had vaginal S. aureus colonization during pregnancy. Among the 23 mothers with vaginal S. aureus colonization, 6 had nasopharyngeal colonization at the age of 1 month. Concordant S. aureus infant carriers were found among them, including MSSA in four mothers, MRSA in one mother, and mixed MRSA/MSSA in one mother. The results of the genetic relatedness of concordant strains from mothers with vaginal S. aureus colonization, mothers with nasopharyngeal S. aureus colonization, and infants with nasopharyngeal S. aureus colonization showed that most of them (83.3%, 5/6) had identical genotypes (ST 59/pulsotype A1/PVL-negative/ SCCmec IV), and all infants were born with vaginal delivery.
Comparison of Clinical Features Between Colonized Infants With Carrier Mothers and Without Carrier Mothers
To understand the influence of maternal carriage on infant S. aureus colonization, three variables, including demographic features, environmental factors, and health conditions, were compared between colonized infants with carrier mothers and those with non-carrier mothers (Table 2). Except for passive smoking exposure (OR 2.06, 95% CI 1.22–3.47, P < 0.01), no other variables were significantly associated with infant colonization with S. aureus in the univariate logistic regression analysis. Furthermore, passive smoking exposure related to infant S. aureus colonization was also found after multiple logistic regression analysis (OR 2.12, 95% CI 1.23–3.66, P < 0.01). Infant exposed to burning incense lighted by the carrier mothers seemed to be negatively associated with S. aureus colonization, but the difference was not statistically significant (OR 0.61, 95% CI 0.36–1.05, P = 0.08).
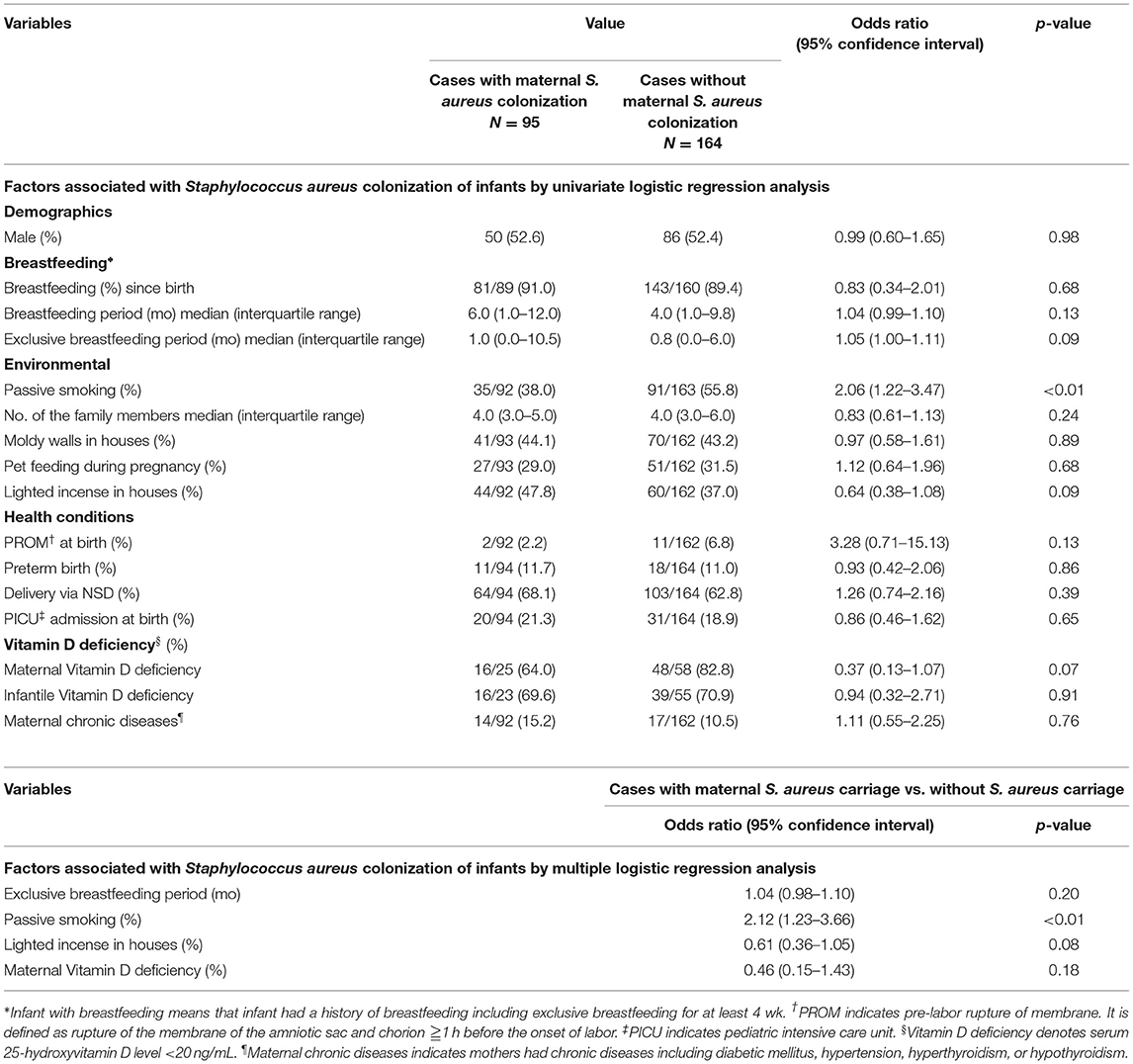
Table 2. Univariate and multivariate analyses of clinical features of infants and mothers by maternal Staphylococcus aureus carrier status.
Discussion
Our cohort showed that 10.2–48.7% and 6.6–30.5% of infants had S. aureus and MRSA carriage between and 2013–2019, respectively. The percentage was lower than that reported in other populations of Taiwanese children (S. aureus in 90%; MRSA in 40% of children aged <2 years) from 2005 to 2010 (14). The difference in the colonization rate may be attributed to the different study locations or periods. In the United States, a decline in the proportion of MRSA among S. aureus isolates (60.4% decrease) or a trend of MRSA infection (52% decrease) has been observed in the pediatric population since 2010 (17, 18).
Among the S. aureus carriage trends during the first five yr of life, the highest prevalence of S. aureus and MRSA carriage was observed at the age of one month, and the trend declined gradually in the first yr of life; however, the rising trend occurred between two and five yr of age. By contrast, an increasing trend of Streptococcus pneumoniae colonization was found during the first three yr of life in our previous report (19). This finding is consistent with those of other longitudinal studies conducted in Taiwan (20). The inverse relationship between S. aureus and S. pneumoniae might be explained by the pneumococcal inhibiting effect on S. aureus, which is mediated by the production of hydrogen peroxide by S. pneumoniae (21).
Results from our study showed that only 3.7% of infants had S. aureus colonization at delivery, and the colonization rate peaked (48.7%) in the first month of life. This finding supports our understanding that the fetus is sterile until shortly before birth and that the neonate becomes colonized with bacteria after birth (22). This suggests that infants acquire S. aureus mainly through horizontal transmission from surrounding carriers, possibly from family members. To clarify the association between maternal and infantile colonized isolates, the genetic relatedness of concurrent S. aureus and MRSA-colonized dyads was investigated. The results showed that 56.8% and 69.6% of concurrent S. aureus and MRSA-colonized dyads had identical genotypes, respectively. In the United States, Jimenez-Truque et al. (1) reported that 43% of S. aureus–colonized maternal–infant dyads had indistinguishable isolates, and Huang et al. (23) also found that half of the paired S. aureus isolates were genetically identical in their cohort. These results demonstrate the important role of maternal–infant horizontal transmission in Staphylococcus or MRSA carriage in early infancy.
Although the colonized infants might have acquired MRSA from their mothers via horizontal transmission, we cannot completely confirm this assumption if maternal colonization status before delivery is not analyzed. Thus, the association between the vaginal Staphylococcus colonization of mothers during pregnancy and nasopharyngeal carriage of infants after birth was investigated. One-fourth of infants (6/23 [26.1%]) born to mothers with vaginal S. aureus colonization had concurrent nasopharyngeal colonization. Although the number of maternal–infant pairs was small in our series, most of the concordant colonized isolates (5/6 [83.3%]) were genetically indistinguishable genotypes. This finding implies that vertical transmission may still occur in a minority of cases even though horizontal spread within family members (particularly from mother to infants) remains the primary method of MRSA acquisition in early infancy. Jimenez-Truque et al. (1) reported that infants born to mothers with S. aureus vaginal colonization were five times more likely to be nasally colonized within two h of birth. However, only 8.2% of pregnant women may be vaginally colonized with S. aureus according to the previous report (24). Therefore, the occurrence of vertical transmission is relatively uncommon and does not lead to a significant risk of S. aureus acquisition in early infancy.
Given the importance of maternal carriage on infantile S. aureus colonization, the clinical characteristics of infants and mothers according to maternal carrier status were compared. We analyzed demographic, environmental, and health-related factors being suggested to influence the infantile S. aureus carriage including gender, breastfeeding, number of family members, history of labor and environmental conditions (i.e., passive smoking or burning incense) (25). The result showed that infants born to smoking carrier mothers were found to be at risk of S. aureus colonization. This finding was consistent with those of previous studies which showed that passive smoking was associated with increased S. aureus colonization (26). The cause of passive smoke exposure associated with the carriage of pathogenic bacteria is not fully understood. One possible explanation is mucociliary transport alteration. Several studies have shown that cigarette smoke may reduce the ciliary beat of respiratory epithelial cells or impair respiratory epithelial ciliogenesis (27). Tobacco smoke may inhibit interleukin-8 and human β-defensin in sinonasal epithelial cell cultures derived from patients with chronic rhinosinusitis, thus suggesting that passive smoke may have a suppressive function on sinonasal innate immunity (28). Therefore, passive smoke exposure may compromise the antibacterial function of leukocytes and increase the risk of colonization by pathogenic S. aureus on respiratory epithelial cells. The influence of lighted incense on infantile S. aureus carriage was also investigated, but the result was not inconsistent with that of passive smoking. The cause of this inconsistency may be due to different toxicants present in incense and tobacco smoke resulting in differential cytotoxic or mutagenic effects on the bacterial community (29). Vallès et al. (30) found that the effect of burning incense exposure on microbiota composition may differ from those of tobacco exposure, and exposure to lighted incense was associated with depletion of the Firmicutes class Bacilli (e.g., Staphylococcus), which was compatible with our study result.
In our study, the most common clone accounting for 54.3% (25/46) of the MRSA isolates among infant–mother pairs were identified as ST 59/PVL-negative/SCCmec IV, namely, “Asian-Pacific” clone. A previous molecular epidemiology study of CA-MRSA in Taiwan revealed that two genotypes accounted for the majority of CA-MRSA strains, including the “Taiwan” clone (ST 59/PVL-positive/SCCmec VT) and an “Asian-Pacific” clone (ST 59/PVL-negative/SCCmec IV) (31). The isolates of both pulsotypes belonging to the ST 59 lineage had a similar genetic background; however, the Asian-Pacific clone was more prevalent in colonizing healthy individuals than the Taiwan clone (29). Among the MRSA carrier surveillance studies in 2001–2002 and 2005–2006, 62–78.3% of colonized isolates belonged to the Asian-Pacific clone, and these findings were similar to our study results (32, 33).
This study has some limitations. First, this is an ongoing project, but the period of this study was limited to 2013–2019; therefore, some enrolled cases could not complete the entire 60-month study course. Second, the number of pregnant women with vaginal Staphylococcus colonization was relatively small; therefore, the role of vertical transmission in infantile MRSA carriage should be interpreted with caution in our series.
In conclusion, most concordant MRSA-colonizing isolates were of indistinguishable genotypes, thus suggesting that maternal–infant horizontal transmission may be the primary source for MRSA acquisition in early infancy. Cigarette smoke may contribute to increased susceptibility to Staphylococcus colonization, and the avoidance of passive smoking could be recommended as an important strategy for the prevention of S. aureus carriage in infants.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Participant privacy prevents public sharing of individual-level data. Requests to access these datasets should be directed to H-JS, YWRhc2hpaDQwMTYmI3gwMDA0MDtnbWFpbC5jb20=.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Keelung Chang Gung Memorial Hospital, Taiwan. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
M-HT designed the study, collected and analyzed the data, and drafted and revised the manuscript. C-YC, K-WS, S-LL, M-CH, T-CY, S-HL, K-WY, and L-CC collected the data and reviewed the manuscript. H-JS did the experiment and collected the data. M-HT and J-LH reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Keelung Chang Gung Memorial Hospital, Taiwan (CMRPG2E0125 and CMRPG2K0311).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the members of the Molecular Infectious Disease Research Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan, and Department of Medical Laboratory, Chang Gung Memorial Hospital, Keelung, Taiwan, for their suggestions and assistance in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.738724/full#supplementary-material
Table S1. Molecular characteristics of methicillin-resistant Staphylococcus aureus isolates among 46 infant-mother pairs at the age of 1 month.
References
1. Jimenez-Truque N, Tedeschi S, Saye EJ, McKenna BD, Langdon W, Wright JP, et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics. (2012) 129:1–8. doi: 10.1542/peds.2011-2308
2. Lebon A, Labout JA, Verbrugh HA, Jaddoe VW, Hofman A, van Wamel WJ, et al. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the generation R study. J Clin Microbiol. (2008) 46:3517–21. doi: 10.1128/JCM.00641-08
3. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. (2003) 290:2976–84. doi: 10.1001/jama.290.22.2976
4. Chen CJ, Wang SC, Chang HY, Huang YC. Longitudinal analysis of methicillin- resistant and methicillin-susceptible Staphylococcus aureus carriage in healthy adolescents. J Clin Microbiol. (2013) 51:2508–14. doi: 10.1128/JCM.00572-13
5. Huang YC, Chen CJ. Community-associated methicillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. Int J Antimicrob Agent. (2011) 38:2–8. doi: 10.1016/j.ijantimicag.2011.01.011
6. Gutierrez K, Halpern MS, Sarnquist C, Soni S, Arroyo AC, Maldonado Y. Staphylococcal infections in children, California, USA, 1985–2009. Emerg Infect Dis. (2013) 19:10–20. doi: 10.3201/eid1901.111740
7. Halablab MA, Hijazi SM, Fawzi MA, Araj GF. Staphylococcus aureus nasal carriage and associated risk factors in individuals in the community. Epidemiol Infect. (2010) 138:702–6. doi: 10.1017/S0950268809991233
8. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. (2004) 39:971–9. doi: 10.1086/423965
9. Leshem E, Maayan-Metzger A, Rahav G, Dolitzki M, Kuint J, Roytman Y, et al. Transmission of Staphylococcus aureus from mothers to newborns. Pediatr Infect Dis J. (2012) 31:360–3. doi: 10.1097/INF.0b013e318244020e
10. Murray PR, Bron EJO, Pfaller MA. Manual of Clinical Microbiology. 6th ed Washington DC: American Society of Microbiology. (1980).
11. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 23rd informational supplement. Document M100-S18. Wayne: CLSI (2013).
12. Huang YC, Ho CF, Chen CJ, Su LH, Lin TY. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin Microbiol Infect. (2008) 14:1167–72. doi: 10.1111/j.1469-0691.2008.02115.x
13. Huang YC, Chen CJ. Nasal carriage of methicillin-resistant Staphylococcus aureus during the first 2 years of life in children in northern Taiwan. Pediatr Infect Dis J. (2015) 34:131–5. doi: 10.1097/INF.0000000000000517
14. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. (2000) 38:1008–15. doi: 10.1128/JCM.38.3.1008-1015.2000
15. Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. (2007) 51:264–74. doi: 10.1128/AAC.00165-06
16. Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter M, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. (1999) 29:1128–32. doi: 10.1086/313461
17. Hultén KG, Mason EO, Lamberth LB, Forbes AR, Revell PA, Kaplan SL. Analysis of invasive community-acquired methicillin-susceptible Staphylococcus aureus infections during a period of declining community acquired methicillin-resistant Staphylococcus aureus infections at a large children's hospital. Pediatr Infect Dis J. (2018) 37:235–41. doi: 10.1097/INF.0000000000001753
18. Spaulding AB, Thurm C, Courter JD, Banerjee R, Gerber JS, Newland JG, et al. Epidemiology of Staphylococcus aureus infections in patients admitted to freestanding pediatric hospitals, 2009-2016. Infect Control Hosp Epidemiol. (2018) 39:1487–90. doi: 10.1017/ice.2018.259
19. Tsai MH, Liao SL, Chiu CY, Shih HJ, Hua MC, Yao TC, et al. Longitudinal investigation of nasopharyngeal pneumococcal carriage in early childhood: The PATCH birth cohort study. PLoS ONE. (2020) 15:e0237871. doi: 10.1371/journal.pone.0237871
20. Chen CH, Kuo KC, Hwang KP, Lin TY, Huang YC. Risk factors and molecular characteristics of methicillin-resistant Staphylococcus aureus nasal colonization among healthy children in southern Taiwan, 2005-2010. J Microbiol Immunol Infect. (2019) 52:929–36. doi: 10.1016/j.jmii.2018.09.003
21. Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. (2006) 188:4996–5001. doi: 10.1128/JB.00317-06
22. Rotimi VO, Dureden BI. The development of the bacterial flora in normal neonates. J Med Microbiol. (1981) 14:51–62. doi: 10.1099/00222615-14-1-51
23. Huang YC, Chao AS, Chang SD, Chen YJ, Peng MT, Sung JH, et al. Association of Staphylococcus aureus colonization in parturient mothers and their babies. Pediatr Infect Dis J. (2009) 28:742–44. doi: 10.1097/INF.0b013e31819c132a
24. Tomlinson MW, Schmidt NM, Rourke JW Jr, McDonald J. Rectovaginal Staphylococcus aureus colonization: is it a neonatal threat? Am J Perinatol. (2011) 28:673–6. doi: 10.1055/s-0031-1276732
25. Chatzakis E, Scoulica E, Papageorgiou N, Maraki S, Samonis G, Galanakis E. Infant colonization by Staphylcoccus aureus: role of maternal carriage. Eur J Clin Microbiol Infect Dis. (2011) 30:1111–17. doi: 10.1007/s10096-011-1199-9
26. Kulkarni R, Antala S, Wang A, Amaral FE, Rampersaud R, LaRussa SJ, et al. Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect Immun. (2012) 80:3804–11. doi: 10.1128/IAI.00689-12
27. Tamashiro E, Xiong G, Anselmo-Lima WT, Kreindler JL, Palmer JN, Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. (2009) 23:117–22. doi: 10.2500/ajra.2009.23.3280
28. Lee WK, Ramanathan M, Spannhake EW, Lane AP. The cigarette smoke component acrolein inhibits expression of the innate immune components IL-8 and human beta-defensin 2 by sinonasal epithelial cells. Am J Rhinol. (2008) 21:658–63. doi: 10.2500/ajr.2007.21.3094
29. Zhou R, An Q, Pan XW, Yang B, Hu J, Wang YH. Higher cytotoxicity and genotoxicity of burning incense than cigarette. Environ Chem Lett. (2015) 13:465–71. doi: 10.1007/s10311-015-0521-7
30. Vallès Y, Inman CK, Peters BA, Wareth LA, Abdulle A, Alsafar H, et al. Incense burning is associated with human oral microbiota composition. Sci Rep. (2019) 9:10039. doi: 10.1038/s41598-019-46353-y
31. Chen CJ, Hsu KH, Lin TY, Hwang KP, Chen PY, Huang YC. Factors associated with nasal colonization of methicillin-resistant Staphylococcus aureus among healthy children in Taiwan. J Clin Microbiol. (2011) 49:131–7. doi: 10.1128/JCM.01774-10
32. Chen CJ, Unger C, Hoffmann W, Lindsay JA, Huang YC, Götz F. Characterization and comparison of 2 distinct epidemic community-associated methicillin-resistant Staphylococcus aureus clones of ST59 lineage. PLoS ONE. (2013) 8:e63210. doi: 10.1371/journal.pone.0063210
Keywords: methicillin-resistant Staphylococcus aureus, children, mother, colonization, smoking
Citation: Tsai M-H, Chiu C-Y, Su K-W, Liao S-L, Shih H-J, Hua M-C, Yao T-C, Lai S-H, Yeh K-W, Chen L-C and Huang J-L (2021) Community-Associated Methicillin-Resistant Staphylococcus Aureus Colonization in a Birth Cohort of Early Childhood: The Role of Maternal Carriage. Front. Med. 8:738724. doi: 10.3389/fmed.2021.738724
Received: 09 July 2021; Accepted: 30 September 2021;
Published: 26 October 2021.
Edited by:
Richard V. Goering, Creighton University, United StatesReviewed by:
Stefan Monecke, Leibniz Institute of Photonic Technology (IPHT), GermanyAna Budimir, University of Zagreb, Croatia
Copyright © 2021 Tsai, Chiu, Su, Liao, Shih, Hua, Yao, Lai, Yeh, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Han Tsai, ZHJ0c2FpMTIwOCYjeDAwMDQwO2dtYWlsLmNvbQ==; Jing-Long Huang, bG9uZyYjeDAwMDQwO2FkbS5jZ21oLm9yZy50dw==
 Ming-Han Tsai
Ming-Han Tsai Chih-Yung Chiu
Chih-Yung Chiu Kuan-Wen Su1,2
Kuan-Wen Su1,2 Kuo-Wei Yeh
Kuo-Wei Yeh Li-Chen Chen
Li-Chen Chen