
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 10 December 2021
Sec. Intensive Care Medicine and Anesthesiology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.738418
Background: Conservative oxygen therapy can prevent both hypoxemia and hyperoxemia, but the effect on the prognosis of patients admitted to the intensive care unit (ICU) remains controversial.
Methods: All controlled studies comparing conservative oxygen therapy and conventional oxygen therapy in adult patients admitted to the ICU were searched. The primary outcome was mortality, and the secondary outcomes were length of ICU stay (ICU LOS), length of hospital stay (hospital LOS), length of mechanical ventilation (MV) hours, new organ failure during ICU stay, and new infections during ICU stay.
Results: Nine trials with a total of 5,759 patients were pooled in our final studies. Compared with conventional oxygen therapy, conservative oxygen therapy did not reduce overall mortality (Z = 0.31, p = 0.75) or ICU LOS (Z = 0.17, p = 0.86), with firm evidence from trial sequential analysis, or hospital LOS (Z = 1.98, p = 0.05) or new infections during the ICU stay (Z = 1.45, p = 0.15). However, conservative oxygen therapy was associated with a shorter MV time (Z = 5.05, p < 0.00001), reduction of new organ failure during the ICU stay (Z = 2.15, p = 0.03) and lower risk of renal replacement therapy (RRT) (Z = 2.18, p = 0.03).
Conclusion: Conservative oxygen therapy did not reduce mortality but did decrease MV time, new organ failure and risk of RRT in critically ill patients.
Systematic Review Registration: identifier [CRD42020171055].
Hypoxemia is life threatening (1, 2) and is related to increasing intensive care unit (ICU) mortality (3). Oxygen administration is a life-saving treatment commonly used in patients admitted to the ICU (4, 5). Unfortunately, although oxygen administration in ICUs is recommended by many guidelines, the most suitable oxygenation target remains unknown (6).
Studies have shown that excess oxygen delivery is very common, and approximately 50% of patients show hyperoxemia, among whom 4% have severe hyperoxemia (7–9). In our previous study, hyperoxia was independently associated with ICU mortality in mechanical ventilation patients [odds ratio (OR) 1.22, 95% confidence interval (CI) 1.12–1.33] (10).
Thus, to prevent hypoxemia and avoid adverse events caused by hyperoxemia, some researchers have studied conservative oxygen therapy, which adheres to the pulse oxygen saturation (SpO2) goal between 88 and 92% with the lowest fraction of inspired oxygen (FiO2). However, the results have remained controversial. In the study by Girardis et al., which included 434 patients, conservative oxygen therapy reduced ICU mortality by approximately 19% (p = 0.01) (11). However, in the study by Mackle et al., conservative oxygenation targets did not show any advantages in ICU mortality over the conventional oxygenation target (35.7 vs. 34.5%) (12).
Therefore, based on the controversial results of the effects of conservative oxygen therapy, we conducted a systematic review with meta-analysis and trial sequence analysis of all published trials aiming to identify the role of conservative oxygen therapy in improving the outcomes of patients admitted to the ICU.
We systematically searched PubMed, Embase, Medline, Cochrane Central Register of Controlled Trails (CENTRAL), and Information Sciences Institute (ISI) Web of Science using the keywords “conservative oxygen therapy” or “conservative oxygenation target” or “oxygenation target” and “critically ill” or “ICU” or “intensive care unit” without limitations on the publication type or language from 1946 to August 2020. A hand search through the reference lists of relevant primary and review articles was also performed for completeness.
Eligible clinical trials were identified based on the following criteria: (1) the subjects enrolled in each study included patients admitted to the ICU; (2) patients were divided into an experimental group, in which conservative oxygen therapy (the target of fraction of inspired oxygen (FiO2) including in room air, or arterial partial pressure of oxygen (PaO2), or arterial oxygen saturation (measured by blood analysis), or peripheral oxygen saturation [measured by a pulse oximeter (SpO2)] were lower than the control group) was applied, and a control group (higher oxygen target); and (3) outcomes included, but were not limited to, mortality at the longest following up time point, length of ICU stay (ICU LOS) (since the start of the study), length of hospital stay (hospital LOS) (since the start of the study), length of mechanical ventilation (MV) hours (since the start of the study), new infection (defined as positive bacterial culture in sputum, urine, or blood),new organ failure (defined as a SOFA score ≥3 for the corresponding organ occurring 48 h or more after ICU admission) during ICU and the rate of renal replacement therapy (RRT). We excluded studies if they were performed on animals or in patients younger than 18 years old or were published as reviews or case reports.
Two investigators (Y-NN and TW) independently screened studies for eligibility according to predefined study selection criteria. Titles and abstracts from the search were examined, and full texts were obtained for all potentially relevant records. Any disagreement was resolved through discussion with a third author (B-ML).
Data were extracted in duplicate by two independent data collectors using a standard form recommended by Cochrane. Authors, publication year, study design, country, NCT No., population, demographic characteristics (age, gender, etc.), disease conditions [the Acute Physiologic and Chronic Health Evaluation III (APACHE III) and Simplified Acute Physiologic Score II (SAPS II)], outcome measures, and study results were extracted. In cases in which data points were missing or ambiguously reported, the corresponding author was contacted by email to obtain the data.
Two authors independently assessed the risk of systematic bias of trials included in the meta-analysis according to the Cochrane Handbook (13). Disagreement during the review process was resolved by consensus through involvement of a third review author. Risk of bias was rated according to the following domains: (1) sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective outcome reporting; and (7) other sources of bias (specifically including baseline imbalance, early stopping and financial bias).
Meta-analysis and forest plots were prepared using the Cochrane systematic review software Review Manager (RevMan; version 5.3.5; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark, 2014). We used the Mantel-Haenszel model to verify the hypothesis and rendered statistical significance as a Z-value and p < 0.05. I2 was used to estimate variation across studies attributable to heterogeneity. A value of p < 0.1 and I2 >50% indicated significance. A random-effects model was applied in the presence of statistical heterogeneity; for continuous data, we calculated the mean difference (MD) and 95% CIs, while for dichotomous data, we calculated the ORs and 95% CIs. We also performed sensitivity analysis to substitute alternative decisions or ranges of values for decisions that were arbitrary or unclear. Trial sequential analysis was performed using TSA viewer software, version 0.9, to correct for cumulative heterogeneous results, decrease type I error and model the potential effects of uncompleted registered studies. Information size was computed assuming an alpha risk of 5% and a beta risk of 20%. Trial sequential analysis 95% CI boundaries that excluded the null (<1.00 or >1.00) were considered statistically significant.
Initially, 1,570 records were identified, of which 1,567 were extracted from electronic databases, and three were extracted from reference list reviews. By screening the titles and abstracts, 1,561 studies were discarded due to duplication (n = 1,297), animal experiments (n = 210) and non-adult patients (n = 52). We researched the full-text articles for the remaining 11 studies, and nine trials were eventually enrolled in our final analysis because one study did not report related outcomes, and one was not designed as expected (Supplementary Figure 1).
All nine studies compared the outcomes of conservative oxygen therapy alone with those of conventional oxygen therapy (11, 12, 14–20). Six studies were randomized, controlled trials (RCTs) (11, 12, 16, 18–20), one study was a retrospective nest cohort analysis (14), and the other two studies were prospective before-after studies (15, 17). Mortality was reported in nine studies (11, 12, 14–20), among which hospital mortality was reported in three studies (11, 14, 15), ICU mortality was reported in four studies (11, 14, 15, 19), 28-day mortality was reported in four studies (14, 17–19), 30-day mortality was reported in one study (15), 90-day mortality was reported in five studies (12, 16, 18–20), and 180-day mortality was reported in one study (15). ICU LOS was presented in six studies (11, 12, 14–16, 18), Hospital LOS was reported in five studies (11, 12, 14–16). MV hours was reported in four studies (12, 14, 15, 19). The rate of new organ failure was recorded in two studies (11, 17), the rate of new infection was recorded in three studies (11, 17, 19), and the rate of renal replacement therapy was reported in five studies (12, 15, 17, 18, 20). Details of each study are summarized in Table 1.
A total of 5,759 patients were pooled from all of the included trials in our final systematic review and meta-analysis, among whom 2,903 patients were treated with conservative oxygen therapy, and 2,856 patients received conventional oxygen therapy. Details of the baseline characteristics of the patients in each enrolled study are shown in Table 2.
Quality assessment of the nine enrolled studies showed that there was no bias in attrition or reporting in nine studies but high bias existed in performance in nine studies and in selection and detection in three studies. No studies were excluded for low quality or dubious decisions in the sensitivity analysis (Supplementary Figures 2, 3).
No significant statistical heterogeneity was found in overall mortality between the conservative and conventional groups (I2 = 46%, χ2 = 14.74, p = 0.06), in ICU LOS (I2 = 25%, χ2 = 6.63, p = 0.25), hospital LOS (I2 = 0%, χ2 = 3.39, p = 0.49), length of MV hours (I2 = 37%, χ2 = 4.78, p = 0.19), or new organ failure during the ICU stay (I2 = 0%, χ2 = 0.05, p = 0.82), or in new infections during the ICU stay (I2 = 2%, χ2 = 3.08, p = 0.38).
No significant difference in overall mortality was found with conservative oxygen therapy compared with conventional oxygen therapy (RR 0.99, 95% CI 0.93–1.06; Z = 0.31, p = 0.75) (Figure 1) or in ICU mortality (RR 0.98, 95% CI 0.64–1.49;Z = 0.10, p = 0.92), (Supplementary Figure 4) hospital mortality (RR 0.90, 95% CI 0.59–1.39; Z = 0.46, p = 0.65), (Supplementary Figure 5) 28-day mortality (RR 0.91, 95% CI 0.76–1.08; Z = 1.07, p = 0.28) (Supplementary Figure 6) or 90-day mortality (RR 1.03, 95% CI 0.92–1.15;Z = 0.48, p = 0.63) (Supplementary Figure 7). We performed subgroup analyses of all of the randomized controlled studies, and no advantages of conservative oxygen therapy were found (RR 0.98, 95% CI 0.86–1.12;Z = 0.26, p = 0.80) (Supplementary Figure 8). Moreover, we performed subgroup analysis (Supplementary Figure 9) and included four studies that defined conservative oxygen therapy as SpO2 88–92%, and the same result was found (RR 1.03, 95% CI 0.95–1.11; Z = 0.65, p = 0.52). The results were confirmed by the TSA test, and the required information size was reached (Figure 2).
Figure 3 shows that the difference was not significant between conservative oxygen therapy and conventional oxygen therapy (MD −0.02, 95% CI −0.24–0.20; Z = 0.17, p = 0.86) in ICU LOS.
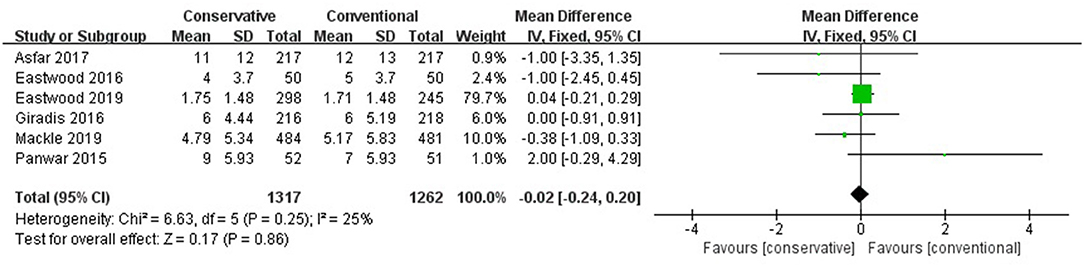
Figure 3. ICU LOS. CI, confidence interval; ICU, intensive care unit; LOS, length of stay; MD, mean difference; SD, standard deviation.
No significant role of conservative oxygen therapy in hospital LOS was found (MD −0.77, 95% CI −1.52– −0.01, Z = 1.98, p = 0.05) (Figure 4).
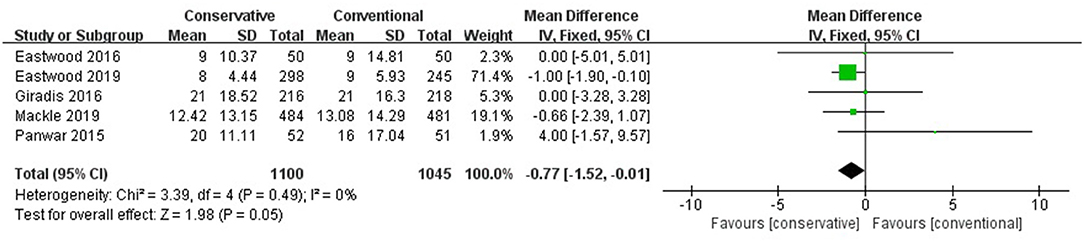
Figure 4. Hospital LOS. CI, confidence interval; LOS, length of stay; MD, mean difference; SD, standard deviation.
Conservative oxygen therapy reduced the MV hours compared with conventional oxygen therapy (MD −2.39, 95% CI −3.31– −1.46; Z = 5.05, p < 0.001) and prolonged the MV-free days (MD 683 683 0.96, 95% CI 0.55–1.37; Z = 4.61, p < 0.001) (Figure 5).
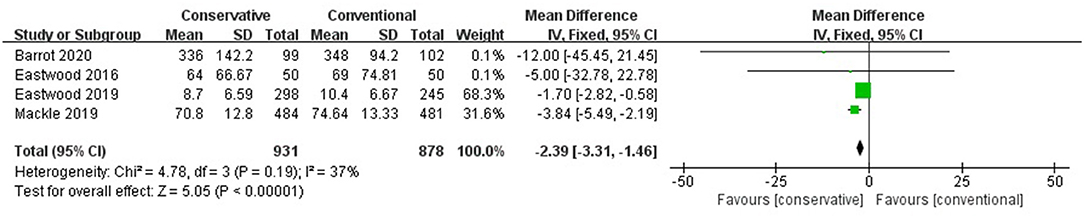
Figure 5. MV hours. CI, confidence interval; MD, mean difference; MV, mechanical ventilation; SD, standard deviation.
Figure 6 shows that differences in new organ failure during the ICU stay existed in the comparison between conservative oxygen therapy and conventional oxygen therapy (RR 0.72, 95% CI 0.54–0.97; Z = 2.15, p = 0.03).
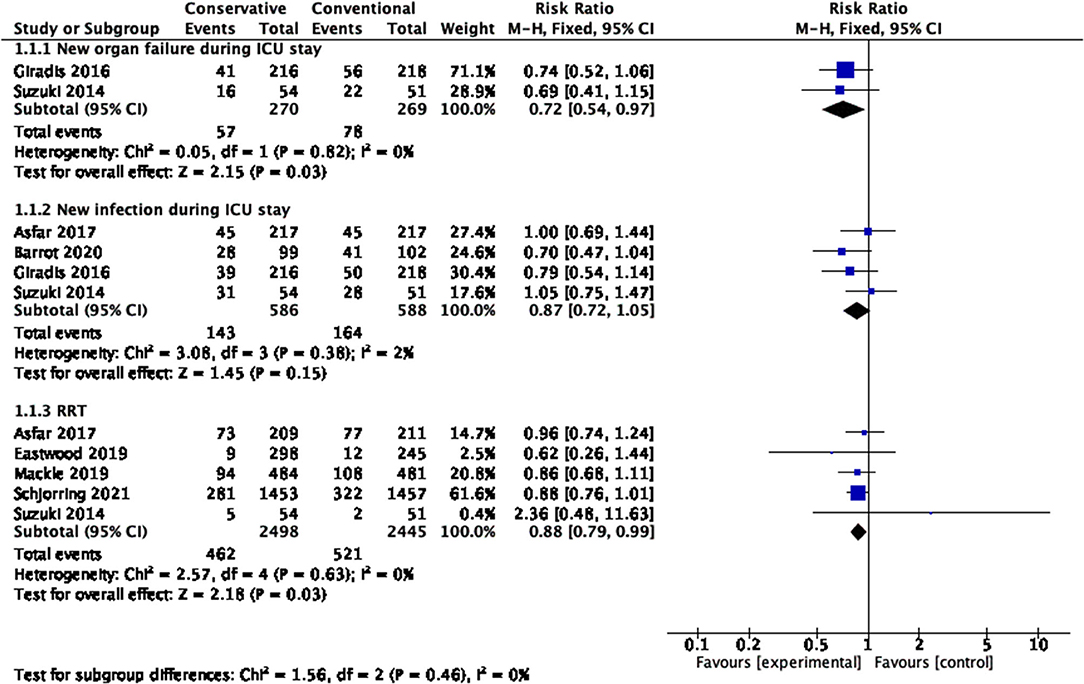
Figure 6. New infection, new organ failure and rate of RRT during the ICU stay. CI, confidence interval; ICU, intensive care unit; RRT, renal replacement therapy; SD, standard deviation.
No significant differences in new infections during the ICU stay existed between conservative oxygen therapy and conventional oxygen therapy (RR 0.87, 95% CI 0.72–1.05; Z = 1.45, p = 0.15) (Figure 6).
Figure 6 shows that conservative oxygen therapy reduced the risk of renal replacement therapy (RR 0.88, 95% CI 0.79−0.99; Z = 2.18, p = 0.03).
In our meta-analysis, we found that conservative oxygen therapy did not decrease the rate of mortality, ICU LOS, hospital LOS or new infections during the ICU stay in critically ill patients. However, conservative oxygen therapy could decrease the MV time, new organ failure during the ICU stay and the risk of RRT.
The advantages of conservative oxygen therapy should be addressed. Conventional therapy would leave 44.5% of patients exposed to hyperoxemia, compared to only approximately 11.4% in the conservative oxygen group (15). The disadvantages of hyperoxemia have been well demonstrated by many studies. First, high inspired oxygen concentrations inhibit the immune system, compromising the ability of macrophages (21), decreasing the abundance of immunoregulatory populations, (22) causing structural changes within alveolar macrophages and leading to serious impairment of their antimicrobial activity (23, 24). Second, pulmonary injury is induced by hyperoxemia. As mentioned above, hyperoxemia can result in decreased mucociliary clearance, atelectasis, inflammation, pulmonary edema, and eventually interstitial fibrosis (25, 26). The combination of immune system compromise and pulmonary injury is related to a higher risk of ventilator-associated pneumonia (VAP). A retrospective, observational study of 503 enrolled patients showed that both hyperoxemia at ICU admission and the percentage of days with hyperoxemia were independently associated with VAP (27). As studies have shown, the rate of VAP is associated with a longer MV period (28). Moreover, two of the enrolled studies showed a trend toward lower use of mandatory MV mode in the conservative oxygen group, which might indicate earlier attempts to wean patients in response to lower FiO2 requirements. This is one of the reasons for the significantly shorter MV hours in the conservative oxygen therapy group (14, 16). Third, every organ, not only the lung, would be damaged by the production of reactive oxygen species (ROS) resulting from high concentrations of oxygen. ROS-mediated stress can lead to cellular necrosis and apoptosis (29). In addition, oxidative stress is responsible for direct damage to biological molecules and indirect injury through the release of cytotoxic products and the mutagenic effects of lipid oxidation (30). ROS-mediated stress and oxidative stress caused by high inspired oxygen concentrations could promote systemic organ failure; otherwise, the decrease of ROS in the conservative oxygen therapy group would lead to less new organ failure during the ICU stay. Hyperoxia exacerbates renal dysfunction, which is mediated by oxygen radical-related injury (31), which is also the one of the reasons that conservative oxygen therapy reduces the rate of RRT.
However, despite the advantages of conservative oxygen therapy, lower mortality, shorter ICU LOS and shorter hospital LOS were not found in our study. We believe that the following reasons might explain this outcome. First, many factors contribute to the mortality of patients. Although conservative oxygen therapy could bring some benefit to patients, many other factors, such as the severity of baseline disease, also contribute significantly to mortality, ICU LOS and hospital LOS (32). Thus, the benefit of conservative oxygen therapy could not be found when combined with so many factors. Meanwhile, in the analysis about mortality in the conservative oxygen therapy and conventional oxygen therapy, the cumulative z score had crossed the trial sequential monitoring boundary, suggesting further trials were not required. Second, conservative oxygen therapy actually exposes patients to higher risk of hypoxia while avoiding hyperoxemia. Hypoxia was also related to higher mortality (33). One of the included studies found that patients in the conservative oxygen group had a significantly higher risk of mesenteric ischemia events and a higher heart rate (19). Moreover, patients were at higher risk of cardiac adverse outcomes, although statistical significance was not attained. This result indicated that there were adverse outcomes of conservative oxygen therapy that could not be ignored and might offset its advantages. More than one study found that the influence of oxygenation on mortality was similar to a “U” shape (34, 35). The lowest mortality was found when the SpO2 was approximately 94 to 98%. Only in the study by Girardis et al. was the SpO2 of conservative oxygen therapy located at the lowest point of the “U” shape. Of all of the included studies, only the study by Girardis et al. also explained why it found the most significant effect of conservative oxygen therapy in reducing ICU mortality in all of the included studies (11). The definition of conservative oxygen therapy in published conservative oxygen therapy studies was not consistent. We recommend that further study carefully consider the target of oxygenation in conservative oxygen therapy. Third, the definitions of conservative oxygen therapy and conventional oxygen therapy overlapped to some extent between the included studies, rendering the advantages of conservative oxygen therapy unclear. Fourth, we found that the effect of conservative oxygen therapy was different in patients with different primary diseases. For example, the advantages of conservative oxygen therapy were obvious in patients with hypoxic ischemia, such as patients with cardiac arrest and hypoxic ischemic encephalopathy (14, 36) but it could add to the mortality of patients with ARDS (37). This result was also consistent with the conclusions of our previous study that hyperoxia will increase mortality, especially in patients with hypoxic ischemia (10). Therefore, we also recommend that further studies explore conservative oxygen therapy in different primary diseases.
In addition, we did not find any advantages of conservative oxygen therapy for new infections during the ICU stay compared with conventional oxygen therapy. The incidence of new infections might have been underestimated because only those ascertained by microbiological samples were recorded (11). Moreover, only two of the enrolled studies reported data about new infections during the ICU stay. Thus, the small sample might also be a limitation.
In one of the included studies, the role of conservative oxygen therapy in increasing MV-free days was found in suspected hypoxic ischemic encephalopathy but not in other diagnoses (12). In our previous study, we found that hyperoxia led to higher mortality in cardiac arrest patients (10). This outcome indicated that patients who have experienced hypoxemia might be more likely to benefit from conservative oxygen therapy. Moreover, in a previous meta-analysis of conservative oxygen therapy in acutely ill patients, more than half of the included studies enrolled patients who experienced hypoxemia due to stroke, myocardial infarction, etc. (37). We believe that this fact was the main reason why a previous analysis found advantages of conservative oxygen therapy in mortality, while our analysis did not.
There are also several limitations in our study that must be addressed. First, high clinical heterogeneity existed in our analysis. Also: (1) the primary diseases of patients included in our enrolled studies were mixed, and conservative oxygen therapy might have more benefit in hypoxic ischemic encephalopathy, but we could not perform the subgroup analysis due to lack of data; (2) the severity of patients who were admitted to the ICU also varied in the included studies; and (3) although all of the studies divided participants into conservative and conventional groups, the actual oxygenation level in each group varied in the included studies. In addition, because of the limit of FiO2 titration, there were episodes in which the oxygenation level of patients was out of the range of target oxygenation levels, which might influence the application of our conclusions.
Compared with conventional oxygen therapy, conservative oxygen therapy had no effects on mortality, ICU LOS or hospital LOS in critically ill patients but could decrease the length of MV hours, new organ failure and risk of RRT.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Y-NN and TW designed the study, drafted the manuscript, and conducted the literature search and data analysis. B-ML and Z-AL made the decision to submit the report for publication. All authors read and approved the final manuscript.
This study was partly supported by the National Key Research and Development Program of China (2016YFC1304303).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.738418/full#supplementary-material
APACHE, The Acute Physiologic and Chronic Health Evaluation; CENTRAL, Cochrane Central Register of Controlled Trails; CI, confidence interval; FiO2, fraction of inspired oxygen; ICU, intensive care unit; ISI, Information Sciences Institute; LOS, length of stay; MD, mean difference; MV, mechanical ventilation; PaO2, partial pressure of arterial oxygen; RCT, randomized, controlled trial; OR, odds ratio; ROS, reactive oxygen species; SpO2, pulse oxygen saturation; SAPS, Simplified Acute Physiologic Score; SD, standard deviation.
1. Eastwood GM, Reade MC, Peck L, Jones D, Bellomo R. Intensivists' opinion and self-reported practice of oxygen therapy. Anaesth Intensive Care. (2011) 39:122–6. doi: 10.1177/0310057X1103900120
2. O'Driscoll BR, Howard LS, Davison AG, BTS guideline for emergency oxygen use in adult patients. Thorax. (2008) 63(Suppl 6):vi1–68. doi: 10.1136/thx.2008.102947
3. Pannu SR, Moreno Franco P, Li G, Malinchoc M, Wilson G, Gajic O. Development and validation of severe hypoxemia associated risk prediction model in 1,000 mechanically ventilated patients*. Crit Care Med. (2015) 43:308–17. doi: 10.1097/CCM.0000000000000671
4. Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. (2000) 161:1450–8. doi: 10.1164/ajrccm.161.5.9902018
5. Rose L, Presneill JJ, Johnston L, Nelson S, Cade JF. Ventilation and weaning practices in Australia and New Zealand. Anaesth Intensive Care. (2009) 37:99–107. doi: 10.1177/0310057X0903700117
6. Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit care. (2014) 18:711. doi: 10.1186/s13054-014-0711-x
7. Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. (2013) 28:647–54. doi: 10.1016/j.jcrc.2013.03.010
8. Rachmale S, Li G, Wilson G, Malinchoc M, Gajic O. Practice of excessive F(IO(2)) and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care. (2012) 57:1887–93. doi: 10.4187/respcare.01696
9. Helmerhorst HJ, Schultz MJ, van der Voort PH, Bosman RJ, Juffermans NP, de Wilde RB, et al. Effectiveness and clinical outcomes of a two-step implementation of conservative oxygenation targets in critically ill patients: a before and after trial. Crit Care Med. (2016) 44:554–63. doi: 10.1097/CCM.0000000000001461
10. Ni YN, Wang YM, Liang BM, Liang ZA. The effect of hyperoxia on mortality in critically ill patients: a systematic review and meta analysis. BMC Pulm Med. (2019) 19:53. doi: 10.1186/s12890-019-0810-1
11. Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. (2016) 316:1583–9. doi: 10.1001/jama.2016.11993
12. Mackle D, Bellomo R, Bailey M. Beasley R. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. (2020) 382:989–98. doi: 10.1056/NEJMoa1903297
13. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. (2021). (accessed February 2021).
14. Eastwood GM, Tanaka A, Espinoza ED, Peck L, Young H, Mårtensson J, et al. Conservative oxygen therapy in mechanically ventilated patients following cardiac arrest: A retrospective nested cohort study. Resuscitation. (2016) 101:108–14. doi: 10.1016/j.resuscitation.2015.11.026
15. Eastwood GM, Chan MJ, Peck L, Young H, Mårtensson J, Glassford NJ, et al. Conservative versus conventional oxygen therapy for cardiac surgical patients: A before-and-after study. Anaesth Intensive Care. (2019) 47:175–82. doi: 10.1177/0310057X19838753
16. Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. (2016) 193:43–51. doi: 10.1164/rccm.201505-1019OC
17. Suzuki S, Eastwood GM, Glassford NJ, Peck L, Young H, Garcia-Alvarez M, et al. Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med. (2014) 42:1414–22. doi: 10.1097/CCM.0000000000000219
18. Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. (2017) 5:180–90. doi: 10.1016/S2213-2600(17)30046-2
19. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. (2020) 382:999–1008. doi: 10.1056/NEJMoa1916431
20. Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. (2021) 384:1301–11. doi: 10.1056/NEJMoa2032510
21. Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas Aeruginosa pneumonia in cystic fibrosis. Mol Med. (2012) 18:477–85. doi: 10.2119/molmed.2012.00024
22. Hanidziar D, Nakahori Y, Cahill LA. Characterization of pulmonary immune responses to hyperoxia by high-dimensional mass cytometry analyses. Sci Rep. (2020) 10:4677. doi: 10.1038/s41598-020-61489-y
23. Morrow DM, Entezari-Zaher T, Romashko J 3rd, Azghani AO, Javdan M, Ulloa L, et al. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia free radical biology & medicine. Free Radic Biol Med. (2007) 42:1338–49. doi: 10.1016/j.freeradbiomed.2007.01.031
24. O'Reilly PJ, Hickman-Davis JM, Davis IC, Matalon S. Hyperoxia impairs antibacterial function of macrophages through effects on actin. Am J Respir Cell Mol Biol. (2003) 28:443–50. doi: 10.1165/rcmb.2002-0153OC
25. Budinger GRS, Mutlu GM. Balancing the risks and benefits of oxygen therapy in critically III adults. Chest. (2013) 143:1151–62. doi: 10.1378/chest.12-1215
26. Fox RB, Hoidal JR, Brown DM, Repine JE. Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis. (1981) 123:521–3. doi: 10.1164/arrd.1981.123.5.521
27. Six S, Jaffal K, Ledoux G, Jaillette E, Wallet F, Nseir S. Hyperoxemia as a risk factor for ventilator-associated pneumonia. Crit Care. (2016) 20:195. doi: 10.1186/s13054-016-1368-4
28. Craven DE, Lei Y, Ruthazer R, Sarwar A, Hudcova J. Incidence and outcomes of ventilator-associated tracheobronchitis and pneumonia. Am J Med. (2013) 126:542–9. doi: 10.1016/j.amjmed.2012.12.012
29. Higuchi Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem Pharmacol. (2003) 66:1527–35. doi: 10.1016/S0006-2952(03)00508-2
30. Motoyama T, Okamoto K, Kukita I, Hamaguchi M, Kinoshita Y, Ogawa H. Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit Care Med. (2003) 31:1048–52. doi: 10.1097/01.CCM.0000055371.27268.36
31. Zwemer CF, Shoemaker JL Jr, Hazard SW 3rd, Davis RE, Bartoletti AG, Phillips CL. Hyperoxic reperfusion exacerbates postischemic renal dysfunction. Surgery. (2000) 128:815–21. doi: 10.1067/msy.2000.109117
32. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. (2006) 34:1297–310. doi: 10.1097/01.CCM.0000215112.84523.F0
33. Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. (2012) 38:91–8. doi: 10.1007/s00134-011-2419-6
34. van den Boom W, Hoy M, Sankaran J, Liu M, Chahed H, Feng M, et al. The search for optimal oxygen saturation targets in critically ill patients: observational data from large ICU databases. Chest. (2020) 157:566–73. doi: 10.1016/j.chest.2019.09.015
35. de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. (2008) 12:R156. doi: 10.1186/cc7150
36. Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy for mechanically ventilated adults with suspected hypoxic ischaemic encephalopathy. Intensive Care Med. (2020) 46:2411–22. doi: 10.1007/s00134-020-06196-y
Keywords: conservative oxygen therapy, mortality, meta-analysis, critically ill, trial sequence analysis
Citation: Ni Y-N, Wang T, Liang B-M and Liang Z-A (2021) The Effect of Conservative Oxygen Therapy in Reducing Mortality in Critical Care Patients: A Meta-Analysis and Trial Sequential Analysis. Front. Med. 8:738418. doi: 10.3389/fmed.2021.738418
Received: 08 July 2021; Accepted: 09 November 2021;
Published: 10 December 2021.
Edited by:
Stefan J. Schaller, Charité University Medicine Berlin, GermanyReviewed by:
Brittany Maggard, University of Louisville, United StatesCopyright © 2021 Ni, Wang, Liang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Wang, dy50MzNAZm94bWFpbC5jb20=; Zong-An Liang, bGlhbmd6YXRnQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.