
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 06 October 2021
Sec. Nephrology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.736098
Background: Immunoglobulin G4-related disease (IgG4-RD) is a systemic immunoreactivity-based fibro-inflammatory disease. Immunoglobulin G4-related kidney disease (IgG4-RKD) is a frequently overlooked diagnosis. This study aimed to describe IgG4-RKD and examine the factors relevant to the renal outcomes of IgG4-RD.
Methods: We studied a prospective IgG4-RKD cohort between January 2012 and December 2020 with close follow-up. Clinicopathologic data at kidney biopsy were collected and analyzed. We aimed to explore independent risk factors for long-term renal outcome and disease relapse. Patients with an eGFR<45 ml/min per 1.73m2 at 12 months were defined as having poor outcomes.
Results: The included 42 patients with IgG4-RKD had a mean age of 58.5 ± 8.7 years (male-to-female ratio = 5:1). The IgG4-RD responder index (RI) was 12.2 ± 3.3. A total of 66.7% of the patients presented with acute on kidney disease or acute on chronic kidney disease. Eight patients (19.0%) showed nephrotic-range proteinuria, and nine (21.4%) had high-titer IgG4-autoantibodies, including antineutrophil cytoplasmic antibody and anti-phospholipase A2 receptor. A kidney biopsy was conducted in 40 patients. Thirty-seven (90.0%) patients were diagnosed with IgG4-related tubulointerstitial nephritis, and 19 (47.5%) of them had concurrent glomerular diseases (membranous nephropathy [MN], n = 3; crescentic glomerulonephritis [CrGN], n = 11; diabetic kidney disease, n = 3; and both MN and CrGN, n = 2). IgG4-RD RI had a close relationship with serum C3 (R = −0.509, P = 0.001), C4 (R = −0.314, P = 0.049) levels, and peripheral blood eosinophil count (PBEC; R = 0.377, P = 0.024), factors that were not included in RI scores. Correlation analysis disclosed that IgG4-RD RI (R = 0.422, P = 0.007), organs involved (R = 0.452, P = 0.003), and C3 (R = −0.487, R = 0.002) were correlated with the percentage decrease of serum creatinine at 1 month. However, multivariate regression analysis failed to identify any clinicopathological parameters that could predict short-term renal restoration and IgG4-RKD relapse. Ten out of 29 variables, of most importance, were identified by the least absolute shrinkage and selection operator (LASSO) regression analysis. By multivariate logistic regression a higher serum IgG4 (OR = 0.671, P = 0.010), IgG1 (OR = 1.396, P = 0.049), IgG3 (OR = 19.154, P = 0.039), and erythrocyte sedimentation rate (ESR; OR = 1.042, P = 0.032) were found to be independent factors for poor long-term outcome. Conventional immunosuppressive medications and/or rituximab were prescribed, and in 83.3% of the patients, the kidney function improved. Repeat kidney biopsies confirmed the remission of interstitial inflammation in two patients under immunosuppressive therapy. However, the disease relapse rate was as high as 31.0%.
Conclusions: We strongly recommend a kidney biopsy in active IgG4-RD, especially when there is proteinuria and renal dysfunction, because concurrent glomerular involvement and active interstitial inflammation should be assessed. A higher serum IgG1, IgG3, and ESR were independent factors for the poor long-term renal outcome; however, elevated IgG4 predicted a good renal prognosis, and appropriate and timely immunosuppressive therapy can help achieve a better prognosis.
Immunoglobulin G4-related disease (IgG4-RD) is characterized by immunoreactivity-based fibro-inflammation and multiple-organ involvement (including the kidney). IgG4-related kidney disease (IgG4-RKD) represents any disease with renal involvement by IgG4-RD (1, 2). The prevalence of IgG4-RKD is reported to be 6.9–27.4% (1, 3, 4). IgG4-RKD has various clinical manifestations, including inflammatory pseudo-tumors, impaired renal function, nephrotic syndrome, and urinary tract obstruction induced by IgG4-related pyelitis or retroperitoneal fibrosis. Currently, the diagnosis of IgG4-RD is based on the diagnostic and classification criteria proposed by the Japanese Rheumatic Association (2011) and the American Rheumatic Association/European Rheumatic Alliance (2019), respectively (5–8). The diagnosis of IgG4-RKD is also based on the criteria defined by these associations and on characteristic renal imaging findings and pathological features (2). A fibro-inflammatory lesion with IgG4-rich plasma cell tissue infiltration is the characteristic histopathological change. In addition, the pathogenesis of IgG4-RD is based on an autoimmune response; however, it mimics the presentation of other autoimmune diseases, which results in overlapping symptoms.
However, the renal outcomes of IgG4-RKD remain unexplained, and the assessment of the systemic inflammatory state in IgG4-RD with the IgG4-RD responder index (RI) inadequately quantifies the degree of renal inflammation (9). An improvement in the renal function in IgG4-RKD indicates that the disease is responsive to steroids; therefore, if the active inflammation can be detected earlier, the prognosis of kidney will be better improved and the transition from acute kidney injury (AKI) to severe chronic kidney disease (CKD) will be reduced. This study aimed to summarize the clinicopathological characteristics and factors relevant to the diagnosis and renal outcomes of patients with IgG4-RKD in a cohort of patients with biopsy-proven IgG4-RKD.
This study was approved by the Committee on Research Ethics of the Peking University First Hospital (2021Y042). A prospective IgG4-RKD cohort was established in 2012. IgG4-RD was diagnosed following the 2011 comprehensive diagnostic criteria (7). As shown in Figure 1, patients who were presumptively diagnosed with IgG4-RD and kidney injury were recruited initially. IgG4-RKD was confirmed using both IgG4-RKD 2011 diagnostic criteria (7) and the Raissian criteria (2). They were enrolled for further clinicopathological analysis. Those who met the above inclusion criteria but were lost to follow-up would be excluded from the study. As of December 2020, 42 patients were included (Figure 1) and followed up regularly at our specialty clinic every 3–6 months.
The pattern and severity of kidney injury were assessed and graded using serum creatinine (SCr) levels and the estimated glomerular filtration rate (eGFR) by the Kidney Disease Improving Global Outcome (KDIGO) clinical practice guidelines for AKI and acute kidney disease (AKD), and the KDOQI guidelines for CKD (10–12). Patients were classified into the AKD and CKD subgroups at diagnosis. A routine blood examination and liver and kidney function tests were performed. Routine urinalysis, microscopic examinations of urinary sediment, determination of the urinary albumin-to-creatinine ratio, and tubular function tests were performed in all patients. Tubular dysfunction was defined as renal glucosuria and had elevated α1-microglobulin levels.
Data relevant to IgG4-RD were collected, comprising a history of concurrent diseases, organ involvement, and the following inflammatory markers: erythrocyte sedimentation rate (ESR); C-reactive protein (CRP) levels; peripheral blood eosinophil count (PBEC); serum levels of IgG subclasses, IgG and IgE; and complement (C)3 and C4 levels. Eosinophilia was diagnosed if the PBEC was >0.50 × 109/μL. Organ involvement was determined by physical examination and radiological findings, such as ultrasonography, CT, MRI, and positron emission tomography (PET). We used the IgG4-RD RI to evaluate systemic disease activity.
Kidney biopsy specimens were collected for immunofluorescence test (IF), and light and electron microscopy analyses. Two experienced renal pathologists made the pathological diagnosis and determined the renal tubulointerstitial injury score (TiIS). The modified renal TiIS was determined by the Banff working group classification standards (13, 14), based on the severity of tubular epithelial cell injury, interstitial edema, interstitial cell infiltration, tubular atrophy, and interstitial fibrosis. The TiIS was scored from 0 to 4; the score was 0 or 1, depending on the presence or absence of tubular cell necrosis and tubulitis. The presence of active inflammation and chronic lesions was assessed by the tubulointerstitial acute injury (Ti-AI) and chronic injury (Ti-CI) scores. Additionally, we analyzed specific pathological markers including immune complex tubular basement membrane (TBM) deposition, an ectopic germinal center in the renal interstitium, storiform fibrosis, and eosinophil aggregation. The aggregation of eosinophils was categorized into mild (0–10) and severe (>10) using the total number of eosinophils in 10 visual fields at 200× magnification.
All patients were followed up regularly at our specialty clinic monthly for the first month and then every 3–6 months after biopsy. We performed complete blood counts, blood tests for biochemistry, ESR, CRP levels, IgG subclasses, complements, and urinalysis. The effect of immunosuppressive therapies and the changes in laboratory parameters were monitored closely. The short-term renal outcome measure was described by the decrease in the SCr level 1 month after the level peaked and classified according to the percentage decrease of the SCr level as follows: unimproved (<20% or in maintenance dialysis), partly improved (20%−40%), and significantly improved (>40%). The long-term outcomes were evaluated based on the eGFR levels during follow-up. We considered patients having a good outcome if they had an eGFR of ≥ 45 ml/min per 1.73 m2 at 12 months. Those who had an eGFR <45 ml/min per 1.73 m2 were considered to have a poor outcome. IgG4-RKD relapse was defined as an elevated SCr that exceeded 30% of the lowest value within 4 weeks. The incidence of extrarenal neoplasms during the observation period was also recorded.
SPSS software version 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses to compare the baseline differences between patients. Continuous variables with a normal distribution, expressed as mean ± SD, were compared between groups using a t-test. Continuous variables with a non-normal distribution were defined as median or quartiles and were compared using the Mann–Whitney U-test between groups. Classified variables were expressed by the number of cases (percentage) and compared using chi-squared test, whereas ordered variables were compared using Wilcoxon rank-sum test. Correlation analysis was used to determine variables related to short-term and long-term renal outcomes. Twenty-nine risk factors including age, gender, IgG4-RD RI, ESR, CRP, total IgG and IgG subclasses, serum C3 and C4 concentration, eosinophil count or eosinophilia, pathological Ti-AI and Ti-CI scores, peak SCr at onset, and the percent of SCr decrease at 1 month after were all included as variables affecting long-term poor renal outcome and disease relapse. Least absolute shrinkage and selection operator (LASSO) regression (SAS software, version 9.4, SAS Institute Inc., Cary, NC, USA) was applied to minimize the potential collinearity and over-fitting of variables. Variables identified by LASSO regression analysis were entered into regression models. The missing values of C3, C4, IgG subclasses, CRP, and ESR were effectively complemented by a median or average data (missing three to four data per variable) for multifactorial prognostic analysis. The multivariate regression was performed to screen predictors of reversible short-term renal recovery. The logistic regression using a backward stepwise elimination was performed to determine independent risk factors for poor renal outcome and relapse. Results of the regression analyses were reported as odds ratios with 95% confidence intervals. Values of P < 0.05 were considered to indicate statistical significance (two-sided).
In the entire IgG4-RKD cohort, 42 patients were included in the study with a male-to-female ratio of 5:1. The mean age of the included cohort was 58.5 ± 8.7 years. The mean number of organs involved was 2.8 ± 1.4. The extra-renal organs involved were the lymph nodes (57.1%), pancreas (28.8%), salivary glands (28.8%), lungs (19.0%), hepatobiliary duct (7.1%), retroperitoneum (7.1%) showing obstructive nephropathy (ON), and large arteries (abdominal aorta, 4.8%). The mean IgG4-RD RI was 12.2 ± 3.3.
Comorbidities included hypertension (23.8%), diabetes (14.4%), hepatitis-B virus infection (4.8%), psoriasis (2.4%), skeletal fluorosis (2.4%), and pneumoconiosis (2.4%). Eight patients (19.0%) were antineutrophil cytoplasmic antibody (ANCA)-positive and met the diagnostic criteria of anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) (15, 16). Four patients had a history of carcinoma, and one each had a thymoma, carcinoma of the ureter, clear cell renal cell carcinoma, and tubular adenocarcinoma. One patient was diagnosed with extranodal marginal zone lymphoma (17). Five patients had monoclonal immunoglobulinemia, namely, IgG-κ (n = 3) and IgG-λ (n = 2).
Laboratory findings are shown in Table 1. All patients had increased SCr levels; the mean peak SCr level was 264.5 ± 145.6 μmol/L. AKD and acute on CKD were diagnosed in 66.7% of the patients. Nine patients had high-titer autoantibodies (six with myeloperoxidase [MPO]-ANCA, two with proteinase 3 [PR3]-ANCA, and one with anti-phospholipase A2 receptor [PLA2R] antibody) and were diagnosed with crescentic glomerulonephritis (CrGN). Eight patients had nephrotic-range proteinuria. They were later pathologically diagnosed with membranous nephropathy (MN), focal segmental glomerular sclerosis, and diabetes glomerulopathy (DG). We found no cases of renal glycosuria and only one case of incomplete renal tubular acidosis. Most patients had mild-to-moderate anemia, whereas 16 patients had eosinophilia (40.0%). The median PBEC before steroid therapy was 0.4 × 109/μL (quartiles: 0.1–0.74).
All patients showed intumescent kidneys. Scar formation was found in two patients. Five patients showed multiple low-density lesions of the kidneys in CT scans. The SUVmax values of high-uptake kidney lesions in the PET-CT examination (n = 6) ranged from 3.7 to 11.1.
The median CRP level was 6.45 mg/L (2.84–24.5; reference range: 0–8 mg/L), and the mean ESR was 83.7 ± 40.3 mm/h. Elevated serum IgG levels were observed in 88.1% of all the patients; IgM and IgE levels were elevated in 2.4 and 75.0% of the patients, respectively. The prevalence of hypocomplementemia was 69.0% for C3 and 50.0% for C4. Increased IgG1, IgG2, IgG3, and IgG4 levels were found in 86.8, 28.9, 52.6, and 94.7% of the patients, respectively (IgG subclasses were not tested in two patients). We found that the MPO–ANCA was IgG1 plus IgG4 in five patients, IgG3 plus IgG4 in one patient, and IgG4 in one patient. PR3-ANCA was IgG4 in one patient, and PLA2R antibody was IgG3 plus IgG4 in another patient.
All IgG4-RKD patients with overlapped AAV were grouped in the AKD subgroup having extremely high ESR and CRP levels compared with the non-AAV patients (Table 1). However, non-AAV AKD patients had a higher IgG4-RD RI, PBEC, organ involvement, and prevalence of low C3 and accelerated ESR than patients in the CKD subgroup. Based on the comparison, IgG4-RKD patients are noted to have eosinophilia with a higher prevalence of AKD and interstitial eosinophil aggregation and significantly elevated IgG4-RD RI, IgG, and IgG4 levels.
A kidney biopsy was conducted in 40 patients. The renal pathology showed typical IgG4-tubulointerstitial nephritis (IgG4-TIN) in most (90.0%) patients (Figure 2); 17 patients (42.5%) were diagnosed with single IgG4-TIN, two with single IgG4-MN, and 19 (47.5%) with IgG4-TIN and concurrent glomerulopathy (Supplementary Tables 1, 2). Manifestations of glomerular involvement mainly included MN, CrGN or glomerular crescent formation, and diabetic glomerulopathy. Other types had focal segmental glomerular sclerosis, endocapillary proliferative glomerulonephritis, Light-chain proximal tubulopathy (LCPT) and IgA nephropathy. Glomerular crescent was common, and 61.5% of these were ANCA-positive. There were eight MN cases; five occurred before IgG4-RD diagnosis, one occurred after IgG4-RD diagnosis, and the other two were detected along with IgG4-RD. The densely distributed interstitial infiltrates consisted of lymphocytes, plasma cells, monocytes/macrophages, and eosinophils. Despite a 40.0% incidence of eosinophilia, we found 77.5% of the patients presenting with interstitial eosinophil infiltration and 83.9% of them (65.0% of the total) had severe aggregation. Repeated renal biopsies revealed elimination of diffusely infiltrated eosinophils, along with a decline in the SCr levels of 17.2–36.9% after steroid administration in two patients (#1 and #2; Table 2).
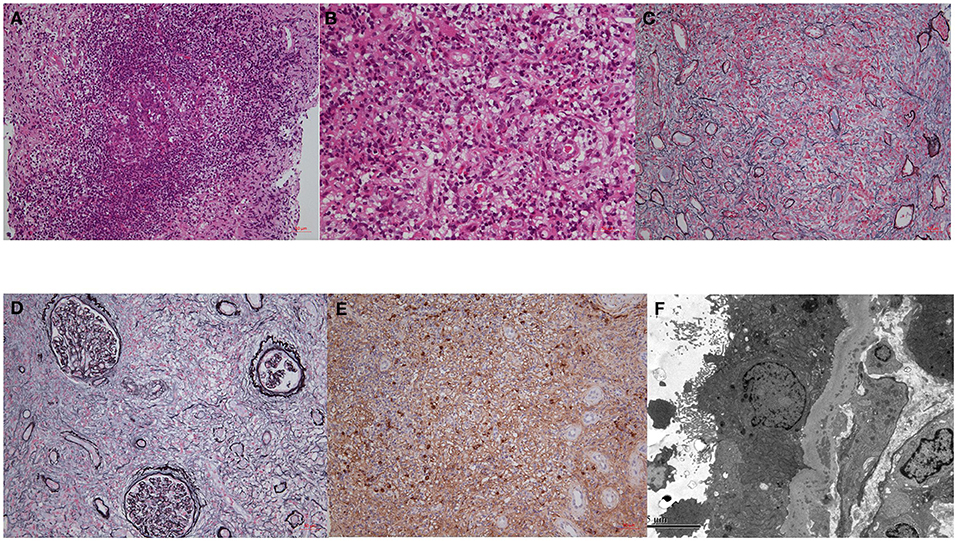
Figure 2. The pathological findings of IgG4-RKD. (A) An ectopic lymphoid tissue; (B) eosinophils infiltration; (C,D) storiform fibrosis; (E) IgG4-possitive staining plasma cells. (F) TBM deposits by EM.
The IF staining features of glomeruli are shown in Supplementary Table 1. Among patients with crescent formation, IgG1, IgG2, and IgG3 were positive in 37.5% of the patients, and IgG4 was positive in 45.0% of the patients. Examination of IF combined with electron microscopy revealed restricted λ-light chain expression in two patients [one in the glomeruli by IF, and the other in the interstitium (17) by EM] and κ-light chain in one. Renal pathology revealed that 19 patients (47.5%) had TBM deposits and 52.6% of them had glomerular lesions. Seventeen patients (42.5%) showed storiform fibrosis during routine light microscopy, whereas six (15.0%) patients showed renal interstitial ectopic lymphoid tissue.
Two patients (#1 and #2) received rebiopsy 4–6 weeks after a strengthened immunosuppressive treatment. The Ti-AI score was extremely lowered. Another two patients (#3 and #4) underwent a repeated biopsy after an ~2-year interval. The initial pathological finding was of MN with TBM deposits. Proteinuria and AKD relapsed several months after steroid withdrawal. The Ti-AI and Ti-CI scores were both elevated, together with interstitial eosinophil infiltration (Table 2).
All patients were initially prescribed prednisone (0.5–1.0 mg/kg/day) for 4–8 weeks, which was regularly tapered by 5 mg every 2 weeks. Six patients (14.3%) with CrGN received methylprednisolone–pulse therapy combined with plasmapheresis. Nine (21.4%) patients had single steroid therapy. Twenty-one patients were given cyclophosphamide (50.0%), mycophenolate mofetil (4.8%), or other conventional immunosuppressive agents (azathioprine or cyclosporine, 4.8%) combined with steroids. Eight patients (19.0%) received a rituximab regimen. For all, prednisone administration was maintained at 5–10 mg/day.
The patients were followed up for 45.4 ± 33.8 months. In 1 month of immunosuppressive therapy, the reversible recovery part of renal function was as follows: (1) unimproved in 14 patients (35.0%), (2) partly improved in 17 patients (40.5%), and (3) significantly improved in nine patients (22.5%). All patients had CKD at 12 months, with CKD stages 3–5 accounting for 50.0, 10.0, and 7.5%, respectively. All patients had remission of hypocomplementemia. Unfortunately, elevated IgG4 level (median at 1.6, quartile: 0.57, 6.0 g/L) remained, involving 47.8% of patients during maintenance immunosuppressive therapy, and the percent of uncontrolled non-IgG4 subclass was found to be 16.7–22.2%. Thirteen patients (31.0%) presented with an IgG4-RKD relapse. Recurrent eosinophilia was present in another four patients (9.5%). Before relapse, six patients (46%) received a single steroid; combination strategies included cyclosporin A, cyclophosphamide, mycophenolate mofetil, azathioprine, and the R-CHOP regimen. Four patients developed new neoplasms (lung cancer, esophageal cancer, and diffuse large B-cell lymphoma). The prevalence of malignancies in the current cohort was 21.4%.
Correlation analysis showed that the clinical parameters including IgG4-RD RI, total IgG, IgG subclass levels, PBEC, and the IgG4/IgG ratio were closely correlated. IgG4-RD RI was also correlated to serum C3 (R = −0.509, P = 0.001), C4 (R = −0.314, P = 0.049) levels, and PBEC (R = 0.377, P = 0.024), factors that were not included in RI scores. The systemic inflammatory indicator ESR was found to correlate with IgG1 (R = 0.350, P = 0.043), and CRP correlated with serum C3 level (R = 0.544, P = 0.001). Pathological Ti-AI had an inverse relationship with serum IgG4 (R = −0.399, P = 0.014). Ti-CI correlated with serum C3 (R = −0.448, P = 0.006) and IgG3 (R = 0.374, P = 0.027). Renal eosinophil infiltration existed when there was eosinophilia (R = 0.447, P = 0.007) and TBM deposit (R = 0.95, P = 0.00). Correlation analysis further disclosed that IgG4-RD RI (R = 0.422, P = 0.007), organs involved (R = 0.452, P = 0.003), and C3 (R = −0.487, R = 0.002) were correlated with the percentage decrease of SCr at 1 month (Table 3). However, multivariate regression analysis failed to identify any clinicopathological parameters that could predict good short-term renal outcome and IgG4-RKD relapse. Comparison displayed in Table 4 revealed that patients with an eGFR ≥ 45 ml/min per 1.73 m2 at 12 months had a significantly higher PBEC and serum IgG4 level, whereas the peak Scr at disease onset was relatively lower.
Ten out of the 29 variables of most importance were identified by LASSO regression analysis. They were included in the multivariate logistic regression, including age, serum IgG subclasses, C3, C4, ESR, CRP, and PBEC. Independent predictors for short-term reversible renal recovery, IgG4-RKD relapse, and long-term poor outcome would be identified. By multivariate logistic regression, a lower serum IgG4 (OR = 0.671, P = 0.010), higher IgG1 (OR = 1.396, P = 0.049), IgG3 (OR = 19.154, P = 0.039), and ESR (OR = 1.042, P = 0.032) were found to be independent factors of poor long-term outcome (Tables 4, 5).
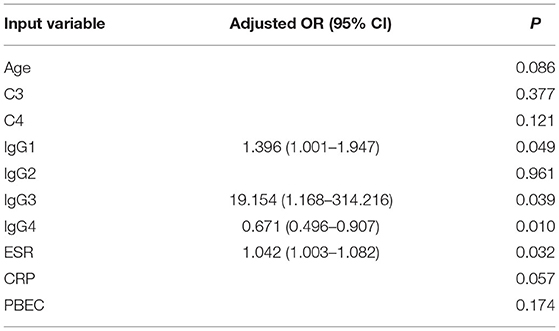
Table 5. The adjusted odds ratio of variables for poor long-term outcome evaluated by eGFR (<45 ml/min per 1.73 m2) at 12 months.
This study includes the clinical data of the largest cohort of patients with biopsy-proven IgG4-RKD at the Peking University Institute of Nephrology. Our study showed that the onset of IgG4-RKD was insidious and that IgG4-TIN was the typical renal manifestation of IgG4-RKD, which was congruent with the findings of previous reports (2). Our results suggested that most (83.3%) of the patients with CKD who underwent kidney biopsy had active tubulointerstitial inflammation and achieved significant restoration in the renal function after receiving immunosuppressive therapies (18). This finding also suggests that the creatinine-based glomerular function assessment method or the tubular dysfunction markers are not sensitive in detecting early interstitial inflammation, although some weak correlations were observed between IgG subclass, hypocomplementemia, and Ti-IS scores. More specific and sensitive indicators are yet to be found. Moreover, we reported a high prevalence and diversity of glomerular diseases in patients with IgG4-RD, especially CrGN or crescent formation, which is critically important and significantly increases the difficulty of clinical evaluation of IgG4-RKD. In other words, the deterioration or recurrence of IgG4-RKD may result from aggravated interstitial inflammation or the occurrence of severe glomerular involvement. For these reasons, biopsy remains the cornerstone of diagnosis to evaluate the renal pathology precisely and minimize missed diagnoses of active inflammation (19). Saeki et al. (20) reported that the revised version of the diagnostic criteria for IgG4-RKD (2020), which adds items pertaining to the extra-renal organ(s) (including “bilateral lacrimal, submandibular, or parotid swelling and imaging findings compatible with type 1 autoimmune pancreatitis or retroperitoneal fibrosis”), helps to improve both the true IgG4-RKD diagnostic sensitivity and specificity. Nevertheless, further investigation of non-invasive indicators reflecting active intrarenal inflammation is still needed (21).
Eosinophilia is a common manifestation of IgG4-RD and is believed to be a sign of an enhanced immune response (19, 22). Renal eosinophilic infiltration is also frequently observed pathologically in IgG4-RKD. It is reported in up to 30.8% of the patients, significantly higher than 9.5% in non-IgG4-RKD (23). Recently, research has shown that eosinophils are involved in the progression of CKD. Rats with eosinophil peroxidase deficiency were reported to have mild renal interstitial fibrosis (24). Zachary et al. (25) observed that elevated PBEC at baseline was a potential predictor of IgG4-RD relapse. Zhang et al. (26) showed that patients with IgG4-RD and eosinophilia had more organ involvement, especially lymph nodes, lacrimal gland, and submandibular gland. Mohapatra et al. (27) reported that eosinophilia with increasing serum IgG4 added diagnostic value at serum IgG4 levels of ≥ two times the upper limit of normal. Takanashi et al. (28) conducted proteomic analysis in IgG4-RD and showed that eotaxin-3 was a new biomarker of disease activity. Our results showed that eosinophilia represented a more active IgG4-RKD and indicated reversible treatment response. Interstitial eosinophilic aggregation was more significant in IgG4-TIN and required immunosuppressive therapy. However, a 35% missed diagnosis rate was noted because patients with obvious eosinophilic infiltration had a normal PBEC. A kidney biopsy could correct a missed diagnosis.
The number of case reports and cohort studies regarding IgG4-RD overlapping with ANCA-positive CrGN, systemic lupus erythematosus (SLE), Sjogren's syndrome, and IgG4-associated MN and TMA have been rising (19, 26, 29–33). Some researchers believe that MN is a special type of IgG4-RKD, regardless of whether PLA2R is negative (34). A study reported 65 IgG4-RD patients with urinary involvement, 7.7% of whom had MN (3). A cohort with type-1 autoimmune pancreatitis and kidney involvement reportedly comprised 26.7% of patients with MN (4). In another IgG4-RD cohort with retroperitoneal involvement, 27.4% of the patients presented with IgG4-TIN and MN (35). The difference is that the present study reported more types of glomerulopathy, especially CrGN induced by IgG4 subtype ANCA and anti-PLA2R. If accompanied by an AAV, patients usually showed different characteristics from that of typical IgG4-TIN, which has an acute onset of AKI with elevated CRP and a rarely detected hypocomplementemia. Martín-Nares et al. (36) also emphasized that a significant number of patients with IgG4-RD were ANCA-positive and more frequently had lymph node and kidney involvement, high IgG1 levels, and high ESR. Wang et al. found similar presentations in four patients with the concurrent crescent formation in AAV and IgG4-TIN (37). Our current study showed that the pathogenicity of IgG4 subtypes specific to ANCA and PLA2R should be classified as IgG4-RKD. Furthermore, we disclosed other patterns of glomerulopathy, such as non-ANCA crescents formation, restricted light chain expression, and concurrent diabetes glomerulopathy, which were also involved in kidney injury.
Even though some IgG4-recognized target antigens have been found (38–41), IgG4 does not bind to activating Fc receptors as a driver of pathogenesis. An increase in the IgG subtypes other than the IgG4 molecules and hypocomplementemia is also frequent in IgG4-RD, but their roles remained unclear. Our study showed that elevated IgG1, IgG2, and IgG3 levels occurred in 86.8, 28.9, 52.6% of the patients, respectively. Low C3 and C4 levels accounted for 69 and 50% of all the patients, respectively. Previous studies with biopsy-proven IgG4-RKD showed that C3 levels were negatively correlated with the renal deposition of C1q, storiform fibrosis, interstitial fibrosis, the number of IgG4-positive plasma cells (42), and deposition of non-IgG4 immunoglobulins in the TBM (43). This was similar to our results that high C3 and IgG3 levels were significantly correlated to the severity of the tubulointerstitial chronic injury. The literature also reports that IgG3 antibodies, such as IgG3-ANCA, can lead to robust complement activation. IgG1 and IgG4 anti-PR3 ANCA can induce degranulation and adhesion of granulocytes (41). Disease severity (44) is associated with an increase in the diversity of autoantibodies in IgG4-RD.
Patients show different characteristics of IgG4-RKD and progression, which correspond to the acceleration or remission of the immune response. Takanashi et al. (28) reported that IgG4-lymphadenopathy represented a phenotype of severe activity and poor prognosis. Miyanaga et al. (45) reported tertiary lymphoid tissue, a potential marker of relapse and drug resistance of IgG4-RKD, in an early stage of IgG4-TIN, related to poor renal prognosis. Chibbar et al. (46) reported recurrent IgG4-related TIN concurrent with chronic active antibody-mediated rejection, despite maintenance immunosuppression. These observations indicate that B- and T-cells are vital to disease pathogenesis. T-follicular helper cells, residing in germinal centers, are elevated in the peripheral blood and affected tissues, promoting B-cell differentiation, expansion, and germinal-center formation. Regulatory T and Th2 cells phenotype stimulate cytokine production (which promotes fibrosis) and an Ig class switches to IgG4 (19, 34). Therefore, conventional immunosuppressive and B-cell-depletion therapies can cause a rapid decline in the production of IgG subclasses and reduce inflammation and fibrosis. However, the risk of disease relapse and tumorigenesis remains a dilemma in clinical treatment (47). In IgG4-RD, serum IgG4 levels decline after B cells depletion but do not normalize, probably because of the long-lived plasma cells that continue to produce it, which result in persistently elevated immunoglobulin. Recently, it is reported that circulating plasmablasts correlate well with disease activity and the number of the organs involved, making them a useful biomarker of IgG4-RD differentiating from mimickers and during disease relapse. The limitation of this study was that we did not measure baseline CD19lowCD20negCD27+CD38hiplasmablasts at diagnosis.
In conclusion, this study indicates that IgG4-RKD patients have complex and diverse clinicopathological manifestations. In the absence of sensitive and specific predictors, pathological kidney findings are still the most reliable basis for diagnosing, assessing, and treating IgG4-RKD. A higher serum IgG1, IgG3, and ESR were independent factors for the poor long-term renal outcome; however elevated IgG4 predicted a good renal prognosis. An appropriate and timely immunosuppressive therapy is helpful in improving renal function restoration.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University First Hospital (2021Y042). The patients/participants provided their written informed consent to participate in this study.
TS and LY participated in research design and data analysis. TS, LY, HW, and SW participated in the performance of the study. TS drafted the manuscript. All authors contributed to the article and approved the final manuscript for submission.
This research was supported by the National Science and Technology Major Projects for major new drugs innovation and development (Grant No. 2017ZX09304028 to TS), National Natural Science Foundation of China (Grant Nos. 91742205 and 81625004 to LY), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant No. 2019-I2M-5-046).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Prof. Min Chen, Dr. Zhiying Li, and Ying Tan for their help with ANCA subclass analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.736098/full#supplementary-material
IgG4-RKD, Immunoglobulin-4–related kidney disease; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; PR3, proteinase 3; PLA2R, phospholipase A2 receptor; AAV, Anti-neutrophil cytoplasmic antibody-associated vasculitis; TBM, tubular basement membrane; AKI, acute kidney injury; AKD, acute kidney disease; CKD, chronic kidney disease; CrGN, crescentic glomerulonephritis; MN, membranous nephropathy; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TI-AI, tubulointerstitial acute injury score; RI, responder index.
1. Saeki T, Kawano M. IgG4-related kidney disease. Kidney Int. (2014) 85:251–7. doi: 10.1038/ki.2013.393
2. Raissian Y, Nasr SH, Larsen CP, Colvin RB, Smyrk TC, Takahashi N, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol. (2011) 22:1343–52. doi: 10.1681/ASN.2011010062
3. Teng F, Lu H, Zheng K, Chen G, Wen Y, Liu Z, et al. Urinary system manifestation of IgG4-related disease: Clinical, laboratory, radiological, and pathological spectra of a Chinese single-centre study. J Immunol Res. (2020) 2020:5851842. doi: 10.1155/2020/5851842
4. Vujasinovic M, Pozzi Mucelli RM, Valente R, Verbeke CS, Haas SL, Löhr JM. Kidney involvement in patients with Type 1 autoimmune pancreatitis. J Clin Med. (2019) 8:258. doi: 10.3390/jcm8020258
5. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. (2015) 385:1460–71. doi: 10.1016/S0140-6736(14)60720-0
6. Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. (2020) 79:77–87. doi: 10.1136/annrheumdis-2019-216561
7. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. (2012) 22:21–30. doi: 10.1007/s10165-011-0571-z
8. Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S, et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol. (2021) 31:529–33. doi: 10.1080/14397595.2020.1859710
9. Carruthers MN, Stone JH, Deshpande V, Khosroshahi A. Development of an IgG4-RD responder index. Int J Rheumatol. (2012) 2012:259408. doi: 10.1155/2012/259408
10. Bomback A, Browne T, Coppo R. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. (2012) 2:1–138. doi: 10.1038/kisup.2012.6. Available online at: http://www.kidney-international.org & 2012.
11. Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. (2017) 13:241–57. doi: 10.1038/nrneph.2017.2
12. Panpan Z, Jizhi Z, Mu W, Ruie F, Xiaowei L, Yamin L, et al. The clinical characteristics of 346 patients with IgG4-related disease. Chin J Intern Med. (2017) 56:644–9. doi: 10.3760/cma.j.issn.0578-1426.2017.09.005
13. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. (2008) 8:753–60. doi: 10.1111/j.1600-6143.2008.02159.x
14. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. (1999) 55:713–23. doi: 10.1046/j.1523-1755.1999.00299.x
15. Sunderkötter CH, Zelger B, Chen KR, Requena L, Piette W, Carlson JA, et al. Nomenclature of cutaneous vasculitis: Dermatologic addendum to the 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. (2018) 70:171–84. doi: 10.1002/art.40375
16. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. Classification Criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Accept Publ Ann Rheum Dis Arthritis Rheum. (2016) 69:35–45. doi: 10.1002/art.39859.2016
17. Wang H, Su T, Kang L, Yang L, Wang S. Diffuse large B cell lymphoma in a preceding IgG4-related disease with kidney restricted lambda light chain expression: Case report and literature review. BMC Nephrol. (2020) 21:315. doi: 10.1186/s12882-020-01975-7
18. Saeki T, Kawano M, Mizushima I, Yamamoto M, Wada Y, Ubara Y, et al. Recovery of renal function after glucocorticoid therapy for IgG4-related kidney disease with renal dysfunction. Clin Exp Nephrol. (2016) 20:87–93. doi: 10.1007/s10157-015-1140-0
19. Maritati F, Peyronel F, Vaglio A. IgG4-related disease: a clinical perspective. Rheumatology (Oxford). (2020) 59:iii123–31. doi: 10.1093/rheumatology/kez667
20. Saeki T, Kawano M, Nagasawa T, Ubara Y, Taniguchi Y, Yanagita M, et al. Validation of the diagnostic criteria for IgG4-related kidney disease (IgG4-RKD) 2011, and proposal of a new 2020 version. Clin Exp Nephrol. (2021) 25:99–109. doi: 10.1007/s10157-020-01993-7
21. Zongfei J, Lingying M, Lijuan Z, Ying S, Rongyi C, Dongmei L, et al. Prognostic factors in IgG4-related disease: A long-term monocentric Chinese cohort study. Clin Rheumatol. (2021) 40:2293–300. doi: 10.1007/s10067-020-05484-8
22. King RL, Tan B, Craig FE, George TI, Horny HP, Kelemen K, et al. Reactive eosinophil proliferations in tissue and the lymphocytic variant of hypereosinophilic syndrome. Am J Clin Pathol. (2021) 155:211–38. doi: 10.1093/ajcp/aqaa227
23. Yoshita K, Kawano M, Mizushima I, Hara S, Ito Y, Imai N, et al. Light-microscopic characteristics of IgG4-related tubulointerstitial nephritis: Distinction from non-IgG4-related tubulointerstitial nephritis. Nephrol Dial Transplant. (2012) 27:2755–61. doi: 10.1093/ndt/gfr761
24. Colon S, Luan H, Liu Y, Meyer C, Gewin L, Bhave G. Peroxidasin and eosinophil peroxidase, but not myeloperoxidase, contribute to renal fibrosis in the murine unilateral ureteral obstruction model. Am J Physiol Ren Physiol. (2019) 316:F360–71. doi: 10.1152/ajprenal.00291.2018
25. Wallace ZS, Mattoo H, Mahajan VS, Kulikova M, Lu L, Deshpande V, et al. Predictors of disease relapse in IgG4-related disease following rituximab. Rheumatology (Oxford). (2016) 55:1000–8. doi: 10.1093/rheumatology/kev438
26. Zhang X, Zhang P, Li J, He Y, Fei Y, Peng L, et al. Different clinical patterns of IgG4-RD patients with and without eosinophilia. Sci Rep. (2019) 9:16483. doi: 10.1038/s41598-019-52847-6
27. Mohapatra S, Charilaou P, Sharma A, Singh DP, Sah RP, Murray D, et al. Significance of peripheral eosinophilia for diagnosis of IgG4-related disease in subjects with elevated serum IgG4 levels. Pancreatology. (2020) 20:74–8. doi: 10.1016/j.pan.2019.11.016
28. Takanashi S, Kikuchi J, Sasaki T, Akiyama M, Yasuoka H, Yoshimoto K, et al. Lymphadenopathy in IgG4-related disease: A phenotype of severe activity and poor prognosis, with eotaxin-3 as a new biomarker. Rheumatology (Oxford). (2021) 60:967–75. doi: 10.1093/rheumatology/keaa648
29. Su T, Yang L, Cui Z, Wang SX, Zhao MH. Concurrent IgG4-related tubulointerstitial nephritis and IgG4 myeloperoxidase-anti-neutrophil cytoplasmic antibody positive crescentic glomerulonephritis: A case report. Medicine (Baltimore). (2017) 96:e6707. doi: 10.1097/MD.0000000000006707
30. Li ZY, Wang X, Xia X, Yu XJ, Wang SX, Chen W, et al. An overlap of antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis and IgG4-related kidney disease. Clin Chim Acta. (2020) 501:12–9. doi: 10.1016/j.cca.2019.11.030
31. Lu H, Cui Z, Zhou XJ, Jin QZ, Yu XJ, Wang SX, et al. Plasma exchange and rituximab treatments in primary membranous nephropathy combined with crescentic glomerulonephritis: A case report. Medicine (Baltimore). (2019) 98:e15303. doi: 10.1097/MD.0000000000015303
32. Yamamoto M, Aochi S, Suzuki C, Nakamura S, Murakami R, Ogawa Y, et al. A case with good response to Belimumab for lupus nephritis complicated by IgG4-related disease. Lupus. (2019) 28:786–9. doi: 10.1177/0961203319840430
33. Ma Y, Chen L, Xu Y, Han Q, Yu B, Yuan Y, et al. Clinical and pathological features of patients with antineutrophil cytoplasmic antibody-associated vasculitides concomitant with IgG4-related disease. Int J Rheum Dis. (2019) 22:2143–50. doi: 10.1111/1756-185X.13726
34. Palmisano A, Corradi D, Carnevali ML, Alberici F, Silini EM, Gatti R, et al. Chronic periaortitis associated with membranous nephropathy: clues to common pathogenetic mechanisms. Clin Nephrol. (2010) 74:485–90. doi: 10.5414/cnp74485
35. Evans RDR, Cargill T, Goodchild G, Oliveira B, Rodriguez-Justo M, Pepper R, et al. Clinical manifestations and long-term outcomes of IgG4-related kidney and retroperitoneal involvement in a United Kingdom IgG4-related disease cohort. Kidney Int Rep. (2019) 4:48–58. doi: 10.1016/j.ekir.2018.08.011
36. Martín-Nares E, Hernandez-Molina G. What is the meaning of ANCA positivity in IgG4-related disease? Rheumatology. (2021) 60:3845–50. doi: 10.1093/rheumatology/keab124
37. Wang GQ, Chen YP, Cheng H, Xu XY, Sun LJ, Dong HR DH. Antineutrophil cytoplasmic antibody and/or antiglomerular basement membrane antibody associated crescentic glomerulonephritis in combination with IgG4-related tubulointerstitial nephritis. Clin Exp Rheumatol. (2019) 37:279–85.
38. Hubers LM, Vos H, Schuurman AR, Erken R, Oude Elferink RP, Burgering B, et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut. (2018) 67:728–35. doi: 10.1136/gutjnl-2017-314548
39. Umehara H, Okazaki K, Kawano M, Tanaka Y. The front line of research into immunoglobin G4-related disease - Do autoantibodies cause immunoglobin G4-related disease? Mod Rheumatol. (2019) 29:214–8. doi: 10.1080/14397595.2018.1558519
40. Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med. (2018) 10:1–11. doi: 10.1126/scitranslmed.aaq0997
41. Du H, Shi L, Chen P, Yang W, Xun Y, Yang C, et al. Prohibitin is involved in patients with IgG4 related disease. PLoS ONE. (2015) 10:e0125331. doi: 10.1371/journal.pone.0125331
42. Wang R, He D, Zhao L, Liang S, Liang D, Xu F, et al. Role of complement system in patients with biopsy-proven immunoglobulin G4–related kidney disease. Hum Pathol. (2018) 81:220–8. doi: 10.1016/j.humpath.2018.07.008
43. Fujisawa Y, Mizushima I, Yamada K, Yamamoto M, Saeki T, Matsui S, et al. Hypocomplementemia is related to elevated serum levels of IgG subclasses other than IgG4 in IgG4-related kidney disease. Mod Rheumatol. (2021) 31:241–8. doi: 10.1080/14397595.2019.1709942
44. Liu H, Perugino CA, Ghebremichael M, Wallace ZS, Montesi SB, Stone JH, et al. Disease severity is linked to an increase in autoantibody diversity in IgG4-related disease. Arthritis Rheumatol. (2020) 72:687–93. doi: 10.1002/art.41140
45. Miyanaga T, Mizuguchi K, Hara S, Zoshima T, Inoue D, Nishioka R, et al. Tertiary lymphoid tissue in early-stage IgG4-related tubulointerstitial nephritis incidentally detected with a tumor lesion of the ureteropelvic junction: A case report. BMC Nephrol. (2021) 22:34. doi: 10.1186/s12882-021-02240-1
46. Chibbar R, Wright GR, Dokouhaki P, Dumanski S, Prasad B, Mengel M, et al. Recurrent IgG4-related tubulointerstitial nephritis concurrent with chronic active antibody mediated rejection: A case report. Am J Transplant. (2018) 18:1799–803. doi: 10.1111/ajt.14758
Keywords: IgG4-related disease, kidney disease, tubulointerstitial nephritis, crescentic nephritis, IgG4 autoantibodies, membranous nephropathy, prognosis
Citation: Su T, Wang H, Wang S and Yang L (2021) Clinicopathological Patterns and Predictors of the Functional Restoration of Immunoglobulin G4-Related Kidney Disease: A Chinese Single-Center Cohort Study. Front. Med. 8:736098. doi: 10.3389/fmed.2021.736098
Received: 04 July 2021; Accepted: 06 September 2021;
Published: 06 October 2021.
Edited by:
Gian Marco Ghiggeri, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Augusto Vaglio, University of Parma, ItalyCopyright © 2021 Su, Wang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Su, dGFvLnN1QGJqbXUuZWR1LmNu; Li Yang, bGkueWFuZ0Biam11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.