
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 25 August 2021
Sec. Intensive Care Medicine and Anesthesiology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.727910
This article is part of the Research Topic Clinical Application of Artificial Intelligence in Emergency and Critical Care Medicine, Volume II View all 19 articles
Object: The fluid management strategy in ARDS is not very clear. A secondary analysis of RCT data was conducted to identify patients with ARDS benefitting from a conservative strategy of fluid management.
Methods: The data of this study were downloaded from the ARDS network series of randomized controlled trials (Conservative Strategy vs. Liberal Strategy in 2006). Based on the clinical feature of patients, within the first 24 h after admission, clustering was performed using the k-means clustering algorithm to identify the phenotypes of ARDS. Survival was analyzed using the Kaplan-Meier survival analysis to assess the effect of the two fluid management strategies on the 90-day cumulative mortality. Categorical/dichotomic variables were analyzed by the chi-square test. Continuous variables were expressed as the mean and standard deviation and evaluated through a one-way ANOVA. A P-value < 0.05 was defined as the statistically significant cut-off value.
Results: A total of 1,000 ARDS patients were enrolled in this unsupervised clustering research study, of which 503 patients were treated with a conservative fluid-management strategy, and 497 patients were treated with a liberal fluid-management strategy. The first 7-day cumulative fluid balance in patients with the conservative strategy and liberal strategy were −136 ± 491 ml and 6,992 ± 502 ml, respectively (P < 0.001). Four phenotypes were found, and the conservative fluid-management strategy significantly improved the 90-day cumulative mortality compared with the liberal fluid-management strategy (HR = 0.532, P = 0.024) in patients classified as “hyperinflammatory anasarca” phenotype (phenotype II). The characteristics of this phenotype exhibited a higher WBC count (20487.51 ± 7223.86/mm3) with a higher incidence of anasarca (8.3%) and incidence of shock (26.6%) at baseline. The furthermore analysis found that the conservative fluid management strategy was superior to the liberal fluid management strategy in avoiding superinfection (10.10 vs. 14.40%, P = 0.037) and returned to assisted breathing (4.60 vs. 16.20%, P = 0.030) in patients classified as “hyperinflammatory anasarca” phenotype. In addition, patients with other phenotypes given the different fluid management strategies did not show significant differences in clinical outcomes.
Conclusion: Patients exhibiting a “hyperinflammatory anasarca” phenotype could benefit from a conservative fluid management strategy.
Acute respiratory distress syndrome (ARDS) refers to acute inflammatory injury of the lung, disruption of the alveolar–capillary barrier and the formation of non-cardiogenic, protein-rich pulmonary oedema (1–4). A conservative fluid management strategy could improve the anasarca and oxygenation index (PaO2/FiO2). In addition, initiating treatment to reduce pulmonary oedema as early as possible could decrease the risk of superinfection (5, 6).
Although lung failure alone can be lethal, death in patients with acute lung injury is usually due to the failure of the non-pulmonary organs. Conservative fluid management strategies may lead to lower intravascular volume and perfusion (7, 8). Wiedemann et al. performed a randomized controlled trial (RCT) to compare conservative and liberal fluid-management strategies in ARDS (9). The results indicated that although the conservative strategy of fluid management improved lung function and shortened the duration of mechanical ventilation, a conservative strategy could not improve the mortality of ARDS. This suggested that not all ARDS patients need dehydration therapy for the improvement of lung function, and the sufficient effective circulating blood volume could also be took into account in parts of ARDS patient. Therefore, the fluid management strategy for ARDS is not very clear.
Artificial intelligence (AI) has found its way into clinical studies in the era of big data. Meanwhile, as increasing number of ARDS clinical trials data is open to public, secondary analysis on these combined datasets provide a powerful way of finding solution to clinical questions with a new perspective (10, 11). When combined with machine learning informatics and clinical trials data, the result will be the development of a precision form of personalized treatment applied to ARDS, which could be a promising way to explore the precise fluid management for specific ARDS population (12).
Based on this clinical problem, the hypothesis for identification of the specific ARDS patients who could benefit from conservative fluid management would be tested through a secondary analysis on RCT data from Wiedemann et al. using a machine learning algorithm (unsupervised clustering).
The data of this study were downloaded from the ARDS network series of randomized controlled trials (Conservative Strategy vs. Liberal Strategy in 2006) (9). A total of 1,000 ARDS patients participated in this study.
Clinical features of ARDS patients were obtained before the start of treatment with a conservative strategy or liberal strategy within the first 24 h after admission. If missing data for a certain feature or sample is more than 5% then we will leave that feature. The other missing data (<5%) were estimated by multiple imputations through the R package, following the process described by Zhang with minor modifications (13). The mice R package conducted three main steps: (1) imputation, (2) analysis, and (3) pooling for missing data. The imputation step identified the characteristic of missing data; then the analysis step provided the predictive mean matching of missing data through modular approach; finally, the pooling step filled up the missing data based on 1,000 imputations iterations (13–15).
To screen suitable clinical features for clustering analysis, we attempted to train several classifiers from scratch. The clear separations and significant statistical results (P < 0.05) were utilized as the criterion for the identification of suitable clinical features for the best classification model.
Clustering was performed using the k-means clustering algorithm implemented in R (k-means package). The best classifications were selected based on clear separations of the consensus heatmaps.
Survival was analyzed using the Kaplan-Meier survival analysis to assess the effect of the two fluid management strategies on the 90-day cumulative mortality. Categorical/dichotomic variables were analyzed by the chi-square test. Continuous variables were expressed as the mean and standard deviation and evaluated through a one-way ANOVA.
A P-value < 0.05 was defined as the statistically significant cut-off value.
All the analyses in this study were conducted using R 4.0.3.
A total of 1,000 ARDS patients were enrolled in this unsupervised clustering research study, of which 503 patients were treated with a conservative fluid-management strategy, and 497 patients were treated with a liberal strategy fluid-management strategy. The first 7-day cumulative fluid balance in patients with the conservative strategy and liberal strategy were −136 ± 491 ml and 6,992 ± 502 ml, respectively (P < 0.001).
After multiclustering, the Acute Physiology and Chronic Health Evaluation III (APACHE III) score, PaO2, central venous pressure (CVP), predicted body weight (PBW), white blood cell count (WBC), platelet count, and the presence or absence of shock and anasarca were finally enrolled in further unsupervised clustering analysis.
The patients were classified as 2 phenotypes to 7 phenotypes through unsupervised clustering analysis, shown in (Figures 1A–F). As the 4-class model showed the clearest separation of the matrix heatmap (Figure 1), 4 phenotypes were utilized in the current study. The numbers of patients in Phenotypes I, II, III and IV were 319, 169, 492 and 11, respectively.
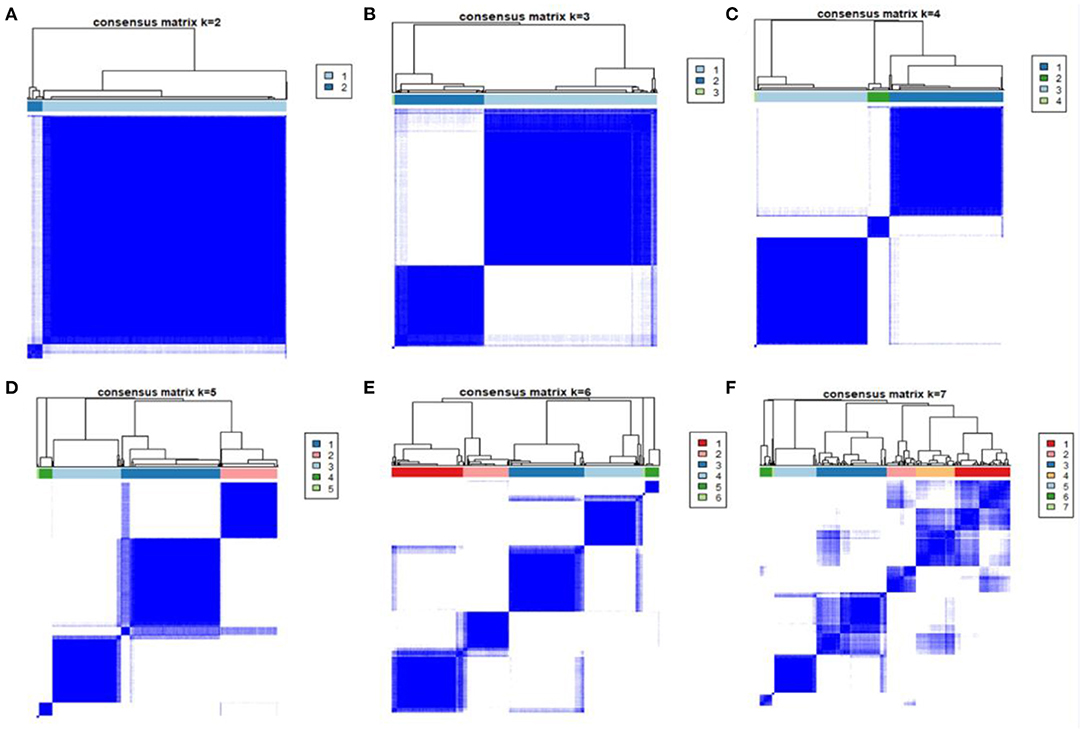
Figure 1. Consensus matrix heatmaps of consensus k-means clustering. Consensus matrix heatmaps of different subgroup numbers (k = 2, 3, 4, 5, 6, 7). When k = 3, the model exhibited the clearest separation of the consensus matrix heatmap.
Phenotype II was identified as the specific population that benefited from the conservative fluid-management strategy because the conservative fluid-management strategy significantly improved the 90-day cumulative mortality compared with the liberal fluid-management strategy (HR = 0.532, P = 0.024), as shown in Figure 2. Regarding secondary outcomes, the conservative fluid management strategy markedly decreased the 90-day mortality compared with the liberal fluid management strategy (25.3 vs. 41.1%, P = 0.030). In addition, the conservative fluid management strategy was superior to the liberal fluid management strategy in avoiding superinfection (10.10 vs. 14.40%, P = 0.037) and returned to assisted breathing (4.60 vs. 16.20%, P = 0.030), as shown in Table 1.
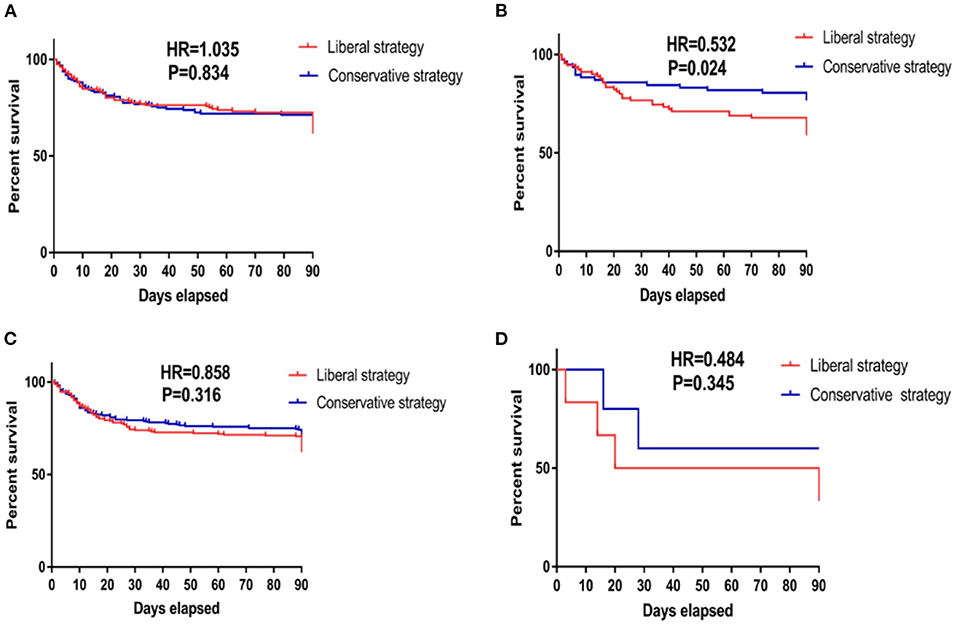
Figure 2. Kaplan–Meier survival curves of 90-day cumulative mortality for patients receiving conservative strategy and liberal strategy among those with the four phenotypes. (A) The survival curves of 90-day cumulative mortality of patients classified as phenotype I. The Kaplan–Meier survival analysis indicated that compared with the liberal strategy, conservative fluid management could not improve the 90-day mortality in patients classified as phenotype I (HR = 1.035, P = 0.843). (B) The survival curves of 90-day cumulative mortality of patients classified as phenotype II. The Kaplan–Meier survival analysis indicated that compared with the liberal strategy, conservative fluid management significantly improved 90-day mortality in patients classified as phenotype II (HR = 0.532, P = 0.024). (C) The survival curves of 90-day cumulative mortality of patients classified as phenotype III. The Kaplan–Meier survival analysis indicated that compared with the liberal strategy, conservative fluid management could not improve the 90-day mortality in patients classified as phenotype III (HR = 0.858, P = 0.316). (D) The survival curves of 90-day cumulative mortality of patients classified as phenotype IV. The Kaplan–Meier survival analysis indicated that compared with the liberal strategy, conservative fluid management could not improve the 90-day mortality in patients classified as phenotype IV (HR = 0.484, P = 0.345). HR, hazard ratio.
Patients with other phenotypes given the different fluid management strategies did not show a significant difference in clinical outcomes, as shown in Figure 2 and Table 1.
For better insight into the characteristics of the phenotypes, features among different phenotypes were compared and evaluated. Phenotype IV was not selected as the main observational cohort due to the small sample size.
Patients classified as phenotype II exhibited a higher WBC (20487.51 ± 7223.86/mm3) and had a higher incidence of anasarca (8.3%) and incidence of shock (26.6%) at baseline, as shown in Tables 2, 3. Therefore, phenotype II was defined as the “hyperinflammatory anasarca” phenotype. Other characteristics of phenotypes are illuminated in Tables 2, 3.
The fluid management strategy for ARDS is not very clear. The current secondary analysis of RCTs identified 4 ARDS phenotypes, and a conservative fluid management strategy significantly improved the 90-day mortality of patients classified as phenotype II compared with a liberal fluid management strategy. In addition, a conservative fluid-management strategy was superior to a liberal fluid-management strategy in avoiding superinfection and returned to assisted breathing. Phenotype II was defined as a “hyperinflammatory anasarca” phenotype due to the higher WBC count with the higher incidence of anasarca and incidence of shock at baseline.
The current study first found that patients exhibiting a “hyperinflammatory anasarca” phenotype could benefit from a conservative fluid management strategy. This specific population showed a higher WBC (20487.51 ± 7223.86/mm3) with a higher incidence of anasarca (8.3%) and incidence of shock (26.6%) at baseline. Distributive shock and oedema due to ARDS-induced systemic inflammatory host responses on cardiovascular systems were marked signs in these patients (16–19). Previous studies uncovered that oedema was an independent risk factor for superinfection. and the anasarca could increase the number of days of mechanical ventilation (2, 20, 21). Our analysis further demonstrated that relieving oedema through a conservative fluid management strategy could effectively avoid superinfection and return to assisted breathing in patients with phenotype II, which could be the main reason to explain why the conservative fluid management strategy improved the mortality of these ARDS populations. Meanwhile, in order to maintain mean arterial pressure ≥ 65 mmHg and sufficient cardiac output to achieve adequate tissue perfusion for important organs, vasopressors are critical and should be used early for patients classified as phenotype II. This strategy is also suggested by Surviving Sepsis Campaign guidelines for septic shock (22, 23).
Individual and detailed situations should be considered to select a suitable fluid management strategy in patients classified as having other phenotypes. As there were no significant differences in clinical outcomes between conservative and liberal fluid management strategies, the detailed therapies should depend on patients' individual morbid conditions. If shock-induced tissue hypoperfusion is a crucial clinical problem in certain patients, a conservative fluid management strategy should be cautiously used in these patients. However, if ARDS-induced shock is reversed, a conservative fluid-management strategy could be considered for the improvement of respiratory failure (24–26).
There are some limitations in this study: prospective validation is required before definitive conclusions regarding therapy can be drawn. Meanwhile, the study is also limited by the fact that the beneficial effect was not externally validated. In addition, specific populations who could benefit from liberal fluid management strategies or other therapeutic methods could not be identified in the current study.
Patients exhibiting a “hyperinflammatory anasarca” phenotype could benefit from conservative fluid management strategies.
The original contributions presented in the study are publicly available. This data can be found here: ARDS network public database (http://www.ardsnet.org/).
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SZ took responsibility for the integrity and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thank you for all the researchers of the ARDS network project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.727910/full#supplementary-material
1. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. (2017) 377:1904–5. doi: 10.1056/NEJMra1608077
2. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. (2018) 319:698–710. doi: 10.1001/jama.2017.21907
3. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
4. Bos LD, Martin-Loeches I, Schultz MJ. ARDS: challenges in patient care and frontiers in research. Eur Respir Rev. (2018) 27:170107. doi: 10.1183/16000617.0107-2017
5. Vignon P, Evrard B, Asfar P, Busana M, Calfee CS, Coppola S, et al. Fluid administration and monitoring in ARDS: which management? Intensive Care Med. (2020) 46:2252–64. doi: 10.1007/s00134-020-06310-0
6. Casey JD, Semler MW, Rice TW. Fluid management in acute respiratory distress syndrome. Semin Respir Crit Care Med. (2019) 40:57–65. doi: 10.1055/s-0039-1685206
7. Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intens Care Med. (2017) 43:155–70. doi: 10.1007/s00134-016-4573-3
8. Monnet X, Teboul JL. My patient has received fluid. How to assess its efficacy and side effects? Ann Intens Care. (2018) 8:54. doi: 10.1186/s13613-018-0400-z
9. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. (2006) 354:2564–75. doi: 10.1016/j.jvs.2006.08.053
10. Zhang Z, Navarese EP, Zheng B, Meng Q, Liu N, Ge H, et al. Analytics with artificial intelligence to advance the treatment of acute respiratory distress syndrome. J Evid Based Med. (2020) 13:301–12. doi: 10.1111/jebm.12418
11. Zhang Z, Zheng B, Liu N, Ge H, Hong Y. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intens Care Med. (2019) 45:856–64. doi: 10.1007/s00134-019-05627-9
13. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. (2016) 4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63
14. Zhang S, Lu Z, Wu Z, Xie J, Yang Y, Qiu H. Determination of a “specific population who could benefit from rosuvastatin”: a secondary analysis of a randomized controlled trial to uncover the novel value of rosuvastatin for the precise treatment of ARDS. Front Med. (2020) 7:598621. doi: 10.3389/fmed.2020.598621
15. Qin X, Li J, Hu W, Yang J. Machine learning K-means clustering algorithm for interpolative separable density fitting to accelerate hybrid functional calculations with numerical atomic orbitals. J Phys Chem A. (2020) 124:10066–174. doi: 10.1021/acs.jpca.0c06019
16. Zhang S, Chu C, Wu Z, Liu F, Xie J, Yang Y, et al. IFIH1 contributes to M1 macrophage polarization in ARDS. Front Immunol. (2021) 11:580838. doi: 10.3389/fimmu.2020.580838
17. Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. (2009) 136:1631–43. doi: 10.1378/chest.08-2408
18. Zhang S, Wu Z, Xie J, Yang Y, Wang L, Qiu H. DNA methylation exploration for ARDS: a multi-omics and multi-microarray interrelated analysis. J Transl Med. (2019) 17:345. doi: 10.1186/s12967-019-2090-1
19. Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. (2019) 40:31–9. doi: 10.1055/s-0039-1683996
20. Chiumello D, Brioni M. Severe hypoxemia: which strategy to choose. Crit Care. (2016) 20:132. doi: 10.1186/s13054-016-1304-7
21. Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. (2017) 195:331–8. doi: 10.1164/rccm.201603-0645OC
22. Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intens Care Med. (2018) 44:1400–26. doi: 10.1007/s00134-018-5175-z
23. Zhang S, Liu F, Wu Z, Xie J, Yang Y, Qiu H. Contribution of m6A subtype classification on heterogeneity of sepsis. Ann Transl Med. (2020) 8:306. doi: 10.21037/atm.2020.03.07
24. Evans RG, Naidu B. Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury? Interact Cardiovasc Thorac Surg. (2012) 15:498–504. doi: 10.1093/icvts/ivs175
25. Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intens Care Med. (2011) 26:116–24. doi: 10.1177/0885066610384192
Keywords: ARDS, conservative fluid management, liberal fluid management, phenotype, hyperinflammatory anasarca
Citation: Xing C-y, Gong W-b, Yang Y-N, Qi X-j and Zhang S (2021) ARDS Patients Exhibiting a “Hyperinflammatory Anasarca” Phenotype Could Benefit From a Conservative Fluid Management Strategy. Front. Med. 8:727910. doi: 10.3389/fmed.2021.727910
Received: 20 June 2021; Accepted: 30 July 2021;
Published: 25 August 2021.
Edited by:
Qinghe Meng, Upstate Medical University, United StatesReviewed by:
Abele Donati, Marche Polytechnic University, ItalyCopyright © 2021 Xing, Gong, Yang, Qi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Zhang, Mzk0ODczOTY3QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.