- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea
- 2Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea
Background: The number of elderly patients with superficial esophageal cancer (SEC) is increasing. We aimed to evaluate the clinical outcomes and prognostic factors of overall survival (OS) in elderly patients undergoing endoscopic submucosal dissection (ESD) or surgical resection for SEC.
Methods: Between January 2001 and May 2020, 290 patients aged ≥65 years who underwent ESD or surgical resection for SEC were evaluated. Their clinical outcomes and prognosis were assessed, and independent risk factors for OS were identified.
Results: The mean patient age (269 men and 21 women) was 70.9 years (range 65–90 years). En bloc, R0, and curative resections were achieved in 94.5%, 90.0%, and 73.4% of the patients, respectively. During the follow-up [mean: 54.6 months (range: 1–210 months)], 79 patients died. The 3-, 5-, and 10-year OS rates were 82.5, 73.1, and 59.7%, respectively. In multivariate analysis, cancer history of the other organs, American Society of Anesthesiologists performance status, and presence of lymphovascular involvement (hazard ratio = 1.852, 1.656, and 1.943, respectively; all P < 0.05) were independent risk factors for poor OS. The high-risk group (≥2 risk factors) showed a significantly lower OS than the low-risk group (≤ 1 risk factor) (P < 0.001).
Conclusions: The three risk factors could be useful in predicting the long-term prognosis of elderly patients with SEC.
Introduction
Esophageal cancer is a major cause of cancer-related deaths worldwide and is the seventh most common malignant tumor (1). Approximately 300,000 patients die of esophageal cancer yearly worldwide (2). The 5-year survival rate is approximately 15–25%. The best results are related to early diagnosis (3); however, only 22% of superficial esophageal cancer (SEC) cases are detectable (4) mainly because SEC shows flat isochromatic features on conventional endoscopy (5), and most SEC or precancerous lesions show no symptoms (6). Recently, the use of Lugol chromoendoscopy and narrow band imaging has improved the detection of SEC (7, 8). With the recent increase in health check-ups, the number of elderly patients diagnosed with SEC is increasing (9).
SEC is a lesion in which tumor infiltration is limited to the basement membrane (Tis), mucosa (T1a), or submucosal layer (T1b) of the esophageal wall (10, 11). SEC can be treated with endoscopic submucosal dissection (ESD), surgical resection, and chemoradiation therapy (12). Surgical resection can be cured and has the advantage of knowledge of the exact stage, but it has high complication and morbidity. The outcome of chemoradiation therapy is favorable; however, it requires a long treatment period, and accurate histologic assessment is impossible (12). ESD is considered a curative treatment option for SEC in some cases, depending on the size, invasion depth, and extent of tumor (13). Therefore, the European Society of Gastrointestinal Endoscopy (ESGE) recommends ESD as the first-line treatment for superficial esophageal squamous cell carcinoma (14). ESD for SEC has a long-term survival equivalent to that of those treated with surgical resection (15). ESD cannot accurately determine whether lymph node metastasis is present; esophagectomy or chemoradiation therapy is therefore recommended when there is a risk of lymph node metastasis. Tumor histology, invasion depth, tumor differentiation, and lymphovascular invasion are known (13) risk factors for lymph node metastasis in SEC.
The life expectancy of western and eastern populations is increasing, and an increasing number of elderly patients are developing esophageal cancer (16). In the Republic of Korea (ROK), the average life expectancy in 2017 was 82.7 years (17); therefore, SEC treatment is important. In elderly patients, a different approach for SEC treatment may be needed because of comorbidities, poor general condition, and limited life expectancy related to aging. As the main cause of mortality in elderly patients with esophageal cancer is non-cancer-related death, survival and maintenance of a good quality of life are important in the management of elderly SEC patients. However, data on the long-term clinical outcomes of elderly SEC patients are relatively insufficient compared to younger patients (18–20). Therefore, we aimed to evaluate the clinical outcomes of elderly SEC patients and the prognostic factors for OS in long-term cohort data.
Methods
Patients and Study Design
We retrospectively reviewed the data of patients aged ≥65 years (21–23) who underwent ESD or surgical resection for SEC at Severance Hospital and Gangnam Severance Hospital between January 2001 and May 2020.
The inclusion criteria were as follows: (1) final pathology result was high-grade dysplasia, or squamous cell carcinoma; (2) tumor limited to the mucosa or submucosa; (3) age ≥ 65 years; and (4) treatment-naive esophageal cancer.
Patients who underwent neoadjuvant therapy (n = 27), patients without data confirming their survival or death (n = 21), and those with insufficient clinical or laboratory information (n = 4) were excluded.
SEC was defined based on the final histopathologic report of the resection specimen, since the tumor was limited to the mucosa or submucosa. ESD was performed for lesions that could be treated endoscopically according to the criteria of The Esophageal Cancer Practice Guidelines 2017 (24).
The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Severance Hospital (IRB number: 4-2020-1493). The requirement for informed consent was waived owing to the retrospective design of this study. All authors had access to the study data and reviewed and approved the final manuscript.
Evaluation of Baseline Patient Characteristics
We assessed the patient's baseline demographic and clinical characteristics, including age, sex, presences of comorbidities, smoking history, alcohol history, cancer history of other organs and use of anticoagulant or antiplatelet medications. Presence of cancer history of other organs does not meant active cancer, but refers to a medical history that has been cured and is not currently receiving treatment.
We also evaluated possible prognostic factors, including the Onodera prognostic nutritional index (PNI) (25), neutrophil-to-lymphocyte ratio (NLR) (26), American Society of Anesthesiologist-performance status (ASA-PS) (27), and Charlson comorbidity index (CCI) (28). CCI was calculated as the sum of the scores assigned for several comorbidities based on the original definition (28). PNI and NLR were calculated based on blood sampling results.
Histological Assessment
For pathologic specimen evaluation, tumor histology, grade of differentiation, tumor size, invasion depth, lymphovascular involvement (LVI), perineural involvement, and presence of tumor in the resection margin were evaluated. The definition of the histology assessment was based on the Japanese Classification of Esophageal Cancer, 11th Edition (11). T1a; Tumor invades mucosa, M1; Carcinoma in situ, M2; Tumor invades lamina proprima mucosae, M3; Tumor invades muscularis mucosae, T1b; Tumor invades submucosa (SM), SM1; Tumor invades the upper third of the submucosal layer, SM2; Tumor invades the middle third of the submucosal layer, SM3; Tumor invades the lower third of the submucosal layer.
Follow-Up
Post-treatment surveillance for recurrence was performed. For the ESD and surgical resection groups, chest computed tomography (CT) was performed every 6 months for 2 years and annually thereafter for 5 years. Endoscopic evaluation was performed every 6 months for 2 years and annually thereafter for the ESD group. Annual endoscopic evaluation after treatment was performed in the surgical resection group. When cancer recurrence was detected, the patients underwent additional chest and abdominal CT scans.
Short-Term Outcomes
Short-term outcomes were evaluated in terms of en bloc resection, R0 resection, curative resection, procedure time, duration of hospital stay, and adverse events. En bloc resection was defined as removal of the lesion in a single piece with a tumor-free margin. R0 resection was defined as en bloc resection with histopathological demonstration of horizontal and vertical margins free of cancer and dysplasia. Curative resection was defined as R0 resection without vascular invasion or lymph node metastasis on histology (29). When ESD is performed for SEC, En bloc resection is considered curative if the tumor is within the mucosa or invade the submucosa up to 200 μm without lymphovascular invasion (sm1) (30). In the ESD group, the procedure time was defined as the time from the start of dissection until the dissection was completed and the lesion was separated, and in the surgical resection group, it was defined as the time from the start of the incision to the time the suture was completed. Clinical signs of bleeding included hematemesis, melena, and hematochezia, and laboratory signs of bleeding were defined as a ≥ 2.0 g/dL decrease in hemoglobin level. Perforation was diagnosed radiologically using chest radiography or CT after the procedure. Pneumonia was diagnosed using chest radiography or CT after the procedure. Post-procedural stricture was defined as a stricture that required endoscopic treatment.
Long-Term Outcomes
Long-term follow-up data were retrospectively collected from medical records. The all-cause mortality data of patients who did not regularly visit our institution were obtained from the National Health Insurance Corporation database. The date when SEC was first treated was defined as the index date, and the date of death from the index date was calculated. The relationship between OS and clinicopathological factors was evaluated. The clinicopathologic factors included patient characteristics (age, sex, smoking history, alcohol history, comorbidities, use of anticoagulants and/or antiplatelet drugs, PNI, NLR, ASA-PS, and CCI), lesion characteristics (tumor size, location, histologic type, gross appearance, circumferential spread, invasion depth, number of lesions, and presence of LVI), en bloc resection, R0 resection, and curative resection. OS was defined as the period from treatment to death from all causes. Follow-up periods were calculated from the date of ESD or surgery.
Statistical Analysis
The patient's demographic, pathologic, and short-term clinical outcome data are summarized as the mean (minimum–maximum) for continuous variables and as numbers with percentages for categorical variables. OS was calculated using the Kaplan–Meier method and compared using the log-rank test. The OS was measured from the index date to the date of death or final follow-up, or the latest confirmation of survival. The relationship between OS and clinicopathologic features of patients was assessed using univariate and multivariate analyses using the Cox proportional hazard model. Hazard ratios (HRs) and 95% confidence intervals (CI) were calculated. When performing subgroup analysis comparing ESD and surgical resection groups, propensity score matching was performed using age, gender, and ASA-PS. The cutoff values of PNI and NLR were the values that maximized the sum of sensitivity and specificity for OS in receiver operating characteristic curve analysis. The value of dividing high- and low-risk groups was based on the sum of sensitivity and specificity, when the correlation between the patient's number of risk factors and the OS was analyzed using the receiver operating characteristic curve. All statistical analyses were performed using the SPSS software version 25.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05.
Results
Patient Characteristics
Among the 342 patients aged ≥65 years who underwent ESD or surgical resection for SEC, 52 were excluded according to our established exclusion criteria. Hence, 290 patients were selected for statistical analysis (Supplementary Figure 1). Of the 290 patients, 116 (40%) patients underwent ESD procedures, while 174 (93 laparoscopic esophagectomy and 81 open esophagectomy) patients underwent surgical resection.
Baseline characteristics of the study population are presented in Table 1. The comparison of baseline characteristics of patients who underwent ESD and those who underwent surgical resection is summarized in Supplementary Table 1. Patients who underwent ESD were significantly older; had higher BMI, serum creatinine level, and neutrophil count; had higher proportions of heavy drinkers, cancer history of the other organs, and use of anticoagulants or antiplatelet drugs; and lower PNI, ASA-PS, CCI, WBC count, and hemoglobin level than those who underwent surgical resection (all P < 0.05).
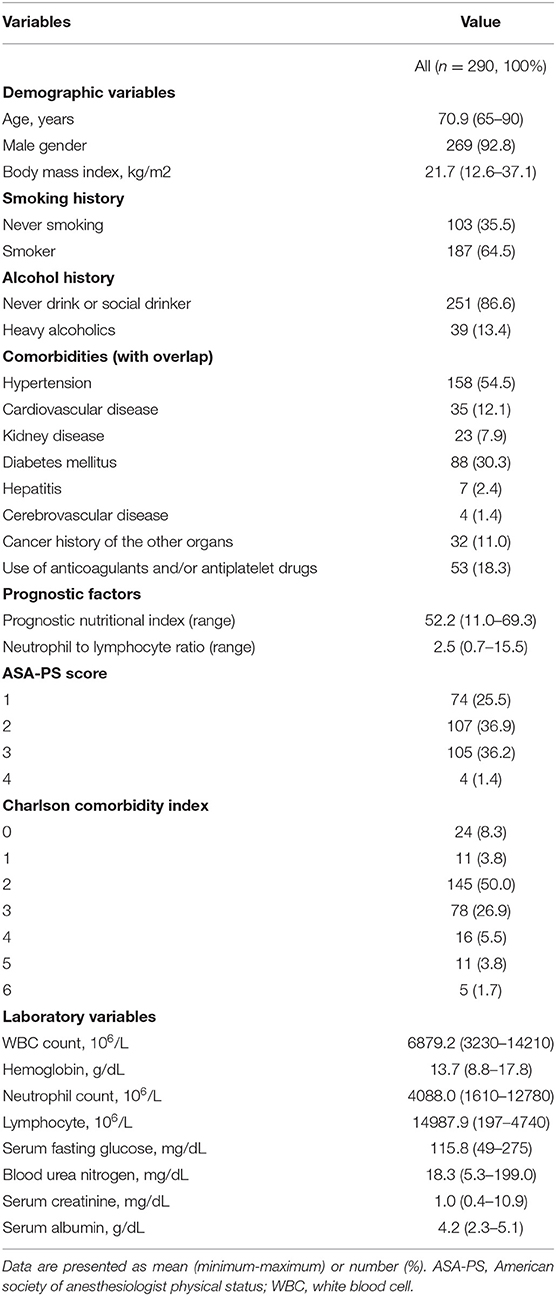
Table 1. Baseline characteristics of the 290 patients aged ≥ 65 years with superficial esophageal cancer.
Lesion Characteristics
The lesion characteristics of the 290 patients are shown in Table 2. Most superficial esophageal neoplasms (n = 252, 86.9%) were squamous cell carcinomas, and the remaining 38 patients (13.1%) were high grade dysplasia, and most (n = 281, 96.9%) occurred in the middle or lower esophagus. Flat lesions were the most common (n = 226, 78.0%), and most (n = 260, 89.7%) were single lesions. The mean tumor size was 22.5 mm (range: 2.0–100.0 mm), and 144 (49.7%) had invaded the submucosal layer. LVI was observed in 32 (11.0%) patients, and perineural involvement was observed in one patient (0.3%).
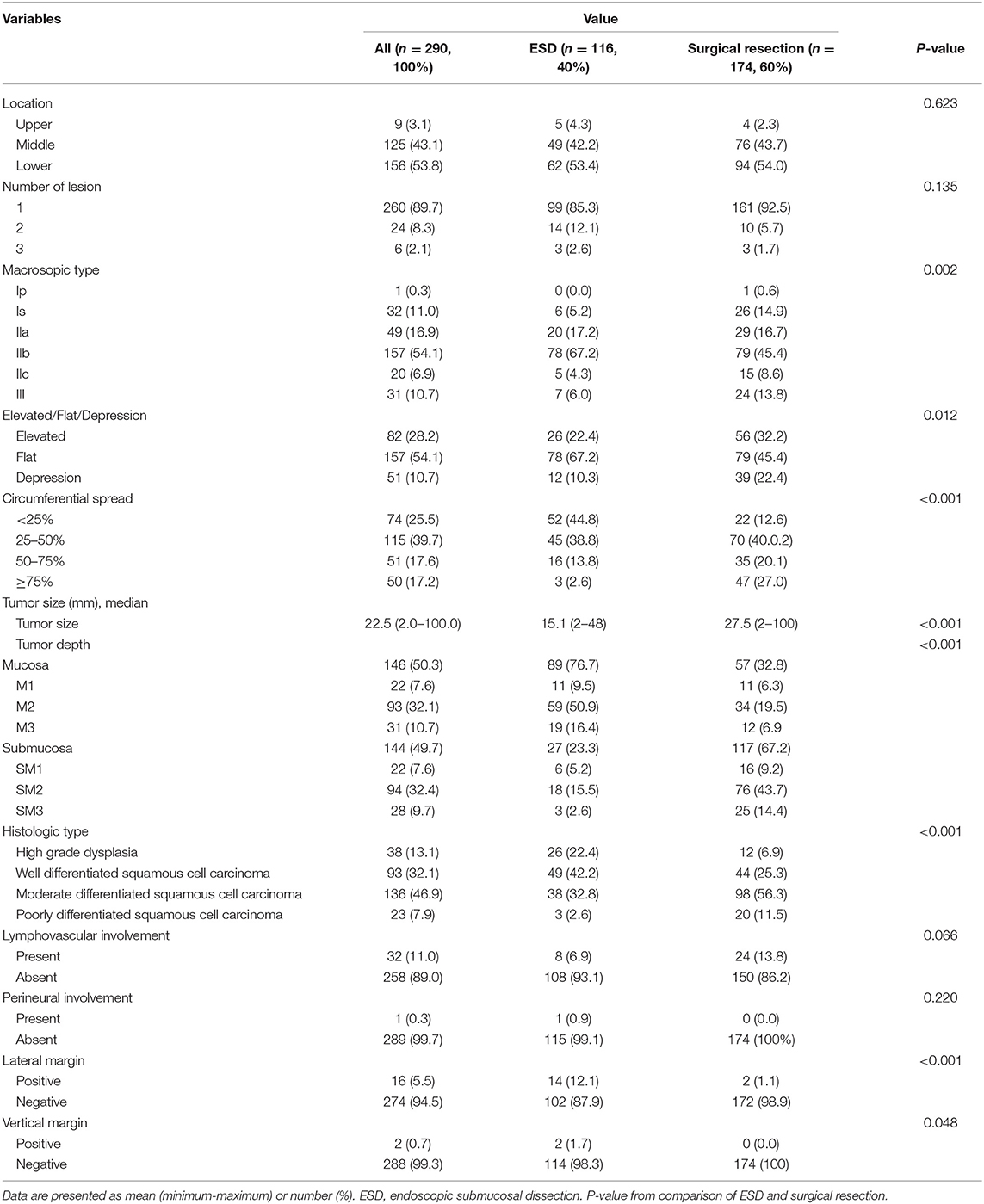
Table 2. Lesion characteristics of the 290 patients aged ≥ 65 years underwent endoscopic submucosal dissection (ESD) or surgical resection for superficial esophageal cancer.
The comparison of lesion characteristics of the 290 patients is summarized in Table 2. Patients who underwent ESD had more flat lesions, a higher proportion of circumferential spread of < 50%, higher proportion of positive resection margin, smaller tumor size, and less submucosal involvement than those who underwent surgical resection (all P < 0.05).
Short-Term Outcomes
The short-term outcomes are shown in Table 3. In the 290 patients, the en bloc and R0 resection rates were 94.5% (n = 274) and 90.0% (n = 261), respectively. Curative resection was achieved in 213 (73.4%) patients. The adverse events were divided into those that occurred 48 h before ESD or surgical resection and those that occurred after 48 h of resection. Perforation occurred in six (2.1%) patients, all of whom underwent ESD, within 48 h after ESD or surgical resection. Adverse events that occurred after 48 h of ESD or surgical resection included bleeding (n = 3, 1.0%), perforation (n = 11, 3.8%), pneumonia (n = 42, 14.5%), and stricture (n = 54, 18.6%). Three (1.0%) patients in the surgical resection group died of adverse events. There were no ESD-related deaths. The mean procedure time and duration of hospital stay were 268.2 min (range: 6–635 min) and 20.4 days (range: 3–165 days), respectively.

Table 3. Short-term clinical outcomes of endoscopic submucosal dissection (ESD) and surgical resection for the 290 elderly patients with superficial esophageal neoplasm.
Long-Term Outcomes and Relationship Between OS and Clinicopathologic Factors
During the follow-up period (mean: 54.6 months, range: 1–210 months), a total of 79 (27.2%) patients died, 23 (7.9%) of whom died of esophageal cancer, and 56 (19.3%) died of other causes. Among the 77 (26.5%) patients who underwent non-curative resection, 29 (10.0%) underwent additional surgical resection, chemotherapy, or radiation therapy. Esophageal cancer recurred in 28 (9.7%) patients during the follow-up period. The mean period until recurrence was 52.8 months. For all patients, the 3-, 5-, and 7-year OS rates were 82.5, 73.1, and 64.9%, respectively, and the 3-, 5-, and 7-year esophageal cancer-related survival rates were 92.7, 89.8, and 89.8%, respectively (Figure 1).
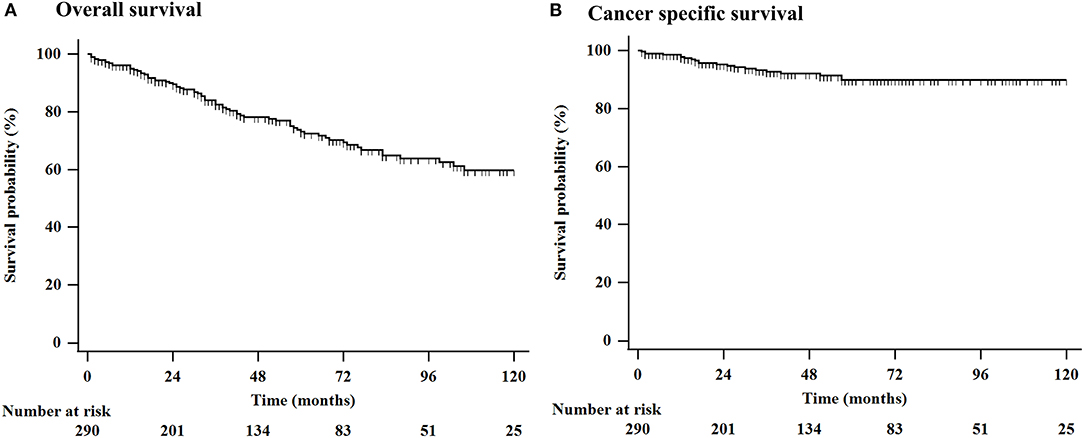
Figure 1. Kaplan-Meier estimation of OS in 290 elderly patients who underwent ESD or surgical resection for superficial esophageal cancer. (A) The 3-, 5-, and 10-year OS rates were 82.5, 73.1, and 59.7%, respectively, and (B) the 3-, 5-, and 10-year esophageal cancer related survival rates were 92.7, 89.8, and 89.8%, respectively. ESD, endoscopic submucosal dissection; OS, overall survival.
When the patients were divided into the ESD and surgical resection groups, the 3-, 5-, and 7-year OS rates in the ESD group were 87.0, 79.1, and 65.0%, respectively, and those in the surgical resection group were 78.7, 67.6, and 64.5%, respectively, with no statistically significant differences (P = 0.606) (Supplementary Figure 2). When analyzed except for patients who had been treated before 2010, the 3-, 5-, and 7-year OS rates in the ESD group were 86.8, 78.9, and 64.3%, respectively, and those in the surgical resection group were 79.0, 66.6, and 64.1%, respectively, with no statistically significant differences (P = 0.562). After performing propensity score matching using age, sex, and ASA-PS, 107 patients were selected each when dividing the ESD and surgical resection group, the 3-, 5-, and 10-year OS rates in the ESD group were 88.0, 81.9, and 56.4%, respectively, and those in the surgical resection group were 80.3, 67.4, and 62.9%, respectively, with no statistically significant differences (P = 0.512).
When the patients were divided into the curative and non-curative resection groups, the 3-, 5-, and 7-year OS rates in the curative resection group were 85.1, 75.8, and 66.9%, respectively, and those in the non-curative resection group were 74.0, 64.7, and 59.8%, respectively, with no statistically significant differences (P = 0.155) (Supplementary Figure 3).
The relationship between OS and clinicopathological factors is shown in Table 4. In univariate analysis, cancer history of the other organs, ASA-PS ≥ 3, and presence of LVI were associated with OS. In multivariate analysis, cancer history of the other organs (HR = 1.852; 95% CI, 1.010–3.397, P = 0.046), ASA-PS ≥3 (HR = 1.656; 95% CI, 1.012–2.710, P = 0.045), and presence of LVI (HR = 1.943; 95% CI, 1.004–3.762, P = 0.049) were independent risk factors for poor OS (Table 5). When calculating the number of risk factors, the correlation with OS through the receiver operating characteristic curve, the sum of sensitivity and specificity is the highest when divided by two. Patients with two or more of these risk factors and those with one or less of these risk factors were considered to be at “high risk” and “low risk,” respectively; accordingly, 268 (92.4%) and 22 (7.6%) patients were in the low-risk and high-risk groups, respectively. The high-risk group showed a significantly lower OS than the low-risk group (P < 0.001). In the low-risk group, the 3-, 5-, and 7-year OS rates were 84.5, 76.3, and 67.6%, respectively. In the high-risk group, these rates were 51.8, 29.6, and 29.6%, respectively (Supplementary Figure 4).
Twenty six (9.0%) of the total patients received additional treatment such as chemotherapy or radiotherapy. When logistic regression analysis was performed to evaluate whether additional treatment was a risk factor, the HR was 0.6 (95% CI, 0.222–1.681, P = 0.340), which was not statistically significant. When dividing patients who received and did not receive additional treatment, the 3-, 5-, and 7-year OS rate in the patients who received additional treatment were 80.0, 72.0, and 72.0%, respectively, and those in the patients who did not receive additional treatment were 82.7, 73.4, and 64.7%, respectively, with no statistical significant differences (P = 0.989).
Subgroup Analysis-Comparison Between ESD and Surgical Resection
A comparison of short-term clinical outcomes is presented in Table 3. The en bloc and R0 resection rates were significantly higher in the surgical resection group (87.1 vs. 99.4%, 77.6 vs. 98.3%, respectively, P < 0.001). However, the curative resection rate was 72.4% in the ESD group and 74.1% in the surgical resection group, with no statistically significant difference (P = 0.672). Perforation and pneumonia that occurred after 48 h of ESD or surgical resection were higher in the surgical resection group (0 vs. 11%, 2.6 vs. 22.4%, respectively, P < 0.05). The mean procedure time and duration of hospital stay were significantly shorter in the ESD group than in the surgical resection group (63.1 vs. 397.6 min, 6.4 vs. 29.1 days, respectively, P < 0.001).
Discussion
Esophageal cancer has a poor prognosis and must be detected and treated early. ESD or surgical resection is performed to cure SEC, and complications after the procedure and long-term prognosis are important issues. Elderly patients have many comorbidities, poor physical status, and shorter life expectancy than younger patients. Therefore, knowing the long-term prognosis or prognostic factors may help determine the treatment plan. To analyze OS after ESD or surgical resection for SEC in elderly patients, a long observation period is mandatory. Our study had a mean observation period of 54.6 months (range: 1–210 months) for 290 patients, which is a significant advantage. We analyzed the long-term outcomes and prognostic factors in elderly patients who underwent ESD or surgical resection for SEC.
In elderly esophageal cancer patients, analysis is necessary because various factors can affect OS. Therefore, we identified the short- and long-term outcomes and prognostic factors of patients aged ≥ 65 years who underwent ESD or surgical resection for SEC. The short-term outcomes of en bloc resection (94.5 vs. 97.1%), R0 resection (90.0 vs. 92.0%), curative resection (71.0 vs. 73.0–90.5%), perforation (3.8 vs. 0.0–12.1%), bleeding (1.0 vs. 2.0%), and stricture (18.6 vs. 5.1–25.9%) were similar to those reported in a previous meta-analysis study (31). The five-year OS (73.1 vs. 87.3%) and disease-specific survival (89.8 vs. 97.7%) rates were lower than those reported in the previous meta-analysis study (mean age: 70.9 vs. 64–71 years) (31).
In our study, cancer history of the other organs, ASA-PS ≥ 3, and presence of LVI were independent prognostic factors in the elderly patients with SEC. In a previous study, CCI ≥ 2 was identified as a prognostic factor in patients with esophageal cancer (20); however, in this study, CCI was not a prognostic factor for esophageal cancer. CCI is a useful assessment tool for comorbidities and a prognostic factor (20, 32); however, it might be insufficient to reflect the functional status or general condition of elderly patients. We found statistically significant differences in the 3-, 5-, and 7-year OS rates between the low-risk and high-risk groups, which may help predict the prognosis of patients.
We performed the subgroup analysis according to treatment methods in elderly patients with SEC. Compared to patients who underwent surgical resection for esophageal cancer, those who underwent ESD were older, more obese, had a history of other cancers, and tended to take anticoagulants or antiplatelet drugs. This difference is presumed to be because of the preference for ESD, if possible, for older patients or those with more comorbidities, as surgical resection is associated with the burden of general anesthesia.
Esophageal cancer lesions in patients who underwent surgery tended to have larger and deeper tumor depths than those in patients who underwent ESD. There was no statistically significant difference in the curative resection rate between ESD and surgical resection; however, late adverse events occurred more frequently and hospital stay and procedure time were longer in the surgical resection group. This difference may have occurred because clinicians tend to choose ESD for patients with favorable tumor characteristics (small lesion, well-differentiated type, and less deep invasion). However, in the long term, there was no statistically significant difference in OS between the ESD and surgical resection groups, even though there was a difference in the baseline or lesion characteristics. Therefore, clinicians can select a procedure based on the lesion or patient condition.
In a meta-analysis (31), the 5-year OS (86.4 vs. 81.8%) and disease-specific survival (97.5 vs. 94.1%) rates of ESD and surgical resection were similar. It is unclear whether ESD or surgical resection is appropriate for elderly SEC patients. However, surgical resection causes more adverse events in elderly patients than ESD. Therefore, it would be better to perform ESD in high-risk patients. If additional surgery is needed after ESD, chemoradiation therapy or follow-up without additional surgery can be considered for patients at high risk rather than surgery. Since it was not a direct comparative analysis, caution should be exercised during interpretation; however, the criteria for the high- and low-risk groups, identified in this study, may not be an absolute standard for determining a treatment plan for elderly patients, but can be used as reference data.
Our study has several clinical implications. Our study involved a large number of patients aged ≥ 65 years who underwent ESD or surgical resection for SEC. In addition, during the follow-up period, the sufficient number of mortality cases (n = 79, 27.2%) might support the reliability of our study. Long-term prognosis and survival analysis were possible because the follow-up period was long, and the mortality rate was not small. Moreover, the median follow-up period of 54.6 months (maximum 210 months) was sufficient to identify long-term outcomes. Finally, we classified patients into low-risk and high-risk groups based on the risk factors we identified. Based on these results, we provided data that can be used as a basis for decision making when there is a concern about whether to perform ESD or surgical resection for SEC in elderly patients.
Our study has some limitations. First, our study had a retrospective design and therefore might have been subject to a potential bias. Patients who underwent neoadjuvant therapy were excluded because it was difficult to determine whether they were SEC patients; however, selection bias may have occurred during this process. Among the elderly patients, those who chose treatments other than ESD or surgical resection due to various underlying diseases or individual circumstances were excluded. Therefore, selection bias may have occurred. Further prospective studies on the prognosis and risk factors of ESD or surgical resection in the elderly are needed to validate our results. Second, this study was not a multicenter and multinational study. It was conducted with patients from two academic teaching hospitals in the ROK. Patients included in this study may not represent the entire elderly population and do not represent all SEC patients. Third, patients who did not undergo ESD or surgical resection and were only followed-up without treatment after esophageal cancer diagnosis were excluded. Some elderly patients, diagnosed with cancer are only followed-up without treatment for various reasons; therefore, for an accurate comparison of clinical outcomes, it may be necessary to compare patients who were observed without treatment with those who underwent ESD or surgical resection. Fourth, the maximum follow-up period was 210 months in our study. There have been advancements in techniques and devices, which may have caused a difference in prognosis and outcomes. Surgical techniques and instruments, endoscopic accessories, hemostasis methods, and drugs have been developed, and the accumulation of the operator's experience may have influenced the outcomes. However, the results were not significantly different when analyzed except for data in the early 2000's, so it seems that the impact was not significant. Fifth, LVI is not a prognostic factor that can be identified before the procedure; it may be difficult for the high-risk group criteria presented in our study to be used to fully predict patient's prognosis before the procedure. Sixth, comparison with the group of young patients was not analyzed.
In conclusion, we found that a history of cancer in other organs, ASA-PS ≥ 3, and presence of LVI were independent risk factors for poor OS in elderly patients undergoing ESD or surgical resection for SEC. These risk factors could be useful in predicting the long-term prognosis of elderly patients with SEC.
Synopsis
The number of elderly patients with superficial esophageal cancer (SEC) is increasing. Cancer history of the other organs, American Society of Anesthesiologists performance status, and lymphovascular involvement were independent risk factors for poor overall survival in elderly patients with SEC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Author Contributions
JC, DJ, and CH: in planning and conducting the study and drafting the manuscript. JC, DJ, CH, JP, SS, SL, and YL: collecting and interpreting data. DJ: guarantor of the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1C1C1013775).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.722141/full#supplementary-material
References
1. Noordzij IC, Curvers WL, Schoon EJ. Endoscopic resection for early esophageal carcinoma. J Thorac Dis. (2019) 11:S713–S22. doi: 10.21037/jtd.2019.03.19
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. (2013) 381:400–12. doi: 10.1016/S0140-6736(12)60643-6
4. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. (2013) 19:5598–606. doi: 10.3748/wjg.v19.i34.5598
5. Lee HD, Chung H, Kwak Y, Choi J, Lee A, Kim JL, et al. Endoscopic submucosal dissection versus surgery for superficial esophageal squamous cell carcinoma: A propensity score-matched survival analysis. Clin Transl Gastroenterol. (2020) 11:e00193. doi: 10.14309/ctg.0000000000000193
6. Wu Y, Zhang H, Zhou B, Han S, Zhang Y. Clinical efficacy of endoscopic submucosal dissection in the treatment of early esophageal cancer and precancerous lesions. J Cancer Res Ther. (2018) 14:52–6. doi: 10.4103/jcrt.JCRT_805_17
7. Goda K, Tajiri H, Ikegami M, Dobashi A, Yoshimura N. Clinical impact of narrow-band imaging magnifying endoscopy for 'basal layer type squamous cell carcinoma' in the esophagus. Dig Endosc. (2011) 23:75–8. doi: 10.1111/j.1443-1661.2011.01121.x
8. Inoue H, Rey JF, Lightdale C. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy. (2001) 33:75–9.
9. Jung HK, Tae CH, Lee HA, Lee H, Don Choi K, Park JC, et al. Treatment pattern and overall survival in esophageal cancer during a 13-year period: a nationwide cohort study of 6,354 Korean patients. PLoS ONE. (2020) 15:e0231456. doi: 10.1371/journal.pone.0231456
10. Malik S, Sharma G, Sanaka MR, Thota PN. Role of endoscopic therapy in early esophageal cancer. World J Gastroenterol. (2018) 24:3965–73. doi: 10.3748/wjg.v24.i35.3965
11. Japanese Japanese Classification of Esophageal Cancer 11th Edition: part I. Esophagus. (2017) 14:1–36. doi: 10.1007/s10388-016-0551-7
12. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. (2019) 16:1–24. doi: 10.1007/s10388-018-0641-9
13. Aadam AA, Abe S. Endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. (2018) 31:doy021. doi: 10.1093/dote/doy021
14. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. (2015) 47:829–54. doi: 10.1055/s-0034-1392882
15. McWhirter JP, Pennington CR. A comparison between oral and nasogastric nutritional supplements in malnourished patients. Nutrition. (1996) 12:502–6. doi: 10.1016/S0899-9007(96)91727-X
16. Kikuchi O, Mouri H, Matsueda K. Endoscopic submucosal dissection for treatment of patients aged 75 years and over with esophageal cancer. ISRN Gastroenterol. (2012) 2012:671324. doi: 10.5402/2012/671324
17. Chang JW, Jung DH, Park JC, Shin SK, Lee SK, Lee YC. Long-term outcomes and prognostic factors of endoscopic submucosal dissection for early gastric cancer in patients aged ≥75 years. Cancers. (2020) 12:3222. doi: 10.3390/cancers12113222
18. Iizuka T, Kikuchi D, Hoteya S. Outcomes of endoscopic submucosal dissection for superficial esophageal cancer in an elderly population: a retrospective single center cohort study. Endosc Int Open. (2019) 7:E355–60. doi: 10.1055/a-0832-8257
19. Hatta W, Gotoda T, Koike T, Masamune A. Management following endoscopic resection in elderly patients with early-stage upper gastrointestinal neoplasia. Dig Endosc. (2020) 32:861–73. doi: 10.1111/den.13592
20. Nakajo K, Abe S, Oda I, Ishihara R, Tanaka M, Yoshio T, et al. Impact of the Charlson Comorbidity Index on the treatment strategy and survival in elderly patients after non-curative endoscopic submucosal dissection for esophageal squamous cell carcinoma: a multicenter retrospective study. J Gastroenterol. (2019) 54:871–80. doi: 10.1007/s00535-019-01583-9
21. Molena D, Stem M, Blackford AL, Lidor AO. Esophageal cancer treatment is underutilized among elderly patients in the USA. J Gastrointest Surg. (2017) 21:126–36. doi: 10.1007/s11605-016-3229-5
22. Skorus UA, Kenig J. Outcome of esophageal cancer in the elderly - systematic review of the literature. Wideochir Inne Tech Maloinwazyjne. (2017) 12:341–9. doi: 10.5114/wiitm.2017.72318
23. Won E, Ilson DH. Management of localized esophageal cancer in the older patient. Oncologist. (2014) 19:367–74. doi: 10.1634/theoncologist.2013-0178
24. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. (2019) 16:25–43. doi: 10.1007/s10388-018-0642-8
25. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
26. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. (2007) 73:215–20. doi: 10.1159/000127412
27. Sedation AURbtASoATFo Non-Anesthesiologists Ab. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. (2002) 96:1004–17. doi: 10.1097/00000542-200204000-00031
28. Charlson ME, Pompei P, Ales KL, MacKenzie CR, A. new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
29. Park CH, Yang D-H, Kim JW, Kim J-H, Kim JH, Min YW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc. (2020) 53:142–66. doi: 10.5946/ce.2020.032
30. Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus april 2012 edited by the Japan esophageal society. Esophagus. (2015) 12:1–30. doi: 10.1007/s10388-014-0465-1
31. Yeh JH, Huang RY, Lee CT, Lin CW, Hsu MH, Wu TC, et al. Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis. Therap Adv Gastroenterol. (2020) 13:1756284820964316. doi: 10.1177/1756284820964316
Keywords: esophageal cancer, endoscopic submucosal dissection, surgical resection, elderly, prognostic factors
Citation: Chang JW, Jung DH, Huh CW, Park JC, Shin SK, Lee SK and Lee YC (2022) Long-Term Outcomes and Prognostic Factors of Superficial Esophageal Cancer in Patients Aged ≥ 65 Years. Front. Med. 8:722141. doi: 10.3389/fmed.2021.722141
Received: 08 June 2021; Accepted: 27 December 2021;
Published: 18 January 2022.
Edited by:
Philip Chiu, The Chinese University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Darrick Li, Yale University, United StatesHyun Jung Lee, Seoul National University, South Korea
Copyright © 2022 Chang, Jung, Huh, Park, Shin, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da Hyun Jung, anVuZ2RoJiN4MDAwNDA7eXVocy5hYw==; Cheal Wung Huh, aHVoY3cmI3gwMDA0MDt5dWhzLmFj
 Jin Won Chang
Jin Won Chang Da Hyun Jung
Da Hyun Jung Cheal Wung Huh2*
Cheal Wung Huh2* Jun Chul Park
Jun Chul Park Sang Kil Lee
Sang Kil Lee