- 1Department of Transplantation, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Microbiology and Infectious Disease Center, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, China
- 3Department of Laboratory, The Third Affiliated Hospital of Southern Medical University, Guangzhou, China
- 4Department of Pathology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 5Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, China
- 6Guangdong Provincial Key Laboratory of Tropical Disease Research, Department of Microbiology, School of Public Health, Southern Medical University, Guangzhou, China
- 7Departments of Urology and Pathology, Harvard Medical School, Massachusetts General Hospital, Boston, MA, United States
Background: Colonization of Cryptococcus rarely occurs in a graft. This study reports a case of malacoplakia and cryptococcoma caused by E. coli and Cryptococcus albidus in a transplanted kidney, with detailed pathology and metagenome sequencing analysis.
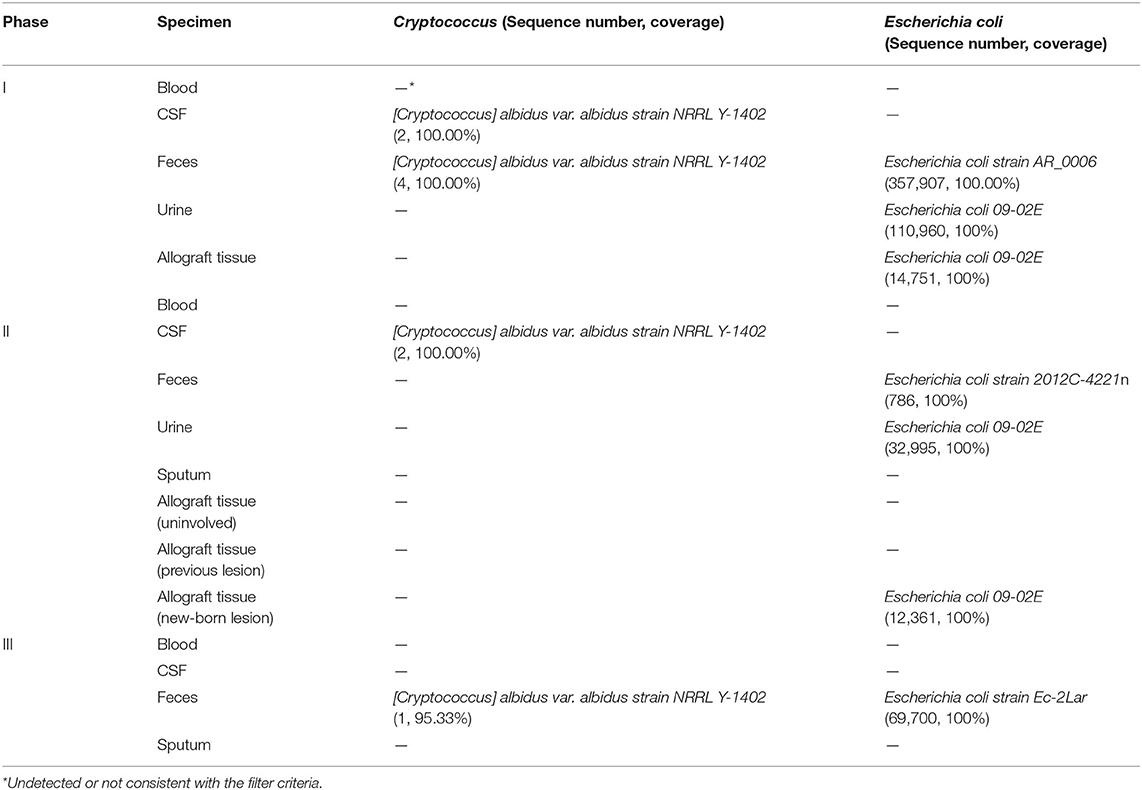
Case Presentation: We presented a case of cryptococcoma and malacoplakia in the genitourinary system including the transplant kidney, bladder, prostate, and seminal vesicles caused by Cryptococcus albidus and Escherichia coli in a renal-transplant recipient. Metagenome sequencing was conducted on a series of samples obtained from the patient at three different time points, which we termed Phase I (at the diagnosis of cryptococcoma), Phase II (during perioperative period of graftectomy, 3 months after the diagnosis), and Phase III (2 months after graftectomy). Sequencing study in the Phase I detected two and four sequences of C. albidus respectively in cerebrospinal fluid (CSF) and feces, with resistant Escherichia coli 09-02E presented in urine and renal mass. A 3-month antibiotic treatment yielded a smaller bladder lesion but an enlarged allograft lesion, leading to a nephrectomy. In the Phase II, two sequences of C. albidus were detected in CSF, while the E. coli 09-02E continued as before. In the Phase III, the lesions were generally reduced, with one C. albidus sequence in feces only.
Conclusions: The existence and clearance of Cryptococcus sequences in CSF without central nervous system symptoms may be related to the distribution of infection foci in vivo, the microbial load, and the body's immunity. Overall, this study highlights the need for enhanced vigilance against uncommon types of Cryptococcus infections in immunocompromised populations and increased concern about the potential correlation between E. coli and Cryptococcus infections.
Introduction
As an opportunistic pathogenic fungus, Cryptococcus is the third most common invasive fungi in solid organ transplantation (SOT) (1). The prevalence of cryptococcosis in this population is 0.2 to 5.8%, with a total mortality rate ranging from 20 to 50% (1, 2). Human immunodeficiency virus-infected patients and SOT recipients are at the highest risk for Cryptococcus infection (3), with Cryptococcus neoformans and Cryptococcus gattii the most common causes of cryptococcosis. Once inhaled, Cryptococcus can disseminate to the whole body or colonize in host tissue through the bloodstream, resulting in diseases such as cryptococcal meningoencephalitis, pulmonary cryptococcosis and cryptococcal granuloma (2, 4, 5). However, Cryptococcus albidus infection is rare, with skin the most commonly involved organ and a mortality rate of around 28% (5/18) (6).
Only one case of localized cryptococcal lesion in transplant kidney has been reported so far (7). Herein we reported the first case of pathology- and metagenome sequencing-proven cryptococcoma caused by C. albidus of a transplanted kidney in a patient presenting with urinary tract infection (UTI) of Escherichia coli and BK polyomavirus viruria. This study sheds light on the correlation between drug-resistant E. coli and Cryptococcus infection. The results suggest that alteration of the immune microenvironment caused by a long-term infection, such as E. coli infection, may be the key reason for the colonization of Cryptococcus in uncommon sites of the body, like an allograft.
Case Description
Clinical History
A 50-year-old male with end-stage renal disease received a left kidney transplant from a deceased male donor who died in a motor vehicle accident in March 2013. After renal transplantation, the patient received a maintenance immunosuppressive regimen consisted of tacrolimus (3.5 mg, bid), mycophenolate mofetil (360 mg, bid), and prednisone (4 mg, qd). At 11 months postoperatively, 1+ to 2+ proteinuria was found on a routine urine examination. The proteinuria was relieved after treatment with Tripterygium glycosides tablets (10 mg, bid). At 15 months postoperatively, the patient developed BKV viruria with a urinary viral load of 1.25 × 107 copies/mL (normal range for reference, <5,000 copies/mL). The viral load was undetectable after the dosage of tacrolimus was reduced to 1.5 mg BID and treating with immunoglobulin (infusion). In April 2016, the patient had a chronic rejection reaction and the 24-h urinary protein quantity increased to 1.13 g/24 h. To maintain the allograft function and suppress proteinuria, the corresponding treatment regimen was methylprednisolone (40 mg) combined with cyclophosphamide (0.2 g) intravenous drip for 3 days/month. After three courses of treatment, the 24-h urinary protein quantity decreased to 0.56 g/24 h. The patient has had recurrent symptoms of UTI such as frequent and urgent urination without obvious inducement since June 2016. Regular outpatient review of urinary examination revealed leukocytes fluctuating from 1+ to 3+. E. coli was detected in the midstream urine culture and intravenous cefoperazone sodium sulbactam (1.5 g, 1/12 h) was given for 1 week. In September 2018, the patients came to the hospital because of cough for 1 day. mNGS of the alveolar lavage fluid indicated pneumosporidiosis and blood tested positive for herpes simplex virus. The pulmonary infection resolved after treated with compound sulfamethoxazole tablets (480 mg, bid). Besides, serum creatinine decreased from 305 μmol/L to 245 μmol/L. However, BKV viruria relapsed with the urinary viral load fluctuated from 2.84 × 105 copies/mL to 3.81 × 107 copies/mL. The immunosuppressive regimen was adjusted to tacrolimus (1.5 mg, bid), mycophenolate sodium enteric-coated tablets (180 mg, bid), and prednisone (4 mg, qd). In November 2018, the patient's serum creatinine was 197 μmol/L, and color Doppler examination of the transplanted kidney and renal vessels showed no significant abnormalities. Regular color Doppler ultrasound examinations of the allograft and transplanted kidney vessels were performed every 6 months after transplantation, all showing neither significant abnormalities nor transplanted kidney masses until the current admission. The patient had not undergone an allograft puncture biopsy within 3 years after renal transplantation. His postoperative serum creatinine level was 190 μmol/L. And the postoperative glomerular filtrate rate (GFR) was summarized in Figure 1. The patient was admitted to hospital in May 2019 because of frequent and urgent urination. The clinical course of the patient is summarized in three phases according to the disease progression.
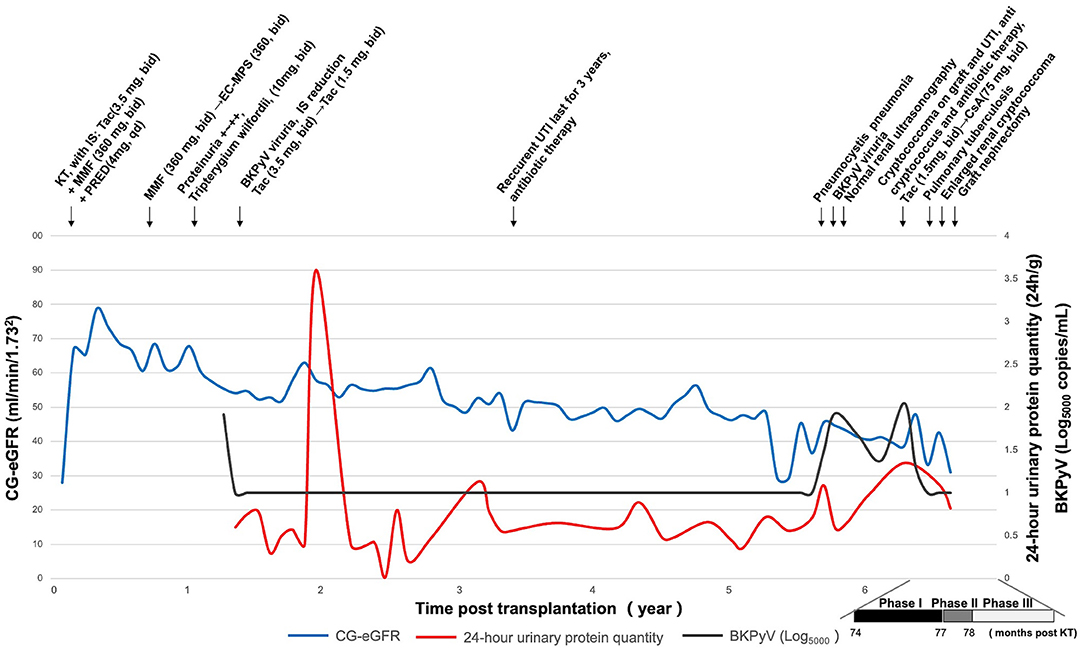
Figure 1. Clinical history of the patient. CG-eGFR, estimated glomerular filtration rate by the Cockcroft-Gault; KT, kidney transplantation; IS, immunosuppressant; Tac, tacrolimus; MMF, mycophenolate mofetil; PRED, prednisone; EC-MPS, mycophenolate sodium enteric-coated tablets; CsA, cyclosporine; BKPyV, BK polyomavirus; UTI, urinary tract infection.
Phase I
In May 2019, the patient was admitted to hospital for recurrent frequent and urgent urination. On admission, his serum creatinine level was 229 μmol/L. Ultrasound and PET/CT showed solid space-occupying lesions in the upper pole of the transplanted kidney and bladder, prostate and seminal vesicles (Figures 2A1–H1). Biopsy of the graft kidney and bladder lesions revealed cryptococcal granulomas, with cystoscopy results provided in Supplementary Figure 1. Extended-spectrum β-lactamase positive E. coli was cultured from both renal graft tissue and midstream urine. To rule out systemic cryptococcosis, a lumbar puncture was performed to collect CSF and CSF opening pressure measured. Cell counts and biochemical parameters in CSF, and CSF opening pressure were normal. Ink staining and Cryptococcus antigen detection of CSF were both negative. No significant abnormalities were observed in brain MRI and chest CT scans. Based on the imaging and pathological findings, the patient was diagnosed with cryptococcoma and malacoplakia of the genitourinary system including transplanted kidney, bladder, prostate and seminal vesicles (Figures 2A1–H1 and Figure 3), accompanied by an UTI of E. coli. The patient was treated with meropenem (0.5 g twice a day), and fluconazole (50 mg twice a day) combined with flucytosine (0.5 g twice a day). Meanwhile, immunosuppression was reduced by conversion from tacrolimus (1.5 mg twice a day) to cyclosporine (100 mg twice a day). Additionally, cyclosporine was later adjusted to 75 mg BID after use of the antifungal drug, fluconazole, which can affect the concentration of cyclosporine. The concentrations of immunosuppressants from renal transplantation to transplant nephrectomy were shown in Supplementary Figure 4. After 10 days' treatment, his renal function improved and the serum creatinine decreased from 229 to 185 μmol/L. In July 2019, his chest X-ray showed a soft tissue shadow in the left hilum, which was diagnosed as pulmonary tuberculosis by bronchoscopy; and the patient was thus treated with isoniazid (300 mg, once daily), ethambutol (750 mg, once daily) and levofloxacin (250 mg, once daily).
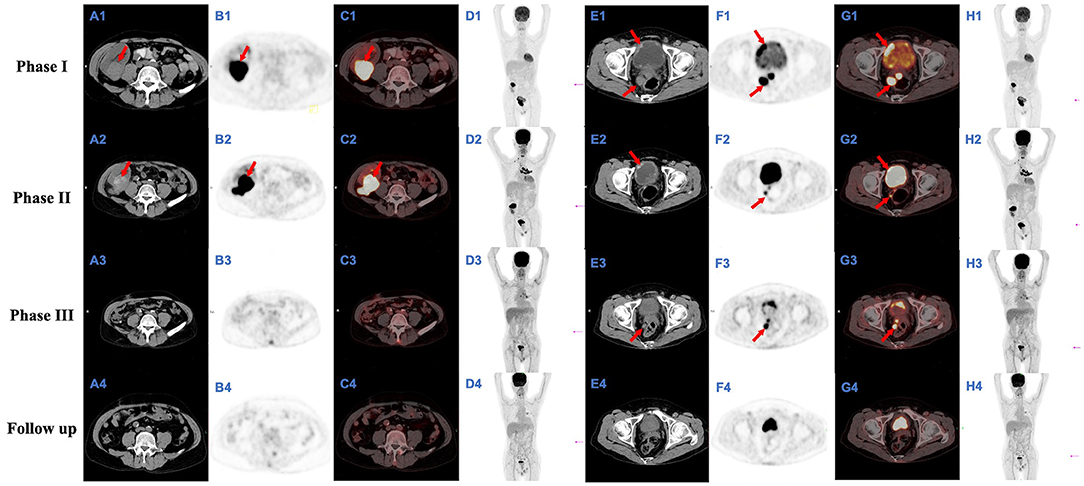
Figure 2. FDG-FDG-PET/CT images. Full-body PET-maximum intensity projection (MIP) (D1–4, H1–4) show several space-occupying hypermetabolic lesions; they also indicate the positions (magenta arrows) of the slice shown (A1–4, B1–4, C1–4, and E1–4, F1–4, G1–4, respectively); an axial CT image (A1–4 and E1–4, respectively), axial PET image (B1–4 and F1–4, respectively), and axial FDG-PET/CT fusion image (C1–4 and G1–4, respectively) from a single plane show a mass of hypermetabolic lesions in the transplanted kidney and bladder respectively; the largest of which (A1–2, B1–2, and C1–2, red arrows), ~4.3 × 4.9 × 4.1 cm and 6.0 × 4.0 × 4.7 cm in size, with maximal standardized uptake value (SUVmax) of 20.3 and 31.0 and average standardized uptake value (SUVave) of 8.8 and 17.4 respectively; the largest of which (E1–3, F1–3, and G1–3, red arrows), ~4.7 × 4.1 × 5.5 cm, 3.8 × 2.3 × 3.6 cm, and 2.8 × 1.4 × 2.5 cm in size, with SUVmax of 21.9, 21.4, and 6.4 and SUVave of 8.7, 8.9, and 4.3 respectively.
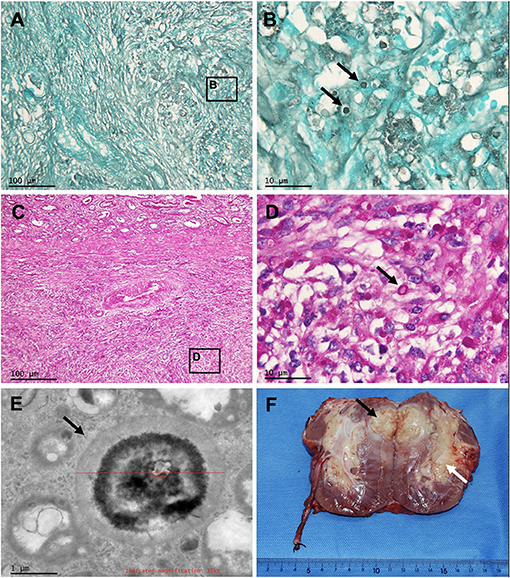
Figure 3. Pathology of the masses of the transplanted kidney, and gross photograph of the allograft nephrectomy specimen. Periodic acid-Schiff staining and Grocott methenamine silver staining of renal mass at 100 × (A,C) and at 1,000 × (B,D) show granuloma caused by Cryptococcus (arrows). Electron microscopy of renal mass (E) shows the Cryptococcus (arrow). (F) Renal cryptococcoma (black arrow) and enlarged renal crptococcoma (white arrow).
Phase II
Follow-up ultrasound and FDG-PET/CT examination in August 2019 showed that although the area of bladder lesion was significantly reduced from 4.7 × 4.1 × 5.5 cm to 3.8 × 2.3 × 3.6 cm, the lesion in the transplanted kidney was enlarged from 4.3 × 4.9 × 4.1 cm to 6.0 × 4.0 × 4.7 cm, with the cryptococcoma and malacoplakia in the upper pole of the transplanted kidney protruding into the adjacent Gerota's fascia (Figures 2A2–H2). Fluconazole was given at 100 mg BID against cryptococcal infection while the anti-Escherichia coli as well as the anti-tuberculosis regimen was maintained, as described in Phase I. The net immunosuppression status of an individual is influenced by the immunosuppression regimen and individual susceptibility and can be assessed by immunosuppressive drug concentrations, peripheral blood leukocyte counts, lymphocyte counts, and viral infection conditions. The peripheral blood leukocyte and lymphocyte counts of this case were lower compared to the average level during uninfected period (Supplementary Figure 5). The presence of pneumosporidiosis, herpes simplex virusemia, and BKV uremia were all suggestive of a low net immunosuppressive status. To stop the disease progression, the patient received a transplant nephrectomy 1 week later.
Phase III
After transplant nephrectomy, the patient's immunosuppressants were discontinued. He was on dialysis three times a week. The anti-tuberculosis treatment was changed to isoniazid (300 mg, once daily), ethambutol (870 mg, once every 2 days) and levofloxacin (250 mg, once every 2 days), while the anti-Cryptococcus treatment was changed to fluconazole (100 mg, twice a day) and flucytosine (0.5 g, three times a day). After 2 months, a follow-up FDG-PET/CT showed that the lesions in the urogenital system and lung were significantly reduced compared with the last examination (Figures 2A3–H3). Six months post-transplant nephrectomy, the lesions in the genitourinary system were eventually eliminated (Figures 2A4–H4).
Pathology
Gross inspection of the transplanted kidney showed that the resected transplant volume was 11 × 9.0 × 5.0 cm with its capsule closely adherent to the surrounding fat. A solid yellow mass of 6.0 × 4.0 × 4.7 cm was found in the upper pole renal parenchyma without breaking through the renal capsule. Another 5.0 × 2.5 × 2.5 cm yellow mass was found in the hilar sinus fat (Figure 3F). The pathological changes of the transplanted kidney lesions were consistent with Cryptococcus infection (Figures 3A–E).
Metagenome Sequencing
Metagenome sequencing revealed that only two and four sequences of C. albidus were respectively detected in the CSF and feces specimens in Phase I. Two sequences of C. albidus were detected in the CSF specimen in Phase II. After transplantation nephrectomy, withdrawal of immunosuppressants and anticryptococcal therapy for 2 months, one sequence of C. albidus was detected in the feces specimen but no sequence in CSF in Phase III. All sequences were typed as [Cryptococcus] albidus var. albidus strain NRRL Y-1402 (Table 1). The type of the E. coli detected in urine and granuloma of the allograft in Phase I and Phase II was E. coli 09-02E (Table 1).
Culture in vitro
In order to understand the interactions between E. coli and Cryptococcus, we cocultured the two microorganisms in vivo. After an 8-h co-culture of E. coli 09-02E filtrate and Cryptococcus neoformans JEC21 (ATCC@96910) in vitro, the Cryptococcus counts in the control group (without E. coli filtrate) and the experimental group (adding 80, 160, 320, 640, and 1,280 μl E. coli filtrate, respectively) were respectively 2.46 ± 0.52 × 105/mL, 2.30 ± 0.57 × 105/mL, 2.83 ± 0.72 × 105/mL, 3.13 ± 0.76 × 105/mL, 3.09 ± 0.61 × 105/mL, and 2.60 ± 0.63 × 105/mL. Comparison between the control group and the experimental Group III (320 μl E. coli filtrate) and Group IV (640 μl E. coli filtrate) showed a statistically significant difference (P < 0.05) (Supplementary Figure 2), indicating that E. coli at these concentrations may stimulate cryptococcal growth.
Discussion
This is the first sequencing study, to our knowledge, of malacoplakia and cryptococcoma of E. coli and C. albidus in the transplanted kidney. Cryptococcus usually attacks the immunocompromised population, resulting in mostly systemic infection (8). Its colonization in the transplanted kidney is extremely rare, with only one case reported before (7). In this case, a 50-year-old male patient with a recurrent UTI of E. coli for 3 years developed cryptococcoma in the transplanted kidney on the 74th month after transplantation. The granuloma disseminated to urogenital organs such as the bladder, prostate, and seminal vesicle. Metagenome sequencing identified the [Cryptococcus] albidus var. albidus strain NRRL Y-1402 as the culprit. And the drug-resistant E. coli 09-02E, first detected in feces from healthy Vietnamese people in 2018, and with unclear pathogenic mechanism and unique urinary system properties (9), arose after the long-term antibiotic use presumably due to selective pressure. Microbiological analysis of kidney transplant preservation fluids was performed prior to transplantation and showed negative results. The patient's recurrent UTI of E. coli began 3 years postoperatively, so the E. coli infection was considered non-donor-derived in this case.
Although co-infection of Cryptococcus and E. coli in the same lesion are rarely detected or reported, we speculated that there is an inevitable relationship between them: long-term repeated antibiotics use leads to dysregulation of bacterial flora drug-resistant strains (10), and promotes the emergence of drug-resistant E. coli and Cryptococcus colonization. In this case, E. coli might have invaded the transplanted kidney prior to Cryptococcus.
Pathological examination revealed granuloma and focal inflammatory cell infiltration and fibrosis in the interstitium. Microbiological culture and metogenomic sequencing results of the transplanted kidney tissues both showed a large number of E. coli. The α-hemolysase released by E. coli can cause renal injury and cicatrization, facilitate the formation of abscesses or granulomas, and block urine excretion in the collecting duct (11), which may be the biological causes of Cryptococcus retention. Studies have shown that E. coli infection alters the immune microenvironment of the infected foci, such as the inflammatory response induced by the activation of cytokines TNF-α, IL-1, IL-6, and IL-8 (11, 12). This immune microenvironment may be the fertile soil for Cryptococcus infection and colonization in E. coli infectious foci.
Interactions between fungi and bacteria are common (13). Our in vitro co-culture result showed that metabolites of E. coli at certain concentration may stimulate cryptococcal growth, suggesting correlated growth between E. coli and Cryptococcus. Urinary susceptible E. coli is an important co-factor of multiple stress factors involved in the generation of melanin which is a necessary pathogenic factor for Cryptococcus, assisting in the removal of oxygen free radicals and averting the onset of oxidative stress response (14). In UTI, the genes involved in the Cu1+ efflox system of E. coli are highly up-regulated, and the copper efflox of E. coli may be the source of the copper intake of Cryptococcus (15), which may facilitate the infection and colonization of Cryptococcus.
Cryptococcus infection within 30 days after transplantation is generally considered donor-derived, and the median time for non-donor-derived Cryptococcus infection is 16–21 months after transplantation (2, 16, 17). Combined with the fact that the patient had no history of pathogen exposure, we suspected that the Cryptococcus spores were accidentally inhaled into the lung rather than donor-derived Cryptococcus infection, traversing pulmonary capillaries into peripheral blood circulation for systemic dissemination. The E. coli infection brought about changes in the transplanted kidney's immune microenvironment that promoted Cryptococcus which is used to manifest transient or latent infection to spread through the blood, to colonize in the transplanted kidney and gradually expand its range. After that, Cryptococcus proceeded down the urinary tract to the bladder or even prostate duct. At the same time, Cryptococcus can breach the blood-brain barrier and enter the CSF. The possible routes of infection were shown in Supplementary Figure 3. However, since pathogenicity was related to Cryptococcus infection foci in vivo, the fungal load in situ and the body's immunity, sequences of C. albidus could be detected in CSF even in an absence of cryptococcal meningoencephalitis symptoms.
The inconsistent outcomes of the lesions in bladder and transplanted kidney may resulted from that the granuloma created barriers around the infection site to prevent drugs entering the renal lesions. In contrast, the bladder lesion was relatively superficial and had long-term exposure to running urine that contained antifungal metabolites. The fluconazole and flucytosine taken by this patient were metabolized by the kidney and excreted from the urine, so the therapeutic effect toward the bladder lesion was significantly superior to that of the transplanted kidney.
Currently, the detection technology of Cryptococcus antigens is based on C. neoformans and C. gattii. The sensitivity of C. albidus detection rate is 75% lower than that of C. neoformans and C. gattii, leading to the false-negative error in preliminary clinical screening (18). This may have contributed to the paradox between pathology-proven cryptococcoma and negative Cryptococcus latex antigen test of CSF and the culture of the blood, urine, CSF, sputum, feces and renal graft tissues in our study. Metagenome sequencing can act as an effective technical complement to pathogen detection in transplant recipients.
Conclusions
In conclusion, this study reported the first sequencing study of cryptococcoma and malacoplakia formed by C. albidus and E. coli in a transplanted kidney. This case suggests a possible synergistic relationship between Cryptococcus colonization and drug-resistant E. coli infection in the transplanted kidney. At the same time, we should be alert to the infection caused by rare Cryptococcus in clinical practice. In addition to traditional diagnostic methods such as culture and immunoassay, metagenome sequencing can be utilized as an auxiliary diagnostic tool.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA719067.
Ethics Statement
This study was approved by Nanfang Hospital Ethical Committee (NFEC-2020-044). Written informed consent was obtained from the patient for the publication of this case report.
Author Contributions
YM, ZY, and WD participated in research design. ZY, YW, and YL participated in the writing of the paper. C-LW, JX, and YM performed critical revision of the manuscript for important intellectual content. ZY, HS, and GC participated in the performance of the research. YM took charge for obtaining funding. XH and JG performed administrative, technical, or material support. RX and WZ performed statistical analysis. YM and C-LW supervised the study. All authors read and approved the final version.
Funding
This study was funded by National Natural Science Foundation of China (Gant No. 82070770), Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010674), the Science and Technology Planning Project of Guangzhou (Grant No. 201803010109), the President Funding of Nanfang Hospital (Grant No. 2018B009, 2018C003), and College Students' Innovative Entrepreneurial Training Plan Program (Grant Nos. X202012121239, 202012121046).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.721145/full#supplementary-material
Supplementary Figure 1. Cystoscopy in Phase I. Pale round-shaped mucosal protrusions on the right side of the bladder, with 3 cm in diameter.
Supplementary Figure 2. Cryptococcus counts after 8 h co-culture of Escherichia coli filtrate in vitro. No E. coli filtrate was added to the control group (group I), and 80 (group II), 160 (group III), 320 (group IV), 640 (group V) and 1,280 (group VI) μl E. coli filtrate was added to the solution of Cryptococcus respectively. *P < 0.05.
Supplementary Figure 3. Possible infection pathway of Cryptococcus ( ) and Escherichia coli (
) and Escherichia coli ( ).
).
Supplementary Figure 4. Dosages and whole blood trough levels of immunosuppressive regimens. MPA, mycophenolic acid.
Supplementary Figure 5. White blood cell counts and lymphocyte counts in peripheral blood of the patient. WBC, white blood cell; LYM, lymphocyte.
References
1. Ponzio V, Chen Y, Rodrigues AM, Tenor JL, Toffaltti DL, Mendina JO, et al. Genotypic diversity and clinical outcome of cryptococcosis in renal transplant recipients in Brazil. Emerg Microbes Infect. (2019) 8:119–129. doi: 10.1080/22221751.2018.1562849
2. Baddley JW, Forrest GN. Cryptococcosis in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. (2019) 33:e13543. doi: 10.1111/ctr.13543
3. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. (2001) 7:375–81. doi: 10.3201/eid0703.010302
4. Fu MS, Coelho C, Leon-Rodriguez CMD, Rossi DCP, Casadevall A. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog. (2018) 14: e1007144. doi: 10.1371/journal.ppat.1007144
5. Ristow LC, Davis JM. The granuloma in cryptococcal disease. PLoS Pathog. (2021) 17:e1009342. doi: 10.1371/journal.ppat.1009342
6. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. (2014) 20:76–98. doi: 10.1111/1469-0691.12360
7. Muranda AZ, Greeff L, Sathekge MM, Meis JF, Cornely OA, Lortholary O. Cryptococcoma of a transplanted kidney in a patient presenting with recurrent urinary tract infection: a case report. BMC Nephrol. (2018) 19:94. doi: 10.1186/s12882-018-0891-8
8. Okurut S, Boulware DR, Olobo J, Meya DB. Landmark clinical observations and immunopathogenesis pathways linked to HIV and Cryptococcus fatal central nervous system co-infection. Mycoses. (2020) 63:840–53. doi: 10.1111/myc.13122
9. Mohsin M, Tanaka K, Kawahara R, Noguchi H, Motooka D, Nakamura S, et al. Whole-genome sequencing and comparative analysis of the genomes of Bacteroides thetaiotaomicron and Escherichia coli isolated from a healthy resident in Vietnam. J Glob Antimicrob Resist. (2020) 21:65–7. doi: 10.1016/j.jgar.2020.02.034
10. Yossi P. Current trends in antimicrobial resistance of escherichia coli. Current topics in microbiology and immunology. (2018) 416:181–211. doi: 10.1007/82_2018_110
11. Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. (2017) 8:1566. doi: 10.3389/fmicb.2017.01566
12. Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The Impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant. (2015) 15:3024–40. doi: 10.1111/ajt.13486
13. Allison DL, Willems H, Jayatilake J, Bruno VM, Peters BM, Shirtliff ME. Candida-Bacteria Interactions: Their Impact on Human Disease. Microbiol Spectr. (2016) 4. doi: 10.1128/microbiolspec.VMBF-0030-2016
14. Esher SK, Zaragoza O, Alspaugh JA. Cryptococcal pathogenic mechanisms: a dangerous trip from the environment to the brain. Mem Inst Oswaldo Cruz. (2018) 113:e180057. doi: 10.1590/0074-02760180057
15. Subashchandrabose S, Mobley HL. Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection. Metallomics. (2015) 7:935–942. doi: 10.1039/c4mt00329b
16. Fishman JA. Infection in organ transplantation. Am J Transplant. (2017) 17:856–79. doi: 10.1111/ajt.14208
17. Pappas PG, Alexander BD, Ands DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. (2010) 50:1101–11. doi: 10.1086/651262
Keywords: cryptococcoma, malacoplakia, Cryptococcus albidus, Escherichia coli 09-02E, transplanted kidney, metagenome sequencing
Citation: Yan Z, Deng W, Wang Y, Liu Y, Sun H, Xia R, Zeng W, Geng J, Chen G, He X, Xu J, Wu C-L and Miao Y (2021) Case Report: Malacoplakia Due to E. coli With Cryptococcus albidus Infection of a Transplanted Kidney in a Patient With Recurrent Urinary Tract Infection. Front. Med. 8:721145. doi: 10.3389/fmed.2021.721145
Received: 06 June 2021; Accepted: 20 August 2021;
Published: 14 September 2021.
Edited by:
Ondrej Viklicky, Institute for Clinical and Experimental Medicine (IKEM), CzechiaReviewed by:
Eva Kieslichová, Institute for Clinical and Experimental Medicine (IKEM), CzechiaSamy Hakroush, University of Göttingen, Germany
Dorota Kamińska, Wroclaw Medical University, Poland
László Wagner, Semmelweis University, Hungary
Copyright © 2021 Yan, Deng, Wang, Liu, Sun, Xia, Zeng, Geng, Chen, He, Xu, Wu and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Lee Wu, Y3d1MiYjeDAwMDQwO21naC5oYXJ2YXJkLmVkdQ==; Yun Miao, bWlhb3l1bmVjaG8mI3gwMDA0MDsxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Ziyan Yan1†
Ziyan Yan1† Xiaolong He
Xiaolong He Yun Miao
Yun Miao