
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 19 November 2021
Sec. Rheumatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.715423
Objectives: To identify ultrasound (US) features associated with the presence of shoulder complaints.
Methods: This observational, case-control study, compared US findings between participants with and without shoulder complaints, matched for age, sex, and dominancy. Data was collected from February 2018 to June 2020. Two-tailed Fisher's and Mann-Whitney U-tests were used, with p-values < 0.05 considered significant.
Results: A total of 202 participants were enrolled (median age 56 years, range 18–70, 155 women), comprising 140 cases and 62 controls. A calcification size ≥6 mm, when age < 56 (p = 0.02), and a distance to tendon insertion ≥6 mm, when age ≥56 (p = 0.009), were only found in symptomatic shoulders. Color Doppler in rotator cuff (RC) tendons predominated in the presence of symptoms (26/140 vs. 2/62, p = 0.003). An algorithm also combining the number of calcifications, tendon echotexture and insertional thickening, osseous irregularity, cuff tears, and subacromial effusion showed a 92% (57/62) specificity for shoulder pain on this study sample.
Conclusion: Calcification diameter of 6 mm or more is associated with shoulder pain in patients younger than 56 years. A distance from calcification to tendon insertion of 6 mm or more is related to pain in older patients. Doppler signal also is associated with shoulder pain. An algorithm based on a set of specific ultrasonographic criteria have a strong association with the presence of symptoms.
Shoulder is one of the most common anatomical sites for musculoskeletal pain, with a higher incidence in women and reaching an annual cumulative incidence of 2.4% among adults aged 45–64 years. Calcific tendinitis and other rotator cuff (RC) disorders are the most frequently diagnosed intrinsic shoulder conditions (1–7). However, shoulder pain may be due to other intrinsic causes or referred pain.
Calcifications have long been associated with pain, but also recognized to be present in asymptomatic shoulders (8). Of interest, the size of calcification is relevant and shoulders with radiological detected calcific deposits of >1.5 cm in length have the highest chance of being symptomatic (9, 10). Calcifications are also more common in women and in subjects aged between 30 and 60 years old (10). Calcifications may be due to a degenerative process, associated with trauma and tissue hypoxia and related to aging, occupation, and the use of the dominant arm, or occur in a viable tissue due to a cell-mediated reactive process, with distinct predictable stages (11). Ultrasound (US) is considered to be reliable in the detection and localization of RC calcifications (12) and US assessment of patients with known calcifications also found larger calcified plaques in symptomatic than in asymptomatic shoulders (13). However, the evaluation of a general population based cohort (women with and without calcified deposits) could not confirm the relationship between pain and calcification size and found calcifications larger than 1 cm to be unusual (14). Thickening, echotexture changes and tears in RC tendons, subacromial bursal widening, and signs of subacromial impingement and acromioclavicular osteoarthritis have been described not only in painful but also in asymptomatic shoulders (15, 16).
Detection of a positive Doppler signal within or close to a calcification was reported to be related with pain (13, 17). The location of a calcification in the medial-lateral direction was suggested to be a prognostic factor (18), with worse outcomes of non-surgical treatment for calcifications more medial and distant from tendon insertion.
The purpose of this study was to identify US features associated with the presence of shoulder complaints.
This was an observational, case-control study, intended to compare US findings between participants with and without shoulder pain. The study was approved by the Ethics Committee of our institution (Approval Letter Ref. n. 413/17). Informed consent was obtained from all individual participants included in the study. Data was collected between February 2018 and June 2020 and anonymously recorded. Some of the participants (127) were included in a previously published study addressing only clinical variables (19).
Patients suffering from shoulder pain, 18–70 years old, referred for unilateral shoulder US in our hospital radiology department were consecutively recruited, until 140 cases were included. The sample size aimed a power of 80% to detect a 35% difference in the proportion of cases between exams with positive and negative findings (20, 21). Controls were randomly selected, and invited to voluntarily participate, between individuals referred to the same radiology department for other US examinations (excluding upper limb); selection aimed to match sociodemographic characteristics, as one control was selected for each two cases already recruited with the same age (decade), sex, and laterality (pain in dominant side or contralateral).
Exclusion criteria (Figure 1; Supplementary Material) were applied to all participants. Patients referred for bilateral shoulder examination were not selected to avoid bias. Controls were not included if they referred shoulder complaints at first interview or scored more than 10 points on a DASH (disabilities of the arm, shoulder, and hand outcome measure) questionnaire (22).
Participants filled a written form, including a DASH questionnaire scored as described in literature (23). Sociodemographic and clinical data were collected by interview; profession was classified according to the International Standard Classification of Occupations (ISCO 08) (24).
A physical exam was performed by one of two rheumatology residents. Active range of motion was registered, and total degrees lost calculated as the sum of the differences to maximum values (180° for abduction and flexion and 90° for internal and external rotation).
The US exam was performed to all participants by a radiologist with 16 years of shoulder US experience, blinded to the results of questionnaires and physical exam. The exam was performed with an Acuson S2000 (Siemens Medical Solutions USA Inc., Issaquah, WA) with a multifrequency linear probe (14L5) operating between 5 and 14 MHz (minimum 11 MHz used). In random participants (n = 15) a second exam was performed by a radiology resident, for an inter-observer reliability assessment. The exam took place with the patient sitting in front of the examiner and followed described procedures (25–27). The long head of the biceps tendon was evaluated with the arm in a neutral position and the forearm on patient's lap. The subscapularis tendon was examined with the arm in external rotation. The supraspinatus tendon was examined with the patient's shoulder in internal rotation, with the hand placed on the back. The infraspinatus and teres minor tendons were evaluated with the patient's hand again on her/his lap, in slight internal rotation. All tendons were scanned on their long and short axis.
Rotator cuff calcifications were defined, as in previous studies (13, 14), as echogenic focus with or without posterior acoustic shadow, identified and measured in two orthogonal planes. The total number was registered and the six largest were described by its largest diameter, location in lateral-medial direction disclosed by the distance to osseous insertion in a direction parallel to tendon fibers, location in a particular tendon or structure, shape geometry, regularity and definition, intensity of acoustic shadow, and grouping.
Changes in tendon echotexture were reported as heterogeneity (variable echogenicity along the tendon) or hypoechogenicity (compared to muscle) and graded (mild—possible change; moderate—definite change). Tendon rupture (17, 25, 26) was classified as full or partial thickness. Subacromial conflict was assessed (28–31).
Color and Power Doppler used a pulse repetition frequency (PRF) of 488 Hz, as previously described (13, 17), with gain set at the highest gain which provided no or only minimal noise, and were graded from 0 to 3. A twinkling artifact was considered when a fast alternance between colors near the limit of spectrum was found.
A Fisher's exact test was used to compare frequencies of categorical variables between groups and a Mann-Whitney U-test was employed to compare distribution of continuous or ordinal data, except when a normal distribution was found (using Shapiro-Wilk's statistics) and an independent-samples t-test could be applied. Spearman's coefficient was used for bivariate correlations.
Findings with significant or near significant (p < 0.10) differences were further investigated in two similar size age subgroups (predefined to be below or above median age) and cutoffs derived from analysis of receiver operating characteristic (ROC) curves were applied.
Regarding reliability, Cohen's Kappa coefficient, and intraclass correlation coefficient (ICC), based on a single measurement, absolute-agreement, two-way mixed-effects model, were used. The influence of possible confounding variables differing between cases and controls was assessed by sensitivity analysis.
An algorithm was constructed which incorporated US significant variables, as major criteria if specificity in the study sample was at least 97%, and as minor criteria otherwise or if criteria was subjective (tendon echotexture changes and thickening); age subgroup or specific location criteria were applied if they provided better significance or specificity. The number of minor criteria required was defined using ROC curves analysis. Confidence intervals (CI) for sensitivity and specificity were calculated by Wald method (32).
All tests used were two-tailed and p-values <0.05 were considered significant.
Data was analyzed using SPSS statistical software, version 24 (IBM corp., Armonk, NY, USA).
From the 243 cases selected, 103 were excluded by predefined criteria (Figure 1). Of particular relevance, chronic inflammatory disease patients were excluded, including systemic lupus erythematosus (n = 10), rheumatoid arthritis (n = 5), ankylosing spondylitis (n = 2), and idiopathic inflammatory bowel disease (n = 2). The sociodemographic and clinical characteristics of the 202 participants (140 cases and 62 controls, median age 56 years) are displayed in Table 1. Cases and controls showed no difference in sex, age, dominancy of examined shoulder, or occupation. In the cases' group, 139 participants had shoulder pain in the previous week (one participant had only complaints of numbness), 123 reported loss of strength, 113 numbness, and 100 loss of mobility. A positive physical exam was more frequent in cases than in controls (127/140 vs. 2/62, p < 0.001). In the cases' group, the median number of days with symptoms was 365 (interquartile range = 745). Only DASH score in the cases' group showed a normal distribution, thus non-parametric statistics were applied in all other situations.
A comparison of condensed US findings is shown in Table 2. Doppler grades (illustrated in Figure 2), subacromial effusion, number of calcifications, tendon tear, echotexture change and insertional thickening, humeral tuberosity cortical irregularity, and interruption of abduction revealed differences between cases and controls. The dimension of calcifications and their distance to tendon insertion deserved detailed assessment. The number of tendons with moderate echotexture changes, effusion thickness, and Doppler grades were low, even in the cases' group.
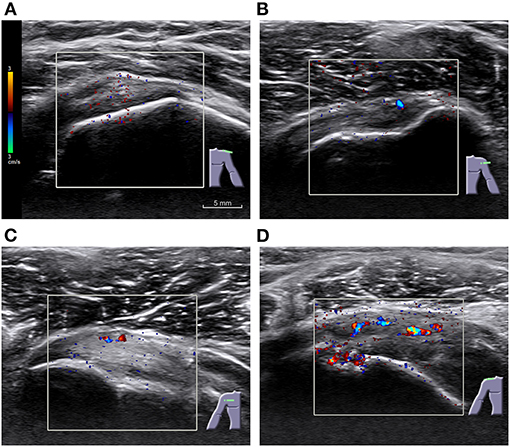
Figure 2. Examples of different grades of Color Doppler in rotator cuff tendons of symptomatic participants. (A) Grade 0, with no significative signal and only minimal scattered noise; (B) grade 1, with one or two points or short lines; (C) grade 2, showing three to six points or short lines; and (D) grade 3, with more than six points, a continuous line, or a bar. A pulse repetition frequency (PRF) of 488 Hz was used. Scale and color coding are identical across images.
A total of 405 calcifications were found, from which 377 were described (Table 3). Calcification diameter ranged from 0.5 to 16.7 mm.
The frequencies of positive findings by age subgroup, with relevant cutoffs applied, are outlined in Table 4.
The presence of calcifications (in any number) did not differ between groups, but three or more calcifications were found more frequently in participants with symptoms. In the younger subgroup of participants (<56 years old), a calcification diameter ≥6 mm was only found in the presence of symptoms. Distance to insertion also showed a higher difference between groups when a 6 mm cutoff was applied, only in older participants, with no difference in the younger subgroup.
Echotexture moderate changes, of at least one RC tendon, were more frequent in cases than in controls, for all ages (with p < 0.001, both for heterogeneity and hypoechogenicity). Some specific tendon echotexture changes also appeared more frequently in the cases' group, but with a lower significance. Insertional thickening of at least one tendon was more common in cases than in controls, but a greater difference was found when isolated supraspinatus and subscapularis were assessed. Infraspinatus thickening was not proved to be different between cases and controls. Also of interest, humeral tuberosity cortical irregularity at the supraspinatus and subscapularis insertion sites was more associated with cases than controls, which was not the case for the infraspinatus insertion.
Rotator cuff tears were more frequent in the cases' group, for all ages. Supraspinatus tendon was the site for 7 of 11 full thickness tears and 22 of 27 partial thickness tears. Any fluid on subacromial bursa (minimum registered positive value was 0.7 mm) was only found in symptomatic participants.
The presence of any sign in both Color and Power Doppler was more frequent in participants with symptoms, with few controls displaying any signal. Doppler predominant locations were supraspinatus tendon (found in 10% of the symptomatic participants) and subscapularis tendon (8.6%). Twinkling artifact was found in 5 of 26 cases and in 1 of 2 controls with color Doppler signal.
The older subgroup (≥56 years old) had a higher calcification number, diameter, and distance to insertion (p < 0.001, Mann–Whitney U-test) and more frequent partial thickness tears and humeral tuberosity cortical irregularities (respectively, p = 0.002 and p = 0.005, Fisher's exact test). No differences between sexes were found in US relevant findings. A positive color Doppler grade was more frequent on the dominant side (26/127, 20.5%) than on the non-dominant side (4/75, 5.3%), p = 0.004.
Color and Power Doppler grades were found to be strongly associated, with a Spearman correlation coefficient of 0.917.
The analysis of DASH scores in the cases' group depicted higher values (independent t-test) in participants with moderate heterogeneity in at least a tendon (M = 57 vs. M = 49, p = 0.01) and with moderate hypoechogenicity in at least a tendon (M = 57 vs. M = 49, p = 0.02). The presence of other US findings did not result in significantly different DASH scores, in the cases' group.
For the presence of humeral tuberosity cortical irregularity, inter-observer agreement was substantial (33), k = 0.72 (0.36–1.0). For the diameter of largest calcification, ICC = 0.98 (0.93–0.99) was obtained, and an ICC = 0.90 (0.72–0.96) was found for the largest distance from calcification to insertion. Reliability analysis for other less frequent findings, including Doppler, resulted in wider, not trustable, CIs.
An algorithm proposal aiming for high specificity, constructed with US findings as major or minor criteria, is showed (Figure 3). Major criteria were meant to provide a positive result alone. The best threshold for the number of minor criteria required, estimated by analysis of ROC curves, was two minor criteria for participants with <56 years and three minor criteria for participants with 56 years or older (Figure 4).
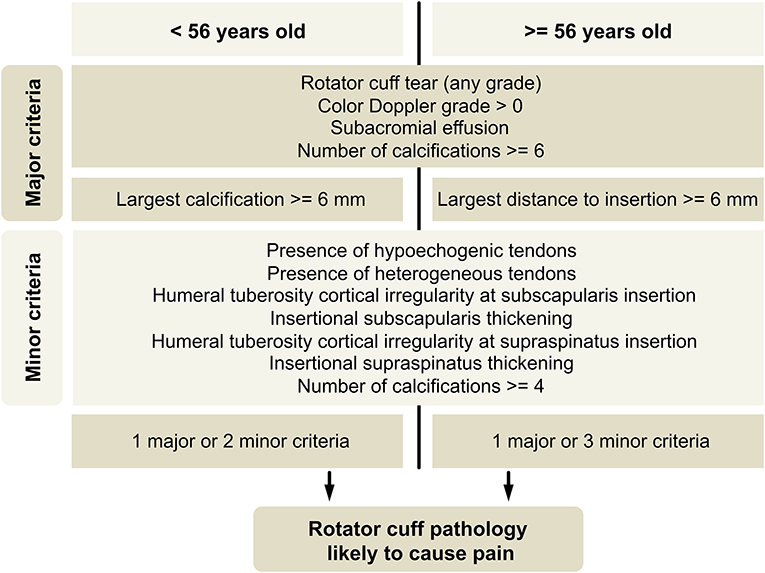
Figure 3. Classification algorithm proposal. Cortical irregularity and insertional thickening related to subscapularis and supraspinatus tendons should be taken as separate criteria. Mild, questionable, heterogeneity, or hypoechogenicity of tendons should not be considered.
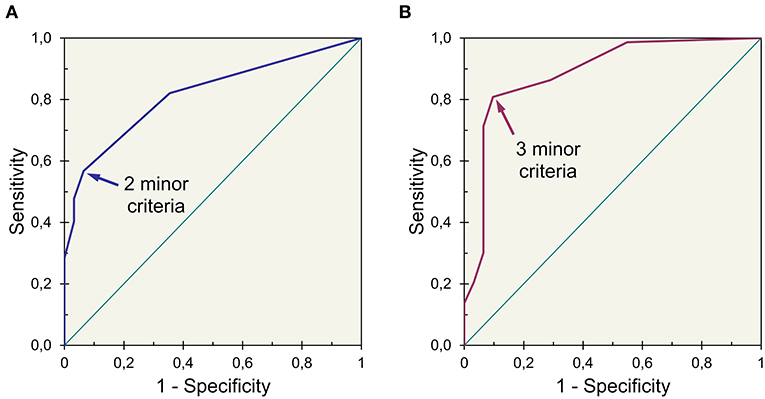
Figure 4. Receiver operating characteristic curves of an index obtained for (A) younger (<56 years) and (B) older (≥56 years) participants showing different thresholds for best accuracy (index established by adding three points for major criteria and one point for minor criteria).
This algorithm provides a classification, in this study sample, with a sensitivity of 69%, 97/140 (95% CI: 61–77%) and a specificity of 92%, 57/62 (95% CI: 85– 99%). If minor criteria based on tendon echotexture changes and insertional thickening are withdrawn, an algorithm with a sensitivity of 59%, 83/140 (95% CI: 51–67%) and a specificity of 94%, 58/62 (95% CI: 88–99%), will result.
Although US findings associated with shoulder pain have been described, substantial overlap exists between painful and asymptomatic shoulders. There are also some discrepancies between studies with different designs. In this study, using calculated thresholds and taking age into account by analysis of two similar size subgroups, it was possible to find differences in painful shoulders concerning calcification size (p = 0.02, age < 56 years) and the presence of color Doppler (p = 0.003), and also regarding calcification distance to insertion, number of calcifications, tendon echotexture and insertional thickening, osseous irregularity, and the presence of RC tear or subacromial effusion. An algorithm based on US findings providing a strong association with the presence of symptoms, with 57/62 (92%) specificity (on the study sample), was constructed.
Our results confirmed and extended the findings of previous reports. On their study, including only participants with proved calcified deposits (13), Le Goff et al. reported a relationship between the presence of symptoms and the size of calcifications, although they did not look for a cutoff. They found thickening of the subacromial bursa to be frequent in symptomatic shoulders, as reported by Aina et al. (34) and in accordance with our results. They also, as described by Chiou et al. (17), found Doppler within or near calcifications to be strongly associated with symptoms. We also found the presence of any grade of color Doppler, related or not to calcifications, to be predictive of symptoms. The PRF that we have used was low, raising concern about a possible overvaluation of the Doppler signal. However, the number of controls with Doppler signal (false positives) was only 3%. We found evidence of twinkling artifact, which could be misleading, in only 5 of the 26 cases with color Doppler signal.
In their US study on a cohort of women (14), Sansone et al. found calcium deposits to be more frequent than previously reported in radiographic observations, and that calcifications, besides supraspinatus, were also frequent in infraspinatus and subscapularis. No relationship between calcifications' size and pain was confirmed by that study, which classified dimensions in only three classes and did not consider the influence of age. We found even more frequent tendon calcifications, present in more than half of the participants, both with and without symptoms, perhaps due to the older age of our sample. The distribution of calcific deposits was comparable among those three tendons and teres minor also showed a non-negligible number of calcifications. In our sample, a higher number of calcifications was related to pain, but not the sole presence of one calcification. We found no association between the presence of pain and calcifications shape or acoustic shadow.
The relevance of the distance from calcification to tendon insertion was not, to our knowledge, previously reported on US. It suggests that, in older people, calcifications located closer to insertion are not necessarily pathological. The greater relevance of calcification size in a younger age subgroup indicates that degenerative changes are less important at this stage, and a different process, such as calcifying tendinitis, can prevail earlier, as hypothesized by Uhtoff et al. (11).
As expected, RC tears were associated with pain, as well as humeral tuberosities irregularities (other than the more common irregularity at insertion of infraspinatus), previously described to be associated with tears (35). Glenohumeral effusion and signs of acromioclavicular osteoarthritis or subacromial conflict were not associated with pain.
The predominance of Doppler in the dominant side suggests that movement or load have a role in the development of vascularization in painful shoulders.
Age, considered an important factor in the development of calcifications, was similar between groups (cases and controls), as a result of the matching process on control selection. This allowed to exclude an effect of age on the case/control comparisons. Likewise, gender and side dominancy distribution were similar between groups.
Our study had several limitations. Shoulder tendinopathy is a relapsing condition; therefore, a history of previous shoulder tendinopathy was more frequent in the cases' group. Thus, the US changes were also due, at least partly, to prior episodes. Professions with potentially less shoulder effort were slightly more frequent in controls, but this difference was not statistically significant. The radiologist who performed the US exams was not fully blinded to the condition of each participant being a case or a control; this limitation is hard to be overcome, as US is a dynamic exam and patients will spontaneously reveal pain on movement. Algorithm minor criteria relying on tendon echotexture changes and thickening are subjective and should be used with caution, as rating could be unreliable; if those criteria are not used, a slightly better specificity and a reduced sensitivity will result. Future work could establish the reliability of Color Doppler grading and tendon changes rating. This classification algorithm will need to be validated in an independent population.
The results here reported cannot be applied to patients with chronic inflammatory diseases, including rheumatic diseases, as they were not included in this study as they tend to show different US characteristics. Indeed, US and Power Doppler in rheumatoid arthritis with symptomatic shoulder frequently shows glenohumeral, subdeltoid bursa, and biceps tendon sheath effusion and/or synovitis and can also display erosions and degenerative changes like osteophytes and RC tear and calcification (36). Regarding polymyalgia rheumatica, subdeltoid bursitis, biceps tenosynovitis, or glenohumeral synovitis are prominent features, and according to the 2012 EULAR/ACR collaborative initiative (37) these findings are useful in discriminating polymyalgia rheumatica from other shoulder conditions (although less useful in discriminating from rheumatoid arthritis).
We hope this work and the US criteria here described will help, when symptoms and physical examination are ambiguous, the diagnostic differentiation between RC/subacromial pathology and other local conditions or referred pain.
In conclusion, calcifications with a diameter of 6 mm or more, in patients younger than 56 years old, or with a distance from tendon insertion of 6 mm or more, in older patients, are associated with pain. The presence of any Doppler signal also is associated with shoulder complaints. An algorithm based on a set of specific, yet less frequent, US criteria, provides a strong association with the presence of symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comissão de Ética do Centro Hospitalar Universitário Lisboa Norte (CHULN) e do Centro Académico de Medicina de Lisboa (CAML)—Approval Letter Ref. n. 413/17. The patients/participants provided their written informed consent to participate in this study.
JJ, SB, and PM: literature research. SB and PM: clinical studies. JJ: statistical analysis. JJ and SB: manuscript editing. All authors: study concepts and design, data acquisition, analysis and interpretation, manuscript drafting and revision for important intellectual content, approval of final version of submitted manuscript. All authors agree to be accountable for the content of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.715423/full#supplementary-material
Supplementary Figure 1. Measurement of the distance from calcification to osseous insertion. The distance (arrow) was measured from the lateral limit of the calcification to the bone contour, in a direction parallel to the tendon fibers.
Supplementary Figure 2. Convexity of coracoacromial ligament (CAL). The displacement (arrow) was measured as the greatest distance from a line joining acromion and coracoid process to the ligament.
Supplementary Table 1. Comprehensive list of exclusion criteria.
Supplementary Table 2. US findings definitions.
Supplementary Table 3. Sensitivity and specificity of individual algorithm criteria.
US, ultrasound; RC, rotator cuff.
1. Djade CD, Porgo TV, Zomahoun HTV, Perrault-Sullivan G, Dionne CE. Incidence of shoulder pain in 40 years old and over and associated factors: a systematic review. Eur J Pain. (2020) 24:39–50. doi: 10.1002/ejp.1482
2. Luime JJ, Koes BW, Hendriksen IJ, Burdorf A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. (2004) 33:73–81. doi: 10.1080/03009740310004667
3. Urwin M, Symmons D, Allison T, Brammah T, Busby H, Roxby M, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. (1998) 57:649–55. doi: 10.1136/ard.57.11.649
4. Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. (2004) 51:642–51. doi: 10.1002/art.20535
5. Hill CL, Gill TK, Shanahan EM, Taylor AW. Prevalence and correlates of shoulder pain and stiffness in a population-based study: the North West Adelaide Health Study. Int J Rheum Dis. (2010) 13:215–22. doi: 10.1111/j.1756-185X.2010.01475.x
6. Greving K, Dorrestijn O, Winters JC, Groenhof F, van der Meer K, Stevens M, et al. Incidence, prevalence, and consultation rates of shoulder complaints in general practice. Scand J Rheumatol. (2012) 41:150–5. doi: 10.3109/03009742.2011.605390
7. van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. (1995) 54:959–64. doi: 10.1136/ard.54.12.959
8. Carnett JB. So-called calcifying subacromial bursitis. Radiology. (1931) 17:505–13. doi: 10.1148/17.3.505
9. Bosworth BM. Calcium deposits in shoulder and subacromial bursitis: survey of 12,122 shoulders. JAMA. (1941) 116:2477–82. doi: 10.1001/jama.1941.02820220019004
10. Louwerens JK, Sierevelt IN, van Hove RP, van den Bekerom MP, van Noort A. Prevalence of calcific deposits within the rotator cuff tendons in adults with and without subacromial pain syndrome: clinical and radiologic analysis of 1219 patients. J Shoulder Elbow Surg. (2015) 24:1588–93. doi: 10.1016/j.jse.2015.02.024
11. Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. (1997) 5:183–91. doi: 10.5435/00124635-199707000-00001
12. Farin PU, Jaroma H. Sonographic findings of rotator cuff calcifications. J Ultrasound Med. (1995) 14:7–14. doi: 10.7863/jum.1995.14.1.7
13. Le Goff B, Berthelot JM, Guillot P, Glémarec J, Maugars Y. Assessment of calcific tendonitis of rotator cuff by ultrasonography: comparison between symptomatic and asymptomatic shoulders. Joint Bone Spine. (2010) 77:258–63. doi: 10.1016/j.jbspin.2010.01.012
14. Sansone V, Consonni O, Maiorano E, Meroni R, Goddi A. Calcific tendinopathy of the rotator cuff: the correlation between pain and imaging features in symptomatic and asymptomatic female shoulders. Skeletal Radiol. (2016) 45:49–55. doi: 10.1007/s00256-015-2240-3
15. Meroni R, Piscitelli D, Valerio S, Boria P, Perin C, De Vito G, et al. Ultrasonography of the shoulder: asymptomatic findings from working-age women in the general population. J Phys Ther Sci. (2017) 29:1219–23. doi: 10.1589/jpts.29.1219
16. Girish G, Lobo LG, Jacobson JA, Morag Y, Miller B, Jamadar DA. Ultrasound of the shoulder: asymptomatic findings in men. AJR Am J Roentgenol. (2011) 197:W713–9. doi: 10.2214/AJR.11.6971
17. Chiou HJ, Chou YH, Wu JJ, Hsu CC, Huang DY, Chang CY. Evaluation of calcific tendonitis of the rotator cuff: role of color Doppler ultrasonography. J Ultrasound Med. (2002) 21:289–95. doi: 10.7863/jum.2002.21.3.289
18. Ogon P, Suedkamp NP, Jaeger M, Izadpanah K, Koestler W, Maier D. Prognostic factors in nonoperative therapy for chronic symptomatic calcific tendinitis of the shoulder. Arthritis Rheum. (2009) 60:2978–84. doi: 10.1002/art.24845
19. Janeiro J, Barreira S, Martins P, Ninitas P, Fonseca JE, Campos J, et al. Palm-up test and range of motion in flexion and external rotation provide best correlation with disability and perceived pain in patients with shoulder complaints. Acta Reumatol Port. (2020) 45:95–103.
20. Miot H. Sample size in clinical and experimental trials. J Vasc Bras. (2011) 10:275–8. doi: 10.1590/S1677-54492011000400001
21. Lwanga SK, Lemenshow S. Sample Size Determination in Health Studies: A Practical Manual. Geneva: World Health Organization (1991).
22. Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum. (2009) 61:623–32. doi: 10.1002/art.24396
23. Santos J, Gonçalves RS. Adaptação e Validação Cultural da Versão Portuguesa do disabilities of the arm shoulder and hand - DASH. Rev Port Ortop Traum. (2006) 14:29–44.
24. International Standard Classification of Occupations (ISCO 08). Vol. I. Geneva: International Labour Office (2012).
25. Papatheodorou A, Ellinas P, Takis F, Tsanis A, Maris I, Batakis N, et al. of the shoulder: rotator cuff and non-rotator cuff disorders. Radiographics. (2006) 26:e23. doi: 10.1148/rg.e23
26. Rutten MJ, Jager GJ, Blickman JG. From the RSNA refresher courses: US of the rotator cuff: pitfalls, limitations, and artifacts. Radiographics. (2006) 26:589–604. doi: 10.1148/rg.262045719
27. Jacobson JA. Shoulder US: anatomy, technique, and scanning pitfalls. Radiology. (2011) 260:6–16. doi: 10.1148/radiol.11101082
28. Farin PU, Jaroma H, Harju A, Soimakallio S. Shoulder impingement syndrome: sonographic evaluation. Radiology. (1990) 176:845–9. doi: 10.1148/radiology.176.3.2202014
29. Daghir AA, Sookur PA, Shah S, Watson M. Dynamic ultrasound of the subacromial-subdeltoid bursa in patients with shoulder impingement: a comparison with normal volunteers. Skeletal Radiol. (2012) 41:1047–53. doi: 10.1007/s00256-011-1295-z
30. Wu CH, Chang KV, Su PH, Kuo WH, Chen WS, Wang TG. Dynamic ultrasonography to evaluate coracoacromial ligament displacement during motion in shoulders with supraspinatus tendon tears. J Orthop Res. (2012) 30:1430–4. doi: 10.1002/jor.22084
31. Dietrich TJ, Jonczy M, Buck FM, Sutter R, Puskas GJ, Pfirrmann CW. Ultrasound of the coracoacromial ligament in asymptomatic volunteers and patients with shoulder impingement. Acta Radiol. (2016) 57:971–7. doi: 10.1177/0284185115610930
32. Sauro J, Lewis JR. Estimating completion rates from small samples using binomial confidence intervals: comparisons and recommendations. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting. Vol 49. Orlando, FL (2005) 2100–3. doi: 10.1177/154193120504902407
33. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. (1977) 33:159–74. doi: 10.2307/2529310
34. Aina R, Cardinal E, Bureau NJ, Aubin B, Brassard P. Calcific shoulder tendinitis: treatment with modified US-guided fine-needle technique. Radiology. (2001) 221:455–61. doi: 10.1148/radiol.2212000830
35. Wohlwend JR, van Holsbeeck M, Craig J, Shirazi K, Habra G, Jacobsen G, et al. The association between irregular greater tuberosities and rotator cuff tears: a sonographic study. AJR Am J Roentgenol. (1998) 171:229–33. doi: 10.2214/ajr.171.1.9648794
36. Stegbauer J, Rump LC, Weiner SM. Sites of inflammation in painful rheumatoid shoulder assessed by musculoskeletal ultrasound and power Doppler sonography. Rheumatol Int. (2008) 28:459–65. doi: 10.1007/s00296-007-0465-8
37. Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. (2012) 71:484–92. doi: 10.1136/annrheumdis-2011-200329
Keywords: shoulder pain, ultrasonography, Doppler, rotator cuff, tendinopathy, calcifications
Citation: Janeiro J, Barreira SC, Martins P, Ninitas P, Campos J and Fonseca JE (2021) Ultrasound Features Associated With Shoulder Complaints: Calcifications Larger Than 6 mm in Young Patients and Positive Doppler Are Associated With Pain. Front. Med. 8:715423. doi: 10.3389/fmed.2021.715423
Received: 26 May 2021; Accepted: 28 October 2021;
Published: 19 November 2021.
Edited by:
Peter Korsten, University Medical Center Göttingen, GermanyReviewed by:
Deng-Ho Yang, Taichung Armed Forces General Hospital, TaiwanCopyright © 2021 Janeiro, Barreira, Martins, Ninitas, Campos and Fonseca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: João Janeiro, anBhamFuZWlyb0BzYXBvLnB0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.