- 1The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education, Chinese National Health Commission and Chinese Academy of Medical Sciences, The State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine, Department of Cardiology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Critical Care Medicine, Yanzhou Branch of Affiliated Hospital of Jining Medical University, Jining, China
- 3Shandong Provincial Clinical Research Center for Emergency and Critical Care Medicine, Institute of Emergency and Critical Care Medicine of Shandong University, Chest Pain Center, Qilu Hospital of Shandong University, Jinan, China
- 4Department of Emergency Medicine, Qilu Hospital of Shandong University, Jinan, China
- 5Key Laboratory of Emergency and Critical Care Medicine of Shandong Province, Key Laboratory of Cardiopulmonary-Cerebral Resuscitation Research of Shandong Province, Shandong Provincial Engineering Laboratory for Emergency and Critical Care Medicine, Qilu Hospital of Shandong University, Jinan, China
- 6Department of Critical Care Medicine, Qilu Hospital of Shandong University, Jinan, China
- 7Department of Critical Care Medicine, Shandong Province Hospital Affiliated to Shandong First Medical University, Shandong First Medical University, Jinan, China
- 8Department of Critical Care Medicine, Liaocheng People's Hospital Affiliated to Shandong First Medical University, Liaocheng, China
- 9Department of Critical Care Medicine, Qilu Hospital of Shandong University, Qingdao, China
- 10Department of Critical Care Medicine, The Second Hospital of Shandong University, Jinan, China
- 11Department of Critical Care Medicine, Jiaxiang People's Hosptial, Jining, China
- 12Department of Clinical Laboratory, Qilu Hospital of Shandong University, Jinan, China
- 13Department of Pharmacology, School of Basic Medical Sciences, Shandong University, Jinan, China
Background: There is little evidence on the changing prevalence, microbiological profile, and outcome of nosocomial Acinetobacter baumannii complex (ABC)-caused bloodstream infection (ABCBSI) specified in intensive care units (ICUs) in long-term studies, especially in China.
Objective: We aimed to investigate changes in incidence, antibiotic resistance, therapy, and prognosis of ABCBSI in ICUs in eastern China during 2009–2018.
Methods: A multicenter retrospective cohort study was conducted, and microbiological and clinical data for patients with ABCBSI acquired in nine adult ICUs in eastern China from 2009 to 2018.
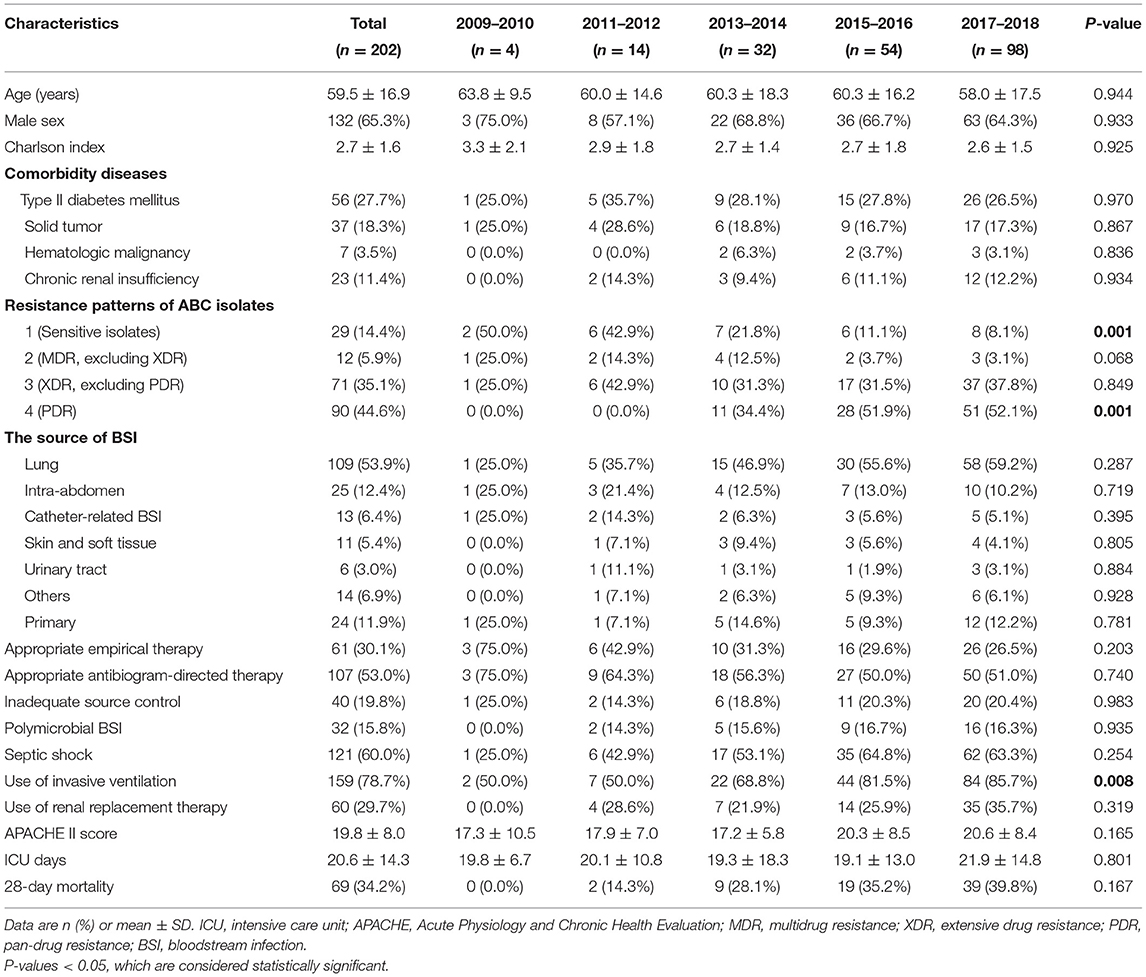
Results: A total of 202 cases were enrolled. For the years 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018, the incidence of ABCBSI increased significantly, as did the percentage of pan-drug-resistant isolates and resistant rates to most of antimicrobial agents; the percentage of drug-sensitive isolates decreased (all P < 0.05). The frequency of treatment with carbapenems and tigecycline increased, and that of cephalosporins decreased. Compared with those in the first years (2009–2012), ABCBSI patients in the lattermost years (2017–2018) were less often treated with appropriate empirical therapy, more often underwent pneumonia-related ABCBSI and mechanical ventilation support, and had higher 28-day mortality rates. Multivariate Cox regression indicated that increase in the degree of ABC antibiotics resistance, pneumonia-related ABCBSI, and septic shock were risk factors of 28-day mortality and associated with significant lower survival days.
Conclusions: The past decade has witnessed a marked increase in the incidence of ABCBSI and in antibiotic resistance, with increasing pneumonia-related infections and worrisome mortality in ICUs in China. Controlling increasing resistance and preventing nosocomial pneumonia may play important roles in combatting these infections.
Introduction
Bloodstream infections (BSIs) are frequent and life-threatening in hospitals (1). Patients in intensive care units (ICUs) are particularly pre-disposed to BSIs (2), with a prevalence of ~15% (3). The Acinetobacter baumannii complex (ABC) has great potential for nosocomial spread (4). It has become an important cause of BSI among patients in ICUs (1, 5), and ABC-caused BSI (ABCBSI) is associated with high mortality (5–7).
Until recently, we had little evidence on the long-term changes in prevalence, microbiological profile, therapy, and outcome of ABCBSI in ICUs over the years. Investigating these dynamic changes has become extremely urgent, especially in China. Although the epidemiology of ABC infection and the antimicrobial susceptibility profiles of ABC isolates vary by regions, years, and even wards all over the world (8–12), the prevalence of ABC nosocomial infection seems extremely challenging in ICUs in China (13–15). Recent reports from Chinese ICUs found that ABC accounted for nearly one-quarter of the ICU-acquired infections (13, 14). The China Antimicrobial Surveillance Network (CHINET) surveillance system also found a large fluctuation in resistance of ABC strains over time in Chinese hospitals (16), which indicates that antibiotic implementation may also have significantly changed over time.
In this study, our aim was to investigate the changes in incidence, antibiotic resistance, antimicrobial treatment, and prognosis of ABCBSI in nine eastern Chinese ICUs over the past decade.
Methods
Study Design and Patient Selection
This was a multicenter retrospective cohort study of ABCBSI in nine mixed adult ICUs in eastern China: Qilu Hospital of Shandong University (two ICUs), Second Hospital of Shandong University, Qingdao Branch of Qilu Hospital, Liaocheng People's Hospital affiliated with Taishan Medical College, Zaozhuang Hospital, Jiaxiang People's Hospital, Shenxian People's Hospital, and Chengwu People's Hospital. We included adult patients (age ≥ 18 years) diagnosed with ICU-acquired ABCBSI from January 2009 to December 2018. ABCBSI was determined according to the surveillance definition of the US Centers for Disease Control and Prevention (17). Patients were included only if blood cultures were obtained 2 days or more after ICU admission (denoting ICU nosocomial infection). The sources of ABCBSI were assessed by study investigators according to clinical symptoms, signs, imaging data, surgical findings, and microbiological evidence. Microbiological evidence refers to the isolation from the source of the same organism that was isolated in blood culture (i.e., A. baumannii) (18). For patients with multiple ABCBSI episodes, only the first episode was included. Patients were excluded if they (1) had a length of ICU stay <72 h; (2) had an incomplete medical history or were lost to follow-up; (3) had their strain isolated within 48 h after admission; or (4) had previously been infected with ABC in another ward or hospital before ICU admission. The patients with coinfection by other pathogens at other sites were not excluded. The study was approved by the ethics committees of each hospital.
Microbiological Studies
Blood cultures were processed at the participating hospitals by the BACTEC system (Becton Dickinson, Franklin Lakes, NJ, USA) or BacT/ALERT 3D system (bioMérieux, Marcy-l'Étoile, France). The blood culture bottles were incubated in the above two blood culture systems until a positive alert was gotten or for a maximum of 5 days. Two or three drops of positive blood culture broth were streaked onto the 5% sheep blood agar plate or MacConkey agar plate, respectively, and all the plates were incubated at 5% CO2 and 35°C. ABC isolates were Gram-negative, non-fermentative, and oxidase-negative coccobacillus using the Gram stain and manual biochemical tests, and they were identified using the VITEK-2 compact system with the GN identification card (bioMérieux, Marcy-l'Étoile, France) or MicroScan WalkAway plus system with the NC50 card (Siemens Healthcare Diagnostics, West Sacramento, CA, USA) according to the manufacturers' manual. The antibiotic susceptibility testing (AST) of ABC isolates was performed on the VITEK-2 compact system with the AST-GN16 card or MicroScan WalkAway plus System with the NC50 card. The strains of Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality controls to ensure the credibility of identification and AST results of ABC isolates.
Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) isolates were defined according to international expert proposals (19). For A. baumannii, antimicrobial categories and agents used to define drug resistance include aminoglycosides (gentamicin, tobramycin, amikacin, and netilmicin), antipseudomonal carbapenems (imipenem, meropenem, and doripenem), antipseudomonal fluoroquinolones (ciprofloxacin and levofloxacin), antipseudomonal penicillins + β-lactamase inhibitors (piperacillin–tazobactam and ticarcillin–clavulanic acid), extended-spectrum cephalosporins (cefotaxime, ceftriaxone, ceftazidime, and cefepime), folate pathway inhibitors (trimethoprim–sulfamethoxazole), penicillins + β-lactamase inhibitors (ampicillin–sulbactam), and tetracyclines (tetracycline, doxycycline, and minocycline). MDR was defined as non-susceptible to at least one agent in three or more antimicrobial categories. XDR was defined as non-susceptible to at least one agent in all but two or fewer antimicrobial categories, and PDR was non-susceptible to all agents listed. The remaining isolates were defined as sensitive isolates. Because polymyxin has not been available in mainland China since 2018, this agent was not tested in the laboratory and was not included in the antimicrobial agents list (19). To better define the resistance patterns and resistance degree of each ABC isolate, we counted the number of sensitive, MDR (excluding XDR), XDR (excluding PDR), and PDR isolates.
Data Collection
Trained study team members collected data by chart review. Demographic and microbiological data, comorbidities, precipitating factors, laboratory data, concurrent infections with other pathogens in the bloodstream, antibacterial agent treatment, and outcome were recorded on standardized case report forms. At the onset of ABCBSI (within 24 h after collection of the first ABC-positive blood sample), laboratory data were collected, and the severity of the initial presentation of ABCBSI was assessed by APACHE II scores. The definition of concurrent infection with (an)other pathogen(s) in the bloodstream was that one or more other pathogens were isolated from cultures of blood samples obtained within 48 h of collection of the first ABC-positive blood sample, irrespective of whether the isolate came from the same or a different blood culture bottle (20). APACHE II scores were calculated according to a previous report. They use 12 acute physiological variables, age, and chronic health status (21). The primary outcome was survival at 28 days after ABCBSI onset. Patients discharged from the hospital were followed up by the medical electronic system or by telephone to determine their survival status. We defined empirical therapy as being an antimicrobial regimen administered within 24 h of extraction of a blood sample and before susceptibility was known; a therapy that was continued or commenced on the day that antibiogram results were reported was considered definitive (antibiogram-directed therapy) (22). Appropriate empirical treatment was defined as prescription of one or more antimicrobial active against the isolate of ABC, in accordance with the standard antimicrobial susceptibility study, in the first 24 h from the onset of the bacteremia and before its diagnosis, with an approved route and dosage appropriate for end organ function. Appropriate definitive treatment was antibiotic active against the isolate of ABC within 24 h after obtaining the antibiogram, with an approved route and dosage appropriate for end organ function (2). Adequate source control was defined as removal of any pre-existing devices thought to be the source of BSI or documented interventions using appropriate decompression, debridement, drainage, and other surgical procedures to control the infection source within 48 h of the onset of BSI (23). Adequacy of source control was assessed independently by a multidisciplinary panel of experts composed of one infectious disease specialists, one intensivist, and a surgeon (all with more than 10 years of experience). Septic shock was diagnosed according to the sepsis-3 definition (24).
The variables included in the 28-day mortality analysis were age, sex, resistance patterns of AB isolates (sensitive, MDR, XDR, and PDR), Charlson Comorbidity Index (25), source of ABCBSI, days from ICU admission to ABCBSI, polymicrobial BSI (26), the severity of infection according to APACHE II scores (27), the presence of septic shock (at ABCBSI diagnosis) (28), appropriate antimicrobial therapy (29), combined antimicrobial treatment (28), invasive procedures (including invasive ventilation and renal replacement therapy) (27, 29), and inadequate source control (30).
Statistical Analyses
SPSS 16.0 (SPSS Inc., IL, USA) was used for data analysis. Categorical data are shown as number (%) and continuous variables as mean ± SD. Chi-square or Fisher's exact-test (two-tailed) was used to compare categorical variables. Unpaired Student's t-test or one-way ANOVA was used to compare continuous variables. Cox proportional hazards regression estimated hazard ratios (HRs) and 95% confidence intervals (CIs) of risk factors for mortality and included all the above potential confounder variables. We set the ordered resistance patterns as a one-degree-of-freedom linear term in the Cox regression model. P < 0.05 was considered statistically significant.
Results
Patient Demographics and the Incidence of ABCBSI by Years
Two hundred and twenty-eight patients presented ABCBSI between 2009 and 2018; we excluded 26 cases: length of stay <72 h (n = 4); incomplete medical history or lost to follow-up (n = 3); and ABC strain isolated within 48 h after admission or previous infection with ABC in another ward or hospital before ICU admission (n = 19). The mean age of enrolled participants (n = 202) was 59.5 ± 16.9 years; 65.3% were male. The mean total length of ICU stay (mean ICU stay) was 20.6 ± 14.3 days, and the overall 28-day mortality was 34.2%. For the years 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018, a significantly increased incidence of ABC infection was observed (99, 285, 498, 672, and 926 per 1,000,000 ICU population, respectively, P < 0.01). The three infection sources of ABCBSI were the lung (53.5%), abdomen and pelvis (11.9%), and catheter-related BSI (6.4%).
Microbiology of ABC Isolates
For the years 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018, the PDR strains increased, and sensitive strains decreased significantly (all P < 0.05), but the proportions of MDR (excluding XDR) and XDR (excluding PDR) strains did not significantly change (Table 1). The rates of sensitivity to ampicillin, ceftazidime, cefepime, imipenem, gentamycin, levofloxacin, and piperacillin–tazobactam decreased significantly (all P < 0.05), with no significant change in rates of intermediate resistance to ceftazidime, cefepime, imipenem, gentamycin, levofloxacin, trimethoprim/sulfamethoxazole, or piperacillin–tazobactam except ampicillin (Figure 1). No ABC isolates were tested for tigecycline resistance until 2012, and for the years 2013–2014, 2015–2016, and 2017–2018, the rates of sensitivity to tigecycline decreased, although not significantly (P > 0.05).
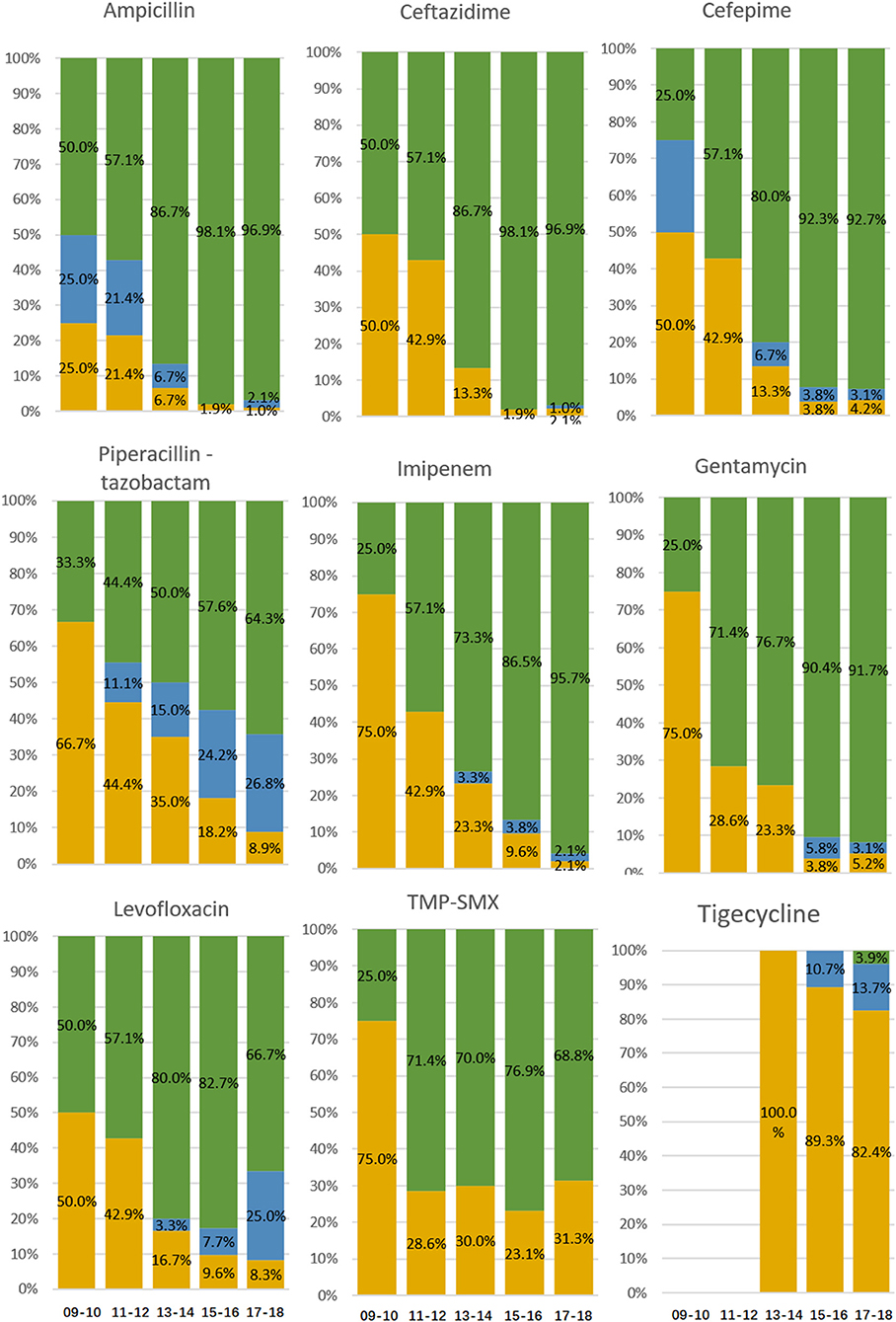
Figure 1. Resistance to antibacterial agents of ABC isolates from patients diagnosed with ICU-acquired ABCBSI. Data are presented as percentage. Green bars, resistant isolates; blue bars, intermediate isolates; yellow bars, sensitive isolates.
Use of Antibiotics, Clinical Characteristics, and Prognosis in ABCBSI Patients
For the years 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018, the frequency of empirical treatment increased with β-lactam/β-lactamase inhibitor, carbapenems, and tigecycline and decreased with cephalosporins and quinolone (Figure 2A); the frequency of antibiogram-directed treatment increased with β-lactam/β-lactamase inhibitor and tigecycline and decreased with cephalosporins (Figure 2B). ABCBSI patients did not differ in age, sex, underlying diseases, the source of ABCBSI, presence of polymicrobial BSI or septic shock, APACHE II scores, and ICU days, but the use of invasive ventilation increased (P < 0.05, Table 1). We found an increased 28-day mortality (0.0, 14.3, 28.1, 35.2, and 39.8%), although it was not significant (Table 1).
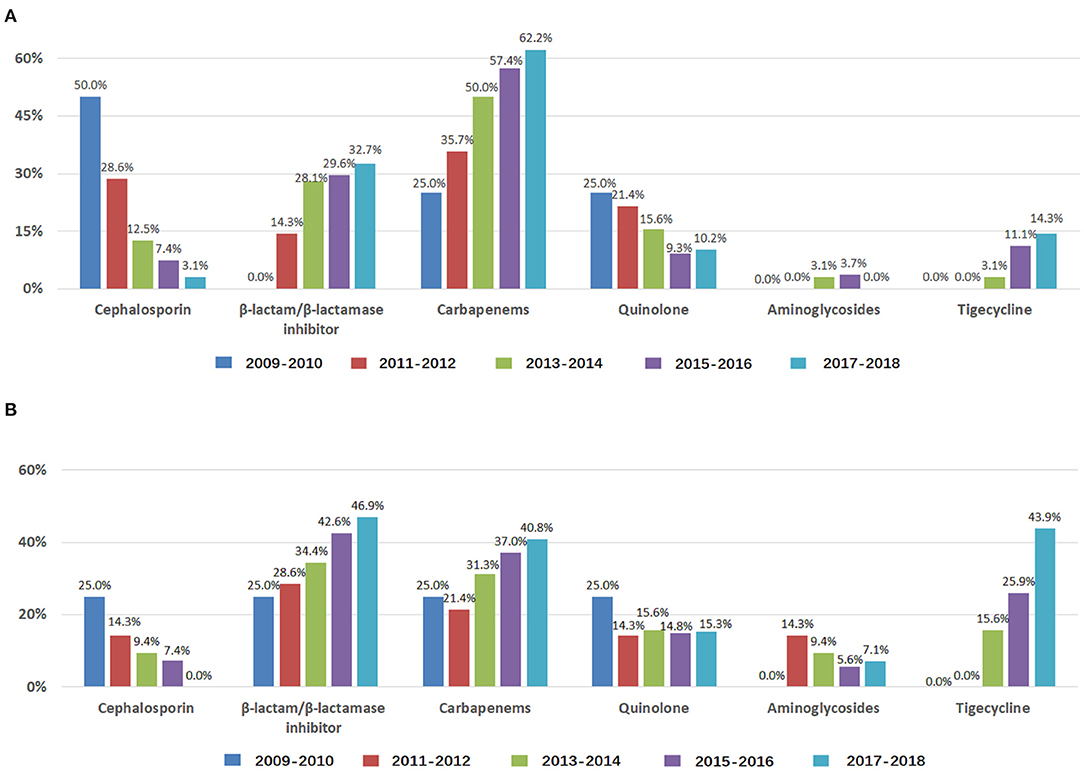
Figure 2. Antibiotics used to treat ABCBSI in ICUs during 2009–2018. Data are presented as percentage. (A) Use of antibiotics for empirical treatment of ABCBSI; (B) Use of antibiotics for antibiogram-directed treatment of ABCBSI.
To better explore this long-term change, we compared the clinical characteristics and prognosis of the patients in the first years (2009–2012) and the last years (2017–2018): ABCBSI patients in the years 2017–2018 were less often treated with appropriate empirical therapy, more often underwent pneumonia-associated ABCBSI and mechanical ventilation support, and had higher 28-day mortality rates (Table 2).
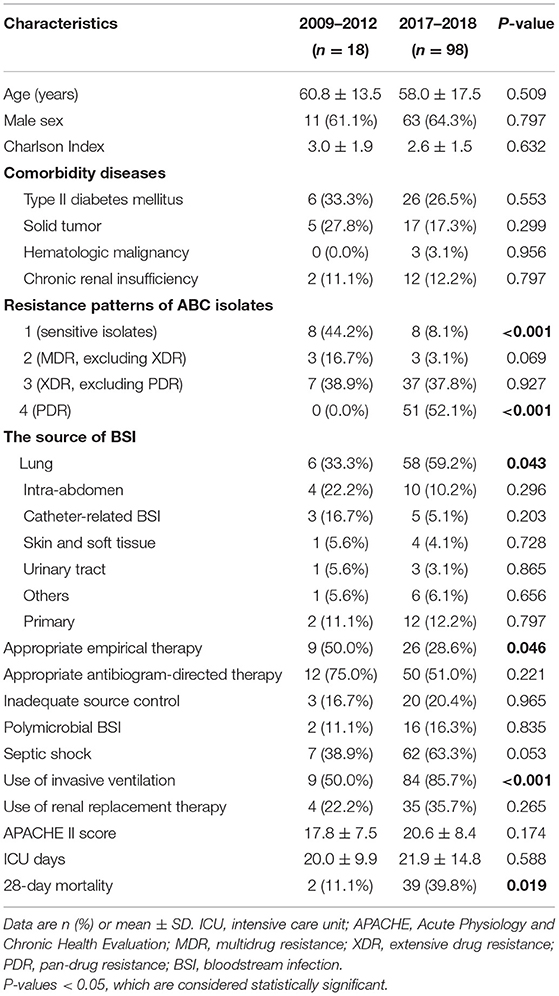
Table 2. Comparison of indicators between years 2009–2012 and years 2017–2018 in patients with ABCBSI.
Univariate and Multivariable Analyses of Factors Associated With 28-Day Mortality in ABCBSI Patients
As compared with survivors, non-survivors more frequently had XDR and PDR strains, less frequently had sensitive isolates, more frequently had comorbidity of diabetes mellitus and lung infection-related ABCBSI, received invasive ventilation, and presented septic shock and higher APACHE II scores (all P < 0.05, Table 3). In the Cox regression model, the increased resistance degree of ABC isolates (HR: 2.51, 95% CI: 1.68–3.75, P-value for trend <0.001), lung infection-related ABCBSI (HR: 3.83, 95% CI: 1.10–13.30), and septic shock (HR: 3.08, 95% CI: 1.55–6.14) were identified as independent risk factors for mortality (Table 3). Kaplan–Meier curves visually compared survival across these three independent risk factors (Supplementary Figure 1).
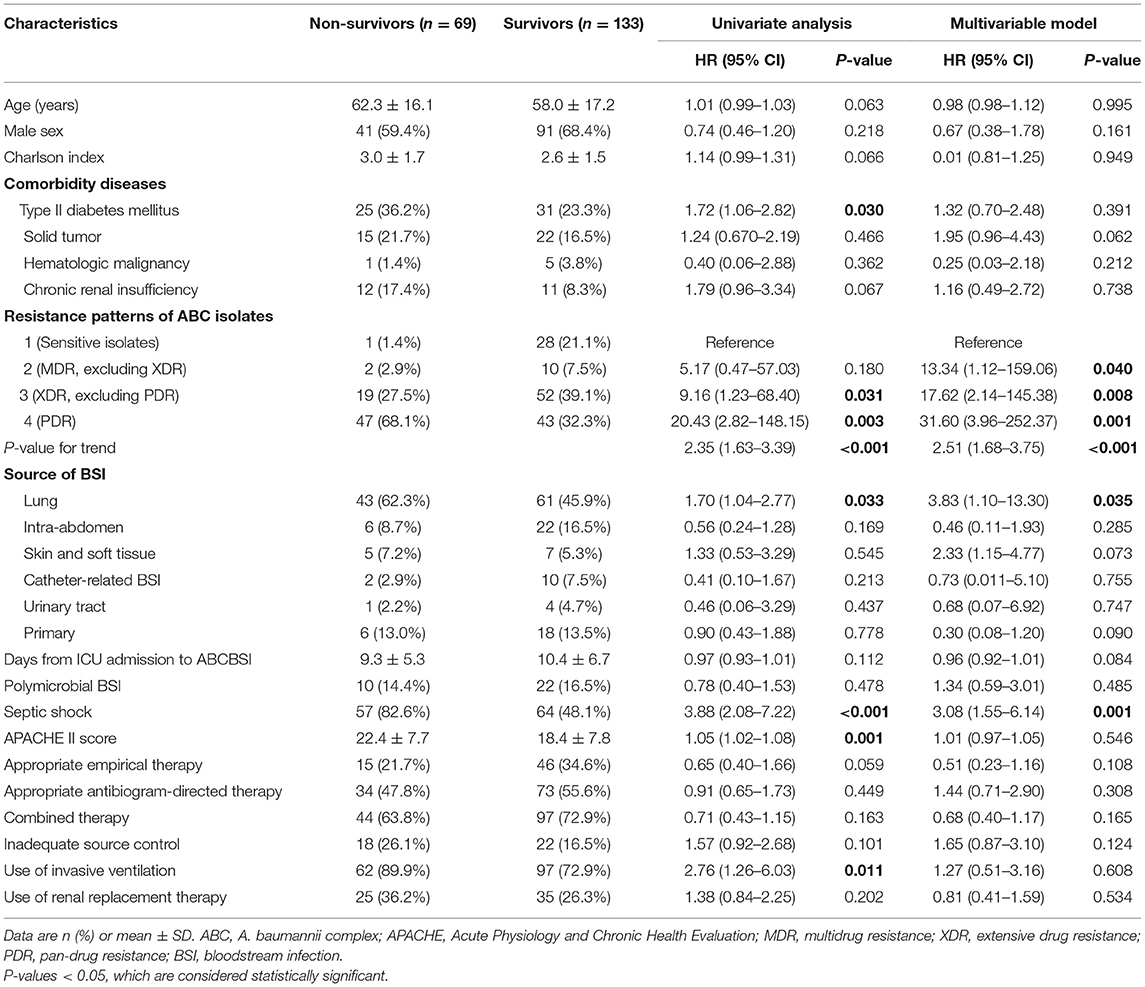
Table 3. Univariate and multivariate Cox regression analyses of the association between different variables and 28-day mortality.
Discussion
This study firstly reported a long-term change in incidence, antibiotic resistance, therapy, and prognosis of nosocomial ABCBSI in ICUs in eastern China. We found that for the years 2009–2018, the incidence of ABC infection increased significantly in the ICUs of eastern China, as did the resistance rates to most antibiotics; the percentage of PDR isolates increased, and sensitive isolates decreased. Compared with those in the years 2009–2012, ABCBSI patients in the years 2017–2018 were less often treated with appropriate empirical therapy, more often underwent pneumonia-related ABCBSI and mechanical ventilation support, and had higher 28-day mortality rates. Increased antibiotics resistance degree of ABC isolates, lung infection-related ABCBSI, and presence of septic shock were risk factors of 28-day mortality.
Although ABC is notorious for its great propensity for epidemic spread and has emerged as an important causative agent of nosocomial infections, the striking 10-fold rise in incidence of nosocomial ABCBSI with worrisome mortality in China ICUs in the past decade is surprising. The increasing incidence of ABCBSI can be interpreted from several aspects. First, the growing drug resistance of A. baumannii means there are progressively fewer therapeutic options and creates competitive advantages for A. baumannii over other pathogens. Data from the China Antimicrobial Resistance Surveillance System (CARSS) and CHINET show that as the prevalence and resistance of A. baumannii increased, the prevalence of Staphylococcus aureus and P. aeruginosa decreased notably (31). Second, infection control depends on active surveillance, contact isolation, healthcare worker compliance with hand hygiene, and aseptic care of vascular catheters and endotracheal tubes (32, 33). Because of human resource shortages, some nursing workers are part-time, low paid, and not well-educated. However, they participate in patient services such as feeding, turnover, metering intake, and output volume; such workers may have low compliance with hand hygiene and disinfection requirements. Third, the characteristics of patients/patient treatment changed over the period of our study. Increased use of invasive ventilation over the last decade has brought about more chance for ABC infection in the lung. Since lung infection was the most common source of BSI, the dominant role of ABC in causing ventilator-associated pneumonia may have contributed to the increasing incidence of ABCBSI (15, 34). Our results suggest the urgent need for increased attention and enhanced health policies to control ABC nosocomial infection in Chinese ICUs to decrease the spread of the organism. Control depends on active surveillance, contact isolation, healthcare worker compliance with hand hygiene, and aseptic care of vascular catheters and endotracheal tubes (32, 33).
A study from one Chinese hospital revealed that the MDR rate of ABCBSI increased significantly from 2010 to 2013 (64.71, 73.47, 75.00, and 83.05%, respectively, P = 0.032) (35). However, in our study, to better define the resistance degree of each ABC isolate, we counted the number of MDR (excluding XDR) isolates and did not find a significant change in its frequency between the years. Instead, the frequency of PDR isolates increased and that of sensitive isolates decreased significantly. Particularly, the rates of resistance to imipenem in Chinese ICUs continually increased (25.0, 57.1, 73.3, 86.5, and 95.7%) by years. In the year 2016, the resistance rate to imipenem was 88.5%, which is higher than that from CHINET data (68.6%) (36), mostly from general wards of Chinese hospitals.
β-Lactamase inhibitor (sulbactam and tazobactam)-based combinations have shown effective antibacterial activity for ABC strains. As shown in our study, the ABC isolates still had 35.7% intermediate and sensitive rates to piperacillin–tazobactam, so β-lactamase inhibitors could have relatively high anti-ABC activity and could be used for MDR treatment, but increasing resistance should also be considered when applied. As compared with colistin, which was unavailable until 2018, tigecycline was commercially available in 2013 in China and serves as the last defense against these persistent bacteria under most circumstances. However, an increasing resistance to tigecycline with the wide use of this agent after the year 2013 was shown and should be paid more attention. Trimethoprim/sulfamethoxazole was little used to treat ABCBSI because there was no intravenous formulation of trimethoprim/sulfamethoxazole available in mainland China; the resistance to trimethoprim/sulfamethoxazole was stable over the years in this study.
The increasing incidence of antibiotic resistance of ABC has become a worldwide challenge in healthcare facilities. A major driver of this emerging issue is the inappropriate use of antimicrobials. Mechanisms of drug resistance of ABC include expression of drug-inactivating enzymes, modifications of the target sites, increased efflux pumps, and decreased membrane permeability (37), on which the use of broad-spectrum antimicrobials exerted dramatic selective pressure. Over the years, we observed increased incidence of imipenem-resistant and pan-drug-resistant ABC, which is consistent with the increased use of carbapenem and tigecycline in Chinese ICUs. In China, ICU physicians often select carbapenems as a first-line empirical antibiotics in patients with sepsis or septic shock because of their broad-spectrum antibacterial activity, effects on bacteria that produce extended-spectrum β-lactamase, and better cost performance. Carbapenems and β-lactamase inhibitors, in combination with other agents, are still commonly used to treat CRAB infections in China (38). The extensive use of carbapenems may contribute to the growing expression of various carbapenemases, which is the hallmark of the XDR phenotype. In addition, the wide use of tigecycline in recent years is an important external factor for the overexpression of multidrug efflux pumps, which is a key molecular mechanism for the decreased susceptibility to tigecycline in Acinetobacter isolates (39, 40). Moreover, the excessive application of antimicrobial agents in agriculture and livestock production, especially in developing countries, also leads to increased exposure to antibiotics and worsens the situation of drug resistance (41, 42). Standardized management of antibiotics, appropriate empirical treatment, and timely de-escalation therapy may be essential for alleviating the rise of antibiotic resistance.
From epidemiological studies, the mortality rates with ABC-associated infections range from 10 to 43% in ICUs, which significantly increases the burden of ICUs (43). The overall 28-day mortality rate of ABCBSI in this study (34.2%) is comparable. However, we showed an increase in mortality (0.0, 14.3, 28.1, 35.2, and 39.8%) over the years. Studies have shown that MDR or XDR isolates were associated with longer hospitalization, increased costs, and an excess attributable mortality rate in ABCBSI patients (44, 45); therefore, they may contribute to a high rate of treatment failure led by the propensity for antimicrobial resistance (46, 47) and high frequency of biofilm forming in higher-resistance ABC (48). Necessary implementation to control the increased resistance may benefit the survival of ABCBSI patients.
In this study, lung infection-related ABCBSI was a risk factor of 28-day mortality. Although ABC can be isolated from various clinical specimens from hospitalized patients, it is commonly isolated in ICUs from intubated patients (49); thus, lower respiratory tract infections were the most common source of ABCBSI acquired in ICUs. Compared to those without pneumonia, the hospital-acquired pneumonia-related ABCBSI group were more frequently treated in ICU, had significantly higher incidence of antibiotic resistance (50), and presented with septic shock in carbapenem-resistant ABCBSI (7). Furthermore, considering nearly 80% of lung infections with ABC were hospital acquired, prevention of nosocomial ABC pneumonia may play an important role to combat these infections and decrease high mortality.
Our study has several limitations and should be interpreted with discretion. First, because molecular studies are not routinely performed and the clinical isolates are not routinely preserved in some of the participating hospitals, we did not investigate the molecular epidemiology of ABC identified by conventional biochemical methods in this study. Second, though the participants were consecutively enrolled, the clinical data were collected retrospectively, which may lead to implied bias. Third, because the study was conducted in adult ICUs in eastern China, the conclusions may not apply to different populations or different clinical situations. Additionally, patients with coinfection by other pathogens at other sites were not specifically identified and excluded, which may cause latent bias to the results.
Conclusions
In summary, the past decade has witnessed a marked increase in incidence of ABCBSI and in antibiotic resistance, with increasing pneumonia-related infections and worrisome mortality in ICUs in eastern China. Considering that increased antibiotic resistance of ABC isolates and lung infection-related ABCBSI were risk factors for death, controlling increasing resistance of ABC and preventing nosocomial pneumonia may play important roles in combatting these infections.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Qilu Hospital of Shandong University, Second Hospital of Shandong University, Qingdao Branch of Qilu Hospital, Liaocheng People's Hospital affiliated with Taishan Medical College, Zaozhuang Hospital, Jiaxiang People's Hospital, Shenxian People's Hospital and Chengwu People's Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XM contributed to manuscript writing and data analysis. JF contributed to information collection. YZ helped in manuscript preparation. WQ, HY, DC, and HL helped in data interpretation. LZ, ZD, JP, WL, HG, JD, CL, and DW participated in data collection. HW contributed to study design and manuscript writing. All authors approved the final manuscript and are responsible for the content.
Funding
This work was supported by the National Natural Science Foundation of China (81873927, 82072231, and 81970319), Taishan Scholars Program of Shandong Province (tsqn202103165 and tsqn202103170), Clinical Research Center of Shandong University (2020SDUCRCC013), and China Post-doctoral Science Foundation (2018M632685).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Xiaorong Yang from Clinical Epidemiology Unit, Qilu Hospital of Shandong University, for his assistance in statistics. We thank Dr. Laura Smales for language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.715213/full#supplementary-material
References
1. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. (2004) 39:309–17. doi: 10.1086/421946
2. Alvarez-Marin R, Navarro-Amuedo D, Gasch-Blasi O, Rodriguez-Martinez JM, Calvo-Montes J, Lara-Contreras R, et al. A prospective, multicenter, and non-interventional case control study about the risk factors associated to acquisition and mortality of Enterobacter species bacteremia. J Infect. (2020) 80:174–81. doi: 10.1016/j.jinf.2019.09.017
3. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. (2009) 302:2323–9. doi: 10.1001/jama.2009.1754
4. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. (2008) 21:538–82. doi: 10.1128/CMR.00058-07
5. Guo N, Xue W, Tang D, Ding J, Zhao B. Risk factors and outcomes of hospitalized patients with blood infections caused by multidrug-resistant Acinetobacter baumannii complex in a hospital of Northern China. Am J Infect Control. (2016) 44:e37–9. doi: 10.1016/j.ajic.2015.11.019
6. Nutman A, Glick R, Temkin E, Hoshen M, Edgar R, Braun T, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect. (2014) 20:O1028–34. doi: 10.1111/1469-0691.12716
7. Russo A, Bassetti M, Ceccarelli G, Carannante N, Losito AR, Bartoletti M, et al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect. (2019) 79:130–8. doi: 10.1016/j.jinf.2019.05.017
8. Lob SH, Hoban DJ, Sahm DF, Badal RE. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int J Antimicrob Agents. (2016) 47:317–23. doi: 10.1016/j.ijantimicag.2016.01.015
9. Falagas ME, Karveli EA, Siempos II, Vardakas KZ. Acinetobacter infections: a growing threat for critically ill patients. Epidemiol Infect. (2008) 136:1009–19. doi: 10.1017/S0950268807009478
10. Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. (2000) 31:690–7. doi: 10.1086/314040
11. Rhomberg PR, Jones RN. Summary trends for the meropenem yearly susceptibility test information collection program: a 10-year experience in the United States (1999-2008). Diagn Microbiol Infect Dis. (2009) 65:414–26. doi: 10.1016/j.diagmicrobio.2009.08.020
12. Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol. (2016) 37:1288–301. doi: 10.1017/ice.2016.174
13. Li Y, Cao X, Ge H, Jiang Y, Zhou H, Zheng W. Targeted surveillance of nosocomial infection in intensive care units of 176 hospitals in Jiangsu province, China. J Hosp Infect. (2018) 99:36–41. doi: 10.1016/j.jhin.2017.10.009
14. Peng H, Tao XB, Li Y, Hu Q, Qian LH, Wu Q, et al. Health care-associated infections surveillance in an intensive care unit of a university hospital in China, 2010-2014: findings of international nosocomial infection control consortium. Am J Infect Control. (2015) 43:e83–5. doi: 10.1016/j.ajic.2015.07.023
15. Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. (2011) 184:140–17. doi: 10.1164/rccm.201102-0349OC
16. Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. (2016) 22 (Suppl. 1):S9–14. doi: 10.1016/j.cmi.2016.01.001
17. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2008) 36:309–32. doi: 10.1016/j.ajic.2008.03.002
18. Mylotte JM, Tayara A, Goodnough S. Epidemiology of bloodstream infection in nursing home residents: evaluation in a large cohort from multiple homes. Clin Infect Dis. (2002) 35:1484–90. doi: 10.1086/344649
19. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
20. Downes KJ, Metlay JP, Bell LM, McGowan KL, Elliott MR, Shah SS. Polymicrobial bloodstream infections among children and adolescents with central venous catheters evaluated in ambulatory care. Clin Infect Dis. (2008) 46:387–94. doi: 10.1086/525265
21. Knaus W, Draper E, Wagner D, Zimmerman J. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. doi: 10.1097/00003246-198510000-00009
22. Pena C, Suarez C, Ocampo-Sosa A, Murillas J, Almirante B, Pomar V, et al. Effect of adequate single-drug vs. combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. (2013) 57:208–16. doi: 10.1093/cid/cit223
23. Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. (2012) 54:1739–46. doi: 10.1093/cid/cis305
24. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
25. Chuang YC, Sheng WH, Li SY, Lin YC, Wang JT, Chen YC, et al. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin Infect Dis. (2011) 52:352–60. doi: 10.1093/cid/ciq154
26. Pavlaki M, Poulakou G, Drimousis P, Adamis G, Apostolidou E, Gatselis NK, et al. Polymicrobial bloodstream infections: epidemiology and impact on mortality. J Glob Antimicrob Resist. (2013) 1:207–12. doi: 10.1016/j.jgar.2013.06.005
27. Amat T, Gutierrez-Pizarraya A, Machuca I, Gracia-Ahufinger I, Perez-Nadales E, Torre-Gimenez A, et al. The combined use of tigecycline with high-dose colistin might not be associated with higher survival in critically ill patients with bacteraemia due to carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. (2018) 24:630–4. doi: 10.1016/j.cmi.2017.09.016
28. Freire MP, de Oliveira Garcia D, Garcia CP, Campagnari Bueno MF, Camargo CH, Kono Magri ASG, et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect. (2016) 22:352–8. doi: 10.1016/j.cmi.2015.12.010
29. Lee YT, Kuo SC, Yang SP, Lin YT, Tseng FC, Chen TL, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. (2012) 55:209–15. doi: 10.1093/cid/cis385
30. Tellor B, Skrupky LP, Symons W, High E, Micek ST, Mazuski JE. Inadequate source control and inappropriate antibiotics are key determinants of mortality in patients with intra-abdominal sepsis and associated bacteremia. Surg Infect (Larchmt). (2015) 16:785–93. doi: 10.1089/sur.2014.166
31. Hu F, Zhu D, Wang F, Wang M. Current status and trends of antibacterial resistance in China. Clin Infect Dis. (2018) 67(Suppl. 2):S128–34. doi: 10.1093/cid/ciy657
32. Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977-2000. Infect Control Hosp Epidemiol. (2003) 24:284–95. doi: 10.1086/502205
33. Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. (2003) 36:1268–74. doi: 10.1086/374847
34. Saharman YR, Karuniawati A, Severin JA, Verbrugh HA. Infections and antimicrobial resistance in intensive care units in lower-middle income countries: a scoping review. Antimicrob Resist Infect Control. (2021) 10:22. doi: 10.1186/s13756-020-00871-x
35. Liu Q, Li W, Du X, Zhong T, Tang Y, Feng Y, et al. Risk and prognostic factors for multidrug-resistant Acinetobacter baumannii Complex Bacteremia: a retrospective study in a tertiary hospital of West China. PLoS ONE. (2015) 10:e0130701. doi: 10.1371/journal.pone.0130701
36. Hu F, Guo Y, Zhu D, Wang F, Jiang X, Xu Y, et al. CHINET surveillance of bacterial resistance across China: report of the results in 2016. Chin J Infect Chemother. (2017) 17:481–91.
37. Eichenberger EM, Thaden JT. Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiotics (Basel). (2019) 8:37. doi: 10.3390/antibiotics8020037
38. Guan X, He L, Hu B, Hu J, Huang X, Lai G, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. (2016) 22(Suppl. 1):S15–25. doi: 10.1016/j.cmi.2015.11.004
39. Peleg AY, Adams J, Paterson DL. Tigecycline efflux as a mechanism for nonsusceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother. (2007) 51:2065–9. doi: 10.1128/AAC.01198-06
40. Ruzin A, Keeney D, Bradford PA. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Antimicrob Chemother. (2007) 59:1001–4. doi: 10.1093/jac/dkm058
41. Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS Med. (2016) 13:e1001974. doi: 10.1371/journal.pmed.1001974
42. Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. (2015) 112:5649–54. doi: 10.1073/pnas.1503141112
43. Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. (2006) 10:R48. doi: 10.1186/cc4869
44. Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, et al. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. (2007) 28:713–9. doi: 10.1086/517954
45. Fu Q, Ye H, Liu S. Risk factors for extensive drug-resistance and mortality in geriatric inpatients with bacteremia caused by Acinetobacter baumannii. Am J Infect Control. (2015) 43:857–60. doi: 10.1016/j.ajic.2015.03.033
46. Ho PL, Ho AY, Chow KH, Lai EL, Ching P, Seto WH. Epidemiology and clonality of multidrug-resistant Acinetobacter baumannii from a healthcare region in Hong Kong. J Hosp Infect. (2010) 74:358–64. doi: 10.1016/j.jhin.2009.10.015
47. Keen EF, III, Murray CK, Robinson BJ, Hospenthal DR, Co EM, Aldous WK. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect Control Hosp Epidemiol. (2010) 31:728–32. doi: 10.1086/653617
48. Zeighami H, Valadkhani F, Shapouri R, Samadi E, Haghi F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect Dis. (2019) 19:629. doi: 10.1186/s12879-019-4272-0
49. Ahmed NH, Hussain T, Biswal I. Antimicrobial resistance of bacterial isolates from respiratory secretions of ventilated patients in a multi-specialty hospital. Avicenna J Med. (2015) 5:74–8. doi: 10.4103/2231-0770.160233
Keywords: Acinetobacter baumannii complex, bloodstream infection, antibiotic resistance, prognosis, eastern China
Citation: Meng X, Fu J, Zheng Y, Qin W, Yang H, Cao D, Lu H, Zhang L, Du Z, Pang J, Li W, Guo H, Du J, Li C, Wu D and Wang H (2021) Ten-Year Changes in Bloodstream Infection With Acinetobacter Baumannii Complex in Intensive Care Units in Eastern China: A Retrospective Cohort Study. Front. Med. 8:715213. doi: 10.3389/fmed.2021.715213
Received: 26 May 2021; Accepted: 05 July 2021;
Published: 05 August 2021.
Edited by:
Guodong Ding, Shanghai Children's Hospital, ChinaReviewed by:
Kanchana Usuwanthim, Naresuan University, ThailandStamatis Karakonstantis, University Hospital of Heraklion, Greece
Copyright © 2021 Meng, Fu, Zheng, Qin, Yang, Cao, Lu, Zhang, Du, Pang, Li, Guo, Du, Li, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wang, d2FuZ2hhbzM0QDEyNi5jb20=
 Xiao Meng1
Xiao Meng1 Yue Zheng
Yue Zheng Hongna Yang
Hongna Yang Wei Li
Wei Li Hao Wang
Hao Wang