- 1Department of Gastroenterology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, China
- 2Department of Oncology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, China
Background: It has been suggested that Helicobacter pylori (H. pylori) infection is associated with hypergastrinemia and proliferation of colorectal mucosa via direct stimulation, dysbiosis of the gut microbiome, and changes in the gut microbiome, all of which may lead to the formation of colorectal polyps. However, the consensus remains lacking regarding whether H. pylori infection is independently associated with colorectal polyps and whether the association differs according to histological type of colorectal polyps. To summarize the current evidence regarding the relationship between H. pylori infection and colorectal polyps, we conducted a meta-analysis of related observational studies according to the histological types of colorectal polyps.
Methods: Observational studies investigating the association between H. pylori infection and colorectal polyps using multivariate analyses were included by search of PubMed, Embase, and Web of Science. A random-effects model was adopted to combine the results.
Results: Seventeen studies that include 322,395 participants were analyzed. It was shown that H. pylori infection was independently associated with overall colorectal polyps (odds ratio [OR]: 1.67, 95% CI: 1.24–2.24, p < 0.001; I2 = 73%). According to the histological type of colorectal polyps, H. pylori infection was independently associated with adenomatous polyps (APs; OR: 1.71, 95% CI: 1.47–1.99, p < 0.001; I2 = 86%), advanced APs (OR: 2.06, 95% CI: 1.56–2.73, p < 0.001; I2 = 0%), and hyperplastic polyps (HPs; OR: 1.54, 95% CI: 1.02–2.30, p = 0.04; I2 = 78%). Evidence based on only one study showed that H. pylori infection was not associated with sessile serrated polyps (SSPs; OR: 1.00, 95% CI: 0.93–1.07, p = 0.99).
Conclusions: Current evidence from case-control and cross-sectional studies suggested that H. pylori infection was independently associated with colorectal APs, advanced APs, and HPs, but not with SSPs. These findings suggested H. pylori infection may be a possible risk factor of colorectal polyp, which is important for the prevention of colorectal polyp in the adult population.
Background
Colorectal polyps refer to protuberance extending from the normally flat colorectal mucosa into the lumen. Clinically, colorectal polyps could be classified based on their histological features and susceptibility to malignant transformation (1). Generally, colorectal polyps could be classified according to the histological types into hyperplastic polyps (HPs), adenomatous polyps (APs), and sessile serrated polyps (SSPs) (2). Among them, APs and some subtypes of HP have been considered as precancerous lesions for colorectal cancer (CRC) (3). As for SSP, the risk factors and epidemiological features are still to be determined (4). Since CRC remains one of the most prevalent malignancies all over the world and the potential role of neoplastic polyps as precancerous lesions (5), it is important to determine the risk factors for colorectal polyps according to the histological features.
Helicobacter pylori (H. pylori) is a common gram-negative bacterium that dwells on the gastric mucosa and can secrete urea enzymes, vacuoles toxins, and cytotoxin-related genes (6, 7). It has been indicated in previous studies that the prevalence of H. pylori infection could be more than 50% in the general population, which has been recognized as the culprit of chronic gastritis, gastric ulcers, and gastric cancer (8, 9). Besides, it has been confirmed that H. pylori infection confers a 1.2–1.6 times greater risk of CRC (10). It has been suggested in preclinical studies that chronic H. pylori infection may cause hypergastrinemia and proliferation of colorectal mucosa via direct stimulation, dysbiosis of the gut microbiome, and changes in the gut microbiome, all of which may lead to the formation of colorectal polyps (10–12). Therefore, it could be hypothesized that H. pylori infection may be a risk factor for colorectal polyps. Accordingly, accumulating studies have been performed to evaluate the potential association between H. pylori infection and colorectal polyps (13–29), whereas the results of these studies are not always consistent. Although a few meta-analyses have shown that H. pylori infection may be associated with the prevalence of colorectal polyps (10, 30–32), particularly of AP (18, 33, 34), analyses according to the histological types of colorectal polyps were rarely performed. Besides, these meta-analyses generally included data derived from univariate analysis (10, 18, 30–34). Moreover, it remains unknown whether the association between H. pylori infection and colorectal polyps remains after adjustment of potential confounding factors, such as age and sex (35). Accordingly, a meta-analysis summarizing the current evidence for the association between H. pylori infection and the prevalence of colorectal polyps is needed. Therefore, in this study, we aimed to perfume a meta-analysis to systematically evaluate the relationship between H. pylori infection and colorectal polyps. Besides, the associations were analyzed according to the different histological types of colorectal polyps in this study. These findings are expected to provide theoretical evidence of H. pylori infection as a possible risk factor of the colorectal polyp and may be important for the prevention of colorectal polyp in the adult population.
Methods
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline (36) and Cochrane's Handbook (37) were followed in this study. The protocol of the meta-analysis was not prospectively registered.
Literature Search
The electronic databases of PubMed, Embase, and Web of Science databases were searched on February 26, 2021, with a keyword-based search strategy as (“Helicobacter pylori” or “Campylobacter pylori” OR “H. pylori” OR “HP” OR “Helicobacter” OR “Helicobacter species” OR “Helicobacter sp.” OR “Helicobacter genus” OR “Campylobacter” OR “Campylobacter infection” OR “Campylobacteriosis” OR “Helicobacter pylori infection” OR “Helicobacter infection” OR “pylori” OR “enterohepatic Helicobacter spp.” OR “Campylobacter spp.”) AND (“polyp” OR “polyps” OR “polypoid-lesion”) AND (“colon” OR “colorectal” OR “colonic” OR “rectum” OR “rectal” OR “colonic-neoplasm” OR “Intestine polyp”). The keyword-based literature search strategy has been well used in previous meta-analyses and may be more sensible for the identification of related studies. Only studies reported in English were considered. References of related articles or reviews were also analyzed.
Study Identification
Studies that fulfilled these criteria were used: (1) observational studies published as full-length papers; (2) included adult population; (3) evaluated the association between H. pylori infection and colorectal polyps, such as overall colorectal polyps or colorectal polyps according to histological types, i.e., AP, advanced AP, HP, and SSP; and (4) reported odds ratios (ORs) for the above associations after adjusting for multiple confounding factors (at least for age and sex). Diagnostic methods for H. pylori infection were in accordance with the strategies applied among the included studies. Determination of overall colorectal polyps and their histological types were based on histological diagnosis with specimens obtained during colonoscopy examination. Reviews, preclinical studies, studies with univariate analysis, and irrelevant studies were not included.
Data Extracting and Quality Evaluation
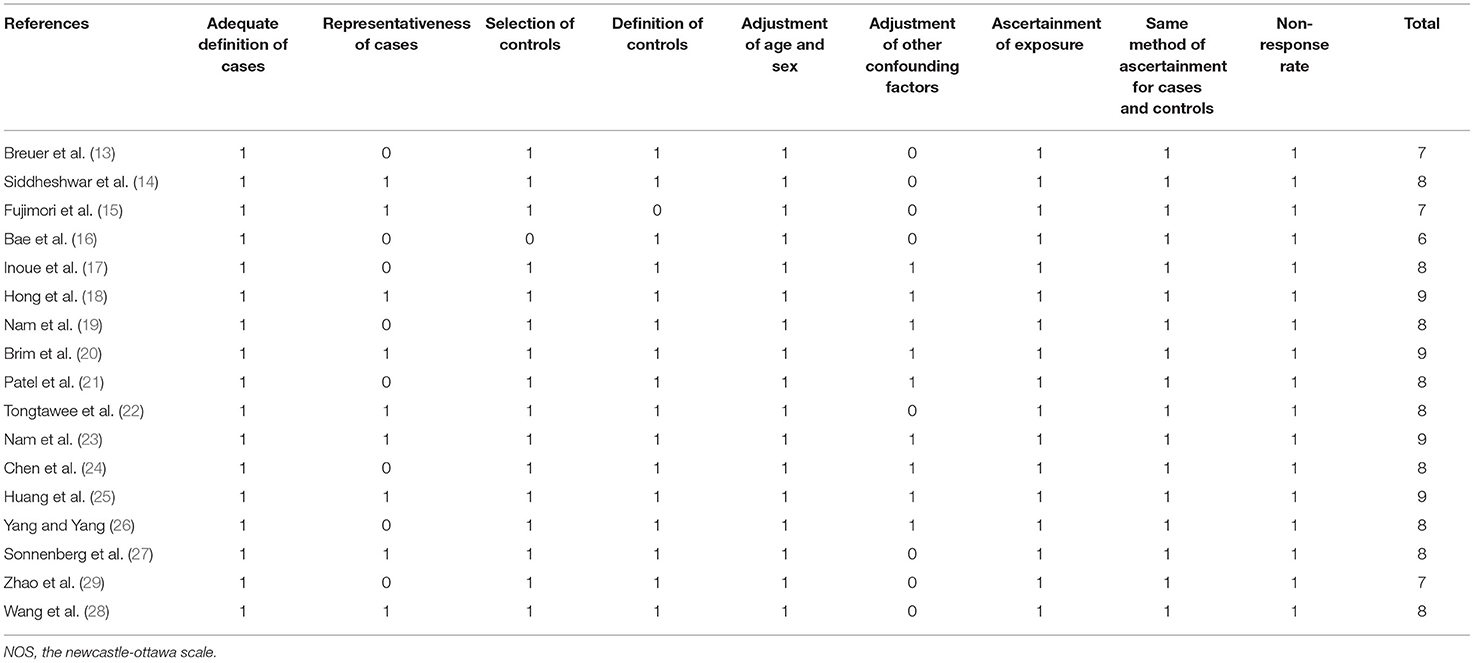
Two authors implemented database search, data extraction, and study quality assessment separately. If disagreements occurred, they were discussed with the corresponding author. These data were recorded: (1) author, study year, and study design; (2) participant characteristics, such as a number of participants included, mean age, and sex; (3) methods for the diagnosis of H. pylori infection and numbers of participants with H. pylori infection; (4) colorectal polyp outcomes reported and numbers of patients with each outcome; and (5) potential confounding factors adjusted in the multivariate analyses. The Newcastle-Ottawa Scale (NOS) (38) was used for study quality evaluation. This scale is rated from 1 to 9 stars and reflected the quality of the study by aspects of participant selection, comparability between groups, and outcome validation.
Statistical Analyses
Odds ratio and the corresponding 95% CIs were extracted for every included study. Then, SEs of ORs were estimated from the 95% CIs or values of p. For normalization of their distribution, ORs were logarithmically transformed (37) and combined. Heterogeneity within the included cohort studies was tested via Cochrane's Q test and the estimation of I2 statistics (39). An I2 > 50% suggests a significant level of heterogeneity. A random-effects model was chosen to combine the ORs by incorporating the potential heterogeneity within studies (37). Sensitivity analyses by sequentially excluding either of the included studies were conducted to clarify the influence of a certain study on the overall results (40). Funnel plots were constructed and were used for the assessment of publication bias (41), which could be further validated by the Egger's regression asymmetry test. The RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and STATA software were involved for statistical analyses.
Results
Database Search
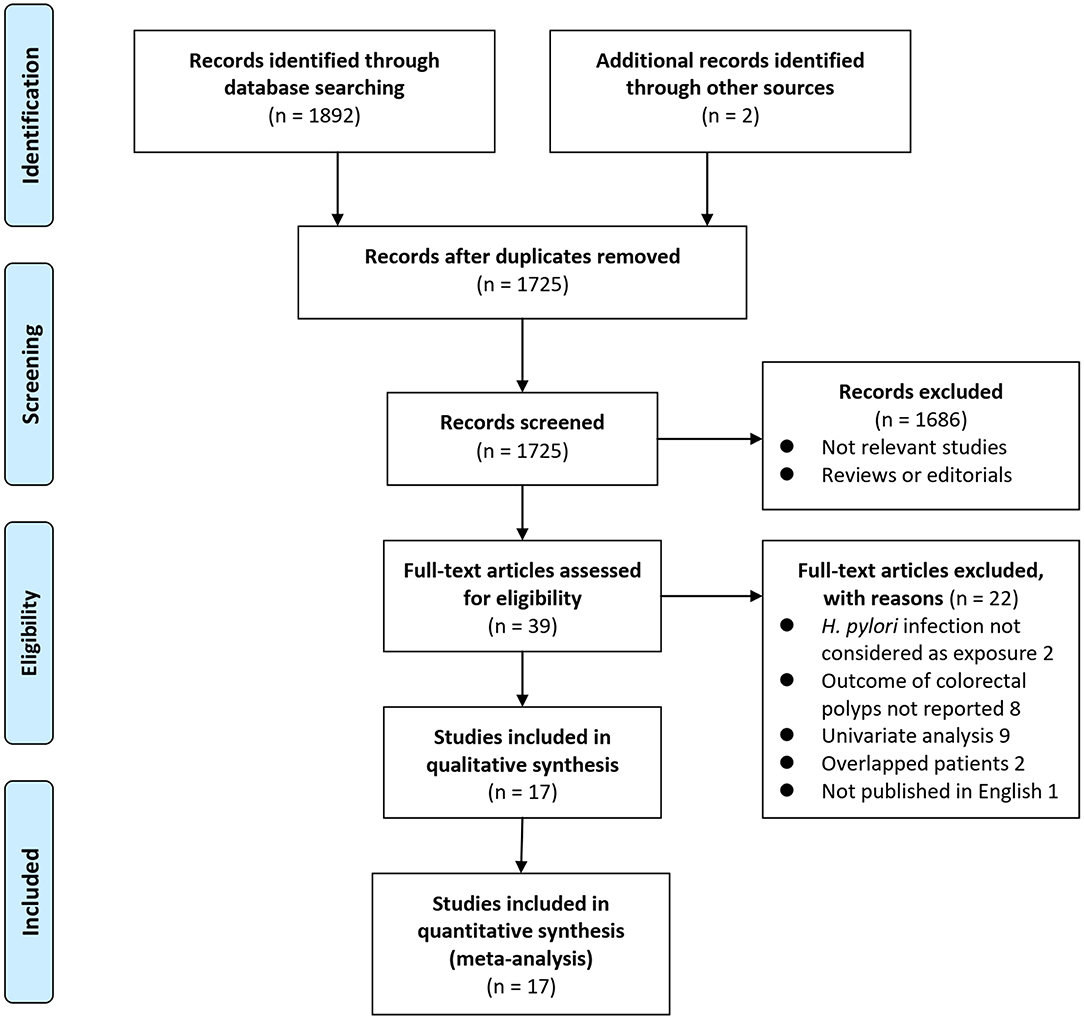
Details of the database search are shown in Figure 1. The first-step database search retrieved 1,725 articles after duplicated studies were excluded. Among them, 1,686 studies were further excluded because they were not related to the purpose of the meta-analysis based on titles and abstracts. Then, for the remaining 39 studies evaluated by full-text reading, 22 were not included for the reasons which are presented in Figure 1, which resulted in 17 studies with multivariate analyses finally included in the meta-analysis (13–29).
Study Characteristics
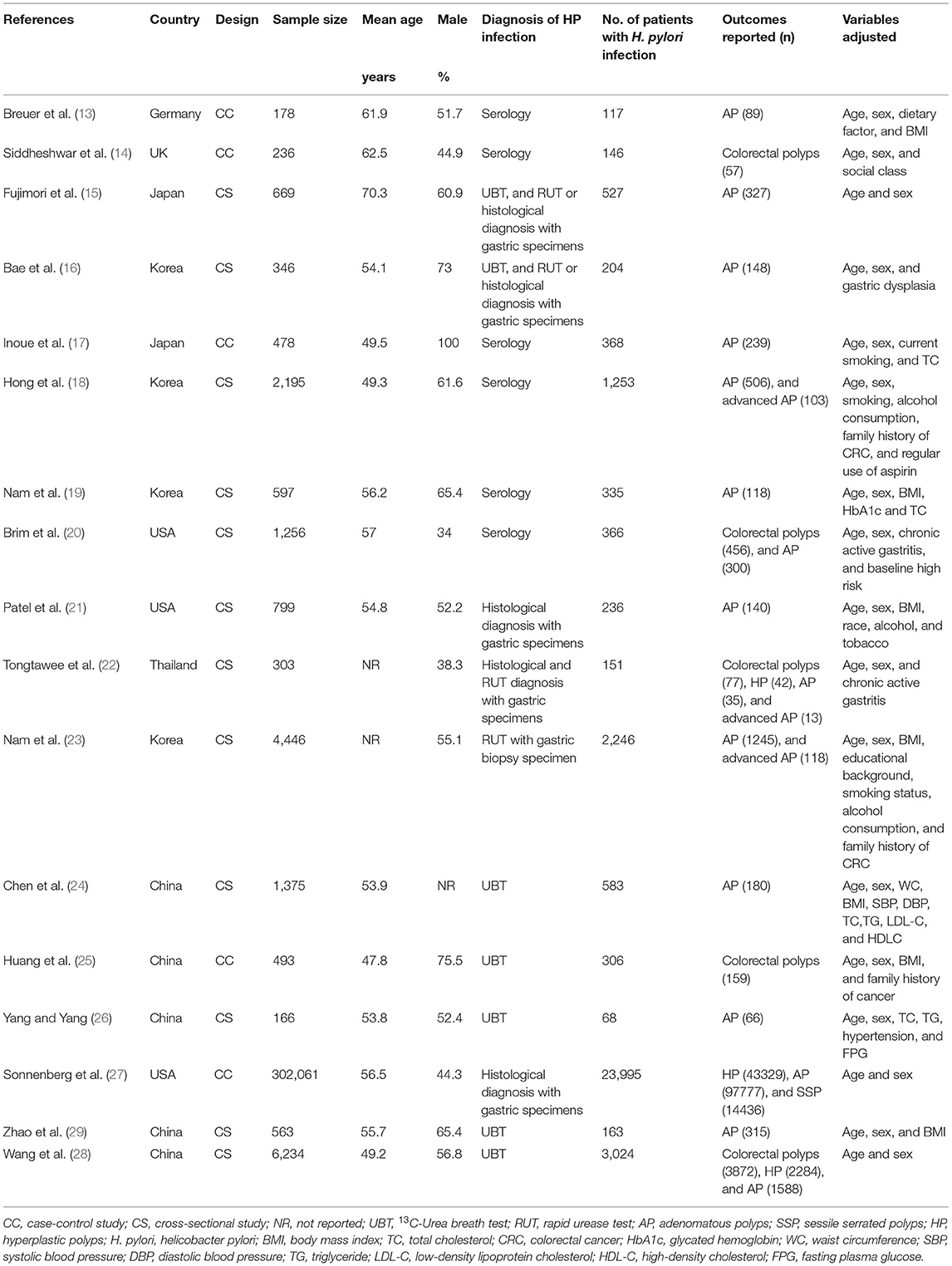
Characteristics of the included studies are shown in Table 1. Overall, 17 studies, i.e., five case-control studies (13, 14, 17, 25, 27) and 12 cross-sectional studies (15, 16, 18–24, 26, 28, 29), with 322,395 participants were analyzed in the meta-analysis. The crude prevalence rates of any colorectal polyps in H. pylori infection and un-infection groups were 23.9% (8153/34088) and 15.0% (43170/288307), respectively. These studies were published between 1999 and 2020. Multiple methods were used for the diagnosis of H. pylori infection, such as serology test, 13C-Urea breath test, rapid urease test, and histological diagnosis with gastric specimens. A total of 34,088 participants had H. pylori infection (10.6%). Outcomes of overall colorectal polyps were reported in five studies (14, 20, 22, 25, 28), AP in 15 studies (13, 15–24, 26–29), advanced AP in three studies (18, 22, 23), HP in three studies (22, 27, 28), and SSP in one study (27). Age, sex, social class, body mass index (BMI), smoking, alcohol drinking, and other potential confounding factors, such as parameters of lipids and glycemic metabolism, were adjusted to a varying degree when the associations between H. pylori infection, and colorectal polyps were reported. The quality of these studies was good, evidenced by six to nine points of the NOS scores (Table 2).
Association Between H. pylori Infection and Colorectal Polyps
Pooled results with a random-effects model showed that H. pylori infection was independently associated with overall colorectal polyps (OR: 1.67, 95% CI: 1.24–2.24, p < 0.001; I2 = 73%; Figure 2A). According to the histological type of colorectal polyps, H. pylori infection was independently associated with AP (OR: 1.71, 95% CI: 1.47–1.99, p < 0.001; I2 = 86%; Figure 2B), advanced AP (OR: 2.06, 95% CI: 1.56–2.73, p < 0.001; I2 = 0%; Figure 2C), and HP (OR: 1.54, 95% CI: 1.02–2.30, p = 0.04; I2 = 78%; Figure 2D). Sensitivity analyses by excluding one study at a time showed consistent results (Table 3). The heterogeneity for the outcomes of overall colorectal polyps, AP, and HP was significantly reduced after excluding the study by Wang et al. (28) (I2 reduced to 25, 68, and 0% for the three outcomes of overall colorectal polyps, AP, and HP), suggesting that this study may be a major determinant of heterogeneity. Evidence based on only one dataset (27) showed that H. pylori infection was not associated with SSP (OR: 1.00, 95% CI: 0.93–1.07, p = 0.99).
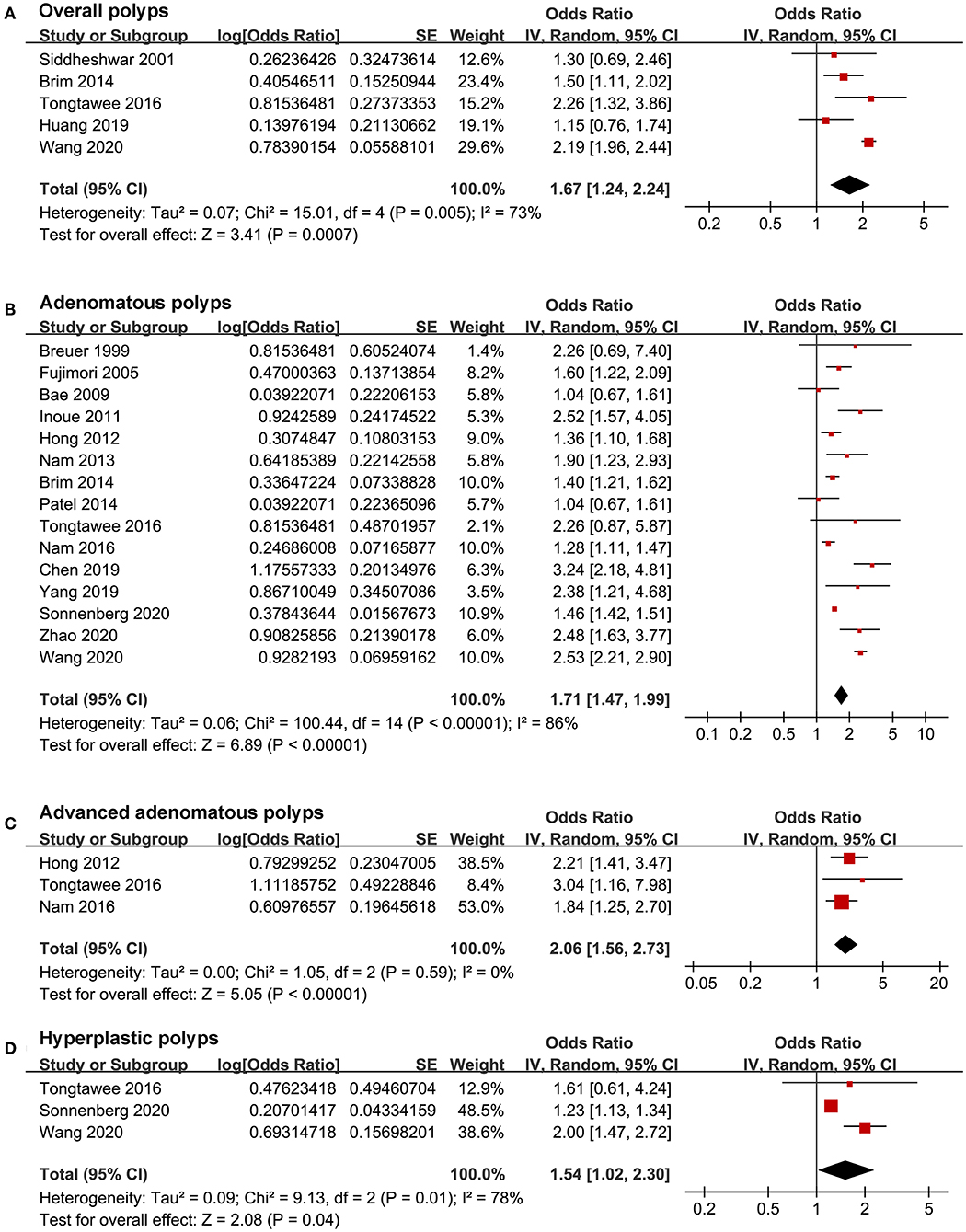
Figure 2. Forest plots for the meta-analysis concerning the association between H. pylori infection and colorectal polyps; (A) the outcome of overall colorectal polyps; (B) the outcome of adenomatous polyps; (C) the outcome of advanced adenomatous polyps; and (D) the outcome of hyperplastic polyps.
Publication Bias
Funnel plots representing the meta-analysis of H. pylori infection and overall colorectal polyps and AP are shown in Figures 3A,B. The plots were symmetrical based on visual inspection, suggesting a low risk of publication bias. Egger's regression test also demonstrated a low risk of publication bias (p for Egger's regression tests = 0.322 and 0.128, respectively). The publication biases underlying the meta-analysis of H. pylori infection and advanced AP and HP were unable to determine since only three studies were included for these two outcomes.
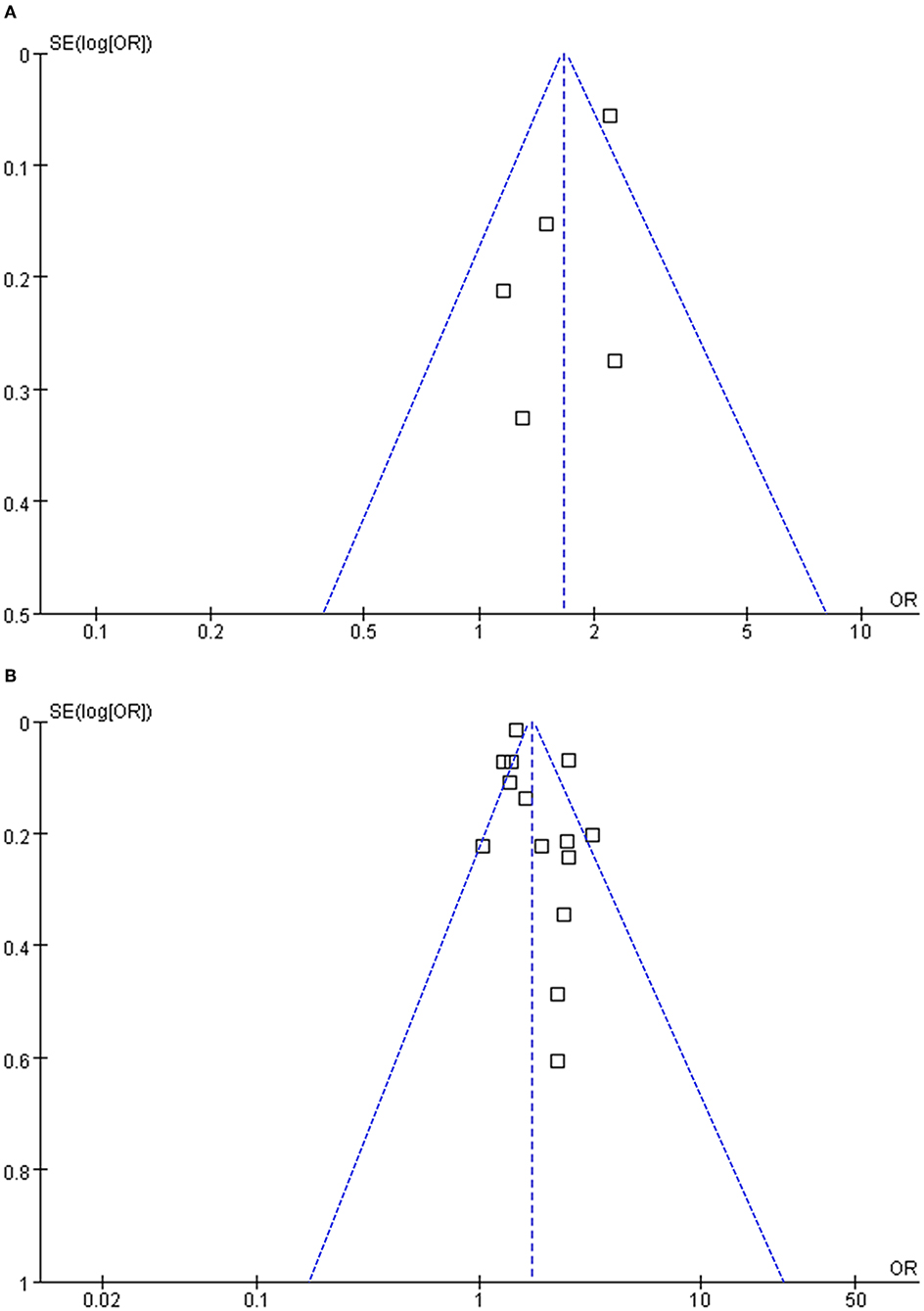
Figure 3. Funnel plots for the meta-analyses; (A) funnel plots for the meta-analysis concerning the association between H. pylori infection and overall colorectal polyps; and (B) funnel plots for the meta-analysis concerning the association between H. pylori infection and adenomatous polyps.
Discussion
In this meta-analysis, we combined the results of seventeen studies that include 322,395 participants and found that H. pylori infection was independently associated with colorectal AP, advanced AP, HP but not with SSP. Although large-scale prospective cohort studies are needed to determine whether H. pylori infection is an independent risk factor for colorectal polyps, these findings highlight the hypothesis that H. pylori infection is likely to be involved in the pathogenesis of colorectal polyp formation.
A few meta-analyses have been performed to evaluate the association between H. pylori infection and colorectal polyps. An early meta-analysis published in 2013 showed that H. pylori infection was associated with colorectal polyps. However, colorectal polyps outcomes with different histological types were combined and data derived from univariate analysis were pooled, which made it difficult to interpret the results (30). Since then, four other meta-analyses were performed to evaluate the association between H. pylori infection and AP. Although these meta-analyses consistently showed that H. pylori infection was associated with AP (OR: 1.49–2.05), all of these studies incorporated data with univariate analyses, which may confound the results (10, 31, 33, 34). To the best of our knowledge, only one previous meta-analysis that includes only studies of the East Asian population investigated the association between H. pylori infection with HP and AP separately (32). The authors showed that H. pylori infection was associated with AP but not with HP, but unfortunately, the results were also based on data of studies with univariate analysis (32). In view of the limitations of previous meta-analyses, we performed this study with an improvement of the study design. Firstly, only studies with data derived from multivariate analyses were included, which minimized the potential influences of confounding factors on the association between H. pylori infection and colorectal polyps. Secondly, the association between H. pylori infection and colorectal polyps was analyzed according to the histological types because of the different clinical and biological characteristics of the lesions. We found that H. pylori infection was independently associated with colorectal AP, advanced AP, HP but not with SSP.
The results of meta-analyses were consistent with the previous hypothesis that H. pylori infection plays important role in the progression of colorectal adenoma formation, advancing and malignant transformation. Several mechanisms may account for the role of H. pylori infection in this process. Firstly, chronic H. pylori infection causes hypergastrinemia, which leads to the proliferation of colorectal mucosa and susceptibility to carcinogenesis (42). Secondly, H. pylori infection induced gastric mucosal atrophy and decrease gastric acid secretion, which subsequently lead to dysbiosis of the gut microbiome and changes in the gut microbiome, an important pathophysiological component of colorectal mucosa carcinogenesis (43, 44). Besides, since H. pylori has been observed to reside in the colorectum and is associated with colorectal neoplasia, a direct carcinogenesis effect of H. pylori infection to colorectal mucosa may also exist (45). Future studies are needed to determine the exact mechanisms and molecular pathways involved. Moreover, data from one study showed that H. pylori infection was not associated with SSP, reflecting that the biological feature of the related risk factors for SSP is different from other colorectal polyps (46), such as AP. Although SSPs are much less common compared to AP, in view of the potential malignant transformative nature of SSP, more studies are needed to determine the risk factors of SSP (47). Currently, it remains unknown why H. pylori infection was not associated with SSP. However, previous studies showed a few epidemiological and biological differences between SSP and other forms of colorectal polyps (48). For example, SSP with dysplasia or invasive carcinoma was associated with advanced age, female sex, and proximal colon (48). However, it has been confirmed that male sex is an independent risk factor for H. pylori infection (49), while women are less likely to have H. pylori infection as compared with men. Future studies are needed to validate whether H. pylori infection is not associated with the formation of SSP.
This study also has limitations. Firstly, significant heterogeneity existed among the included studies. Results of sensitivity analysis showed that the heterogeneity for the outcomes of overall colorectal polyps, AP, and HP was significantly reduced after excluding the study by Wang et al. (28), suggesting that this study may be a major determinant of heterogeneity. In particular, the prevalence of colorectal polyps was highest in the HP negative group in the study by Wang et al. (28) among the included studies, suggesting that patients in this study may have a higher risk for colorectal polyps as compared to those in other studies, which lead to the significant heterogeneity. Besides, the meta-analysis was based on data from the study level but not from individual patients, which prevented further analyses on the influence of patient characteristics on the outcome, such as the ethnicity, age, sex, and comorbidities of the participants. In addition, diagnosis of H. pylori infection varied among the included studies, and the association between H. pylori infection with colorectal polyps according to the different H. pylori infection diagnostic strategies remains unknown. Moreover, although only studies with multivariate analyses were included, we were unable to exclude the possibility that there may be residual factors that may still confound the association. Furthermore, studies evaluating the association between H. pylori infection with advanced AP, HP, and SSP are limited. Therefore, the results for these outcomes should be validated in future studies. Finally, only case-control and cross-sectional studies were included, and no prospective cohort studies are available regarding the association between H. pylori infection and colorectal polyps. Accordingly, it is still unknown whether H. pylori infection is an independent risk factor for colorectal polyps.
In conclusion, this meta-analysis showed that H. pylori infection is independently associated with colorectal polyps, and the associations are consistent for AP, advanced AP, HP but not for SSP. Large-scale prospective cohort studies are warranted to determine whether H. pylori infection is an independent risk factor for colorectal polyps.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DLu and QizW designed the study. DLu and MiW performed literature search, study identification, data extraction, and study quality evaluation. DLu, XK, QiaW, JW, DLi, and MeW performed statistical analyses and interpreted the results. DLu drafted the manuscript. All authors revised the manuscript and approved the submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Herszenyi L. The “Difficult” colorectal polyps and adenomas: practical aspects. Dig Dis. (2019) 37:394–9. doi: 10.1159/000495694
2. Oines M, Helsingen LM, Bretthauer M, Emilsson L. Epidemiology and risk factors of colorectal polyps. Best Pract Res Clin Gastroenterol. (2017) 31:419–24. doi: 10.1016/j.bpg.2017.06.004
3. Pickhardt PJ, Pooler BD, Kim DH, Hassan C, Matkowskyj KA, Halberg RB. The natural history of colorectal polyps: overview of predictive static and dynamic features. Gastroenterol Clin North Am. (2018) 47:515–36. doi: 10.1016/j.gtc.2018.04.004
4. Pai RK, Bettington M, Srivastava A, Rosty C. An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas. Mod Pathol. (2019) 32:1390–415. doi: 10.1038/s41379-019-0280-2
5. Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. (2016) 11:967–76. doi: 10.2147/CIA.S109285
6. Santos MLC, de Brito BB, da Silva FAF, Sampaio MM, Marques HS, Oliveira ESN, et al. Helicobacter pylori infection: Beyond gastric manifestations. World J Gastroenterol. (2020) 26:4076–93. doi: 10.3748/wjg.v26.i28.4076
7. Wroblewski LE, Peek RM. Helicobacter pylori, Cancer, and the gastric microbiota. Adv Exp Med Biol. (2016) 908:393–408. doi: 10.1007/978-3-319-41388-4_19
8. Leja M, Grinberga-Derica I, Bilgilier C, Steininger C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter. (2019) 24(suppl. 1):e12635. doi: 10.1111/hel.12635
9. Eshraghian A. Epidemiology of Helicobacter pylori infection among the healthy population in Iran and countries of the Eastern Mediterranean Region: a systematic review of prevalence and risk factors. World J Gastroenterol. (2014) 20:17618–25. doi: 10.3748/wjg.v20.i46.17618
10. Choi DS, Seo SI, Shin WG, Park CH. Risk for colorectal neoplasia in patients with Helicobacter pylori infection: a systematic review and meta-analysis. Clin Transl Gastroenterol. (2020) 11:e00127. doi: 10.14309/ctg.0000000000000127
11. Ryoo SK, Kim TJ, Kim ER, Hong SN, Kim YH, Chang DK. Helicobacter pylori infection and the development of advanced colorectal neoplasia. J Clin Gastroenterol. (2020) 54:696–700. doi: 10.1097/MCG.0000000000001273
12. Qing Y, Wang M, Lin YM, Wu D, Zhu JY, Gao L, et al. Correlation between Helicobacter pylori-associated gastric diseases and colorectal neoplasia. World J Gastroenterol. (2016) 22:4576–84. doi: 10.3748/wjg.v22.i18.4576
13. Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, et al. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion. (1999) 60:210–5. doi: 10.1159/000007661
14. Siddheshwar RK, Muhammad KB, Gray JC, Kelly SB. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol. (2001) 96:84–8. doi: 10.1111/j.1572-0241.2001.03355.x
15. Fujimori S, Kishida T, Kobayashi T, Sekita Y, Seo T, Nagata K, et al. Helicobacter pylori infection increases the risk of colorectal adenoma and adenocarcinoma, especially in women. J Gastroenterol. (2005) 40:887–93. doi: 10.1007/s00535-005-1649-1
16. Bae RC, Jeon SW, Cho HJ, Jung MK, Kweon YO, Kim SK. Gastric dysplasia may be an independent risk factor of an advanced colorectal neoplasm. World J Gastroenterol. (2009) 15:5722–6. doi: 10.3748/wjg.15.5722
17. Inoue I, Mukoubayashi C, Yoshimura N, Niwa T, Deguchi H, Watanabe M, et al. Elevated risk of colorectal adenoma with Helicobacter pylori-related chronic gastritis: a population-based case-control study. Int J Cancer. (2011) 129:2704–11. doi: 10.1002/ijc.25931
18. Hong SN, Lee SM, Kim JH, Lee TY, Choe WH, Lee SY, et al. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci. (2012) 57:2184–94. doi: 10.1007/s10620-012-2245-x
19. Nam KW, Baeg MK, Kwon JH, Cho SH, Na SJ, Choi MG. Helicobacter pylori seropositivity is positively associated with colorectal neoplasms. Korean J Gastroenterol. (2013) 61:259–64. doi: 10.4166/kjg.2013.61.5.259
20. Brim H, Zahaf M, Laiyemo AO, Nouraie M, Perez-Perez GI, Smoot DT, et al. Gastric Helicobacter pylori infection associates with an increased risk of colorectal polyps in African Americans. BMC Cancer. (2014) 14:296. doi: 10.1186/1471-2407-14-296
21. Patel S, Lipka S, Shen H, Barnowsky A, Silpe J, Mosdale J, et al. The association of H. pylori and colorectal adenoma: does it exist in the US Hispanic population? J Gastrointest Oncol. (2014) 5:463–8. doi: 10.3978/j.issn.2078-6891.2014.074
22. Tongtawee T, Kaewpitoon S, Kaewpitoon N, Dechsukhum C, Leeanansaksiri W, Loyd RA, et al.Helicobacter pylori associated gastritis increases risk of colorectal polyps: a hospital based-cross-sectional study in Nakhon Ratchasima Province, Northeastern Thailand. Asian Pac J Cancer Prev. (2016) 17:341–5. doi: 10.7314/APJCP.2016.17.1.341
23. Nam JH, Hong CW, Kim BC, Shin A, Ryu KH, Park BJ, et al. Helicobacter pylori infection is an independent risk factor for colonic adenomatous neoplasms. Cancer Causes Control. (2017) 28:107–15. doi: 10.1007/s10552-016-0839-x
24. Chen C, Mao Y, Du J, Xu Y, Zhu Z, Cao H. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol. (2019) 19:14. doi: 10.1186/s12876-018-0918-4
25. Huang L, Wu L, Qiao Q, Fang L. Correlation between colon polyps and metabolic syndrome and HP infection status. Gastroenterol Res Pract. (2019) 2019:3916154. doi: 10.1155/2019/3916154
26. Yang W, Yang X. Association between Helicobacter pylori infection and colorectal adenomatous polyps. Gastroenterol Res Pract. (2019) 2019:7480620. doi: 10.1155/2019/7480620
27. Sonnenberg A, Turner KO, Genta RM. Associations between gastric histopathology and the occurrence of colonic polyps. Colorectal Dis. (2020) 22:814–7. doi: 10.1111/codi.14968
28. Wang M, Kong WJ, Zhang JZ, Lu JJ, Hui WJ, Liu WD, et al. Association of Helicobacter pylori infection with colorectal polyps and malignancy in China. World J Gastrointest Oncol. (2020) 12:582–91. doi: 10.4251/wjgo.v12.i5.582
29. Zhao XX, Liu MH, Wang RL, Tian T. Effect of gender and age on the correlation between Helicobacter pylori and colorectal adenomatous polyps in a Chinese Urban Population: A Single Center Study. Gastroenterol Res Pract. (2020) 2020:8596038. doi: 10.1155/2020/8596038
30. Rokkas T, Sechopoulos P, Pistiolas D, Kothonas F, Margantinis G, Koukoulis G. The relationship of Helicobacter pylori infection and colon neoplasia, on the basis of meta-analysis. Eur J Gastroenterol Hepatol. (2013) 25:1286–94. doi: 10.1097/MEG.0b013e328363d3cd
31. Wu Q, Yang ZP, Xu P, Gao LC, Fan DM. Association between Helicobacter pylori infection and the risk of colorectal neoplasia: a systematic review and meta-analysis. Colorectal Dis. (2013) 15:e352–64. doi: 10.1111/codi.12284
32. Guo Y, Li HY. Association between Helicobacter pylori infection and colorectal neoplasm risk: a meta-analysis based on East Asian population. J Cancer Res Ther. (2014) 10:263–6. doi: 10.4103/0973-1482.151482
33. Wang F, Sun MY, Shi SL, Lv ZS. Helicobacter pylori infection and normal colorectal mucosa-adenomatous polyp-adenocarcinoma sequence: a meta-analysis of 27 case-control studies. Colorectal Dis. (2014) 16:246–52. doi: 10.1111/codi.12290
34. Wang JL, Liang X, Xu J, Chen YX, Fang JY. Helicobacter pylori infection increases the risk of colorectal adenomas: an updated meta-analysis. Clin Lab. (2018) 64:1163–70. doi: 10.7754/Clin.Lab.2018.180115
35. Papastergiou V, Karatapanis S, Georgopoulos SD. Helicobacter pylori and colorectal neoplasia: Is there a causal link? World J Gastroenterol. (2016) 22:649–58. doi: 10.3748/wjg.v22.i2.649
36. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
37. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. London (2011).
38. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-Analyses. (2010). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
39. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
40. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
41. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
42. Hartwich A, Konturek SJ, Pierzchalski P, Zuchowicz M, Labza H, Konturek PC, et al. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Colorectal Dis. (2001) 16:202–10. doi: 10.1007/s003840100288
43. Lam SY, Yu J, Wong SH, Peppelenbosch MP, Fuhler GM. The gastrointestinal microbiota and its role in oncogenesis. Best Pract Res Clin Gastroenterol. (2017) 31:607–18. doi: 10.1016/j.bpg.2017.09.010
44. Park CH, Eun CS, Han DS. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest Res. (2018) 16:338–45. doi: 10.5217/ir.2018.16.3.338
45. Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol. (2007) 5:51. doi: 10.1186/1477-7819-5-51
46. Meester RGS, van Herk M, Lansdorp-Vogelaar I, Ladabaum U. Prevalence and clinical features of sessile serrated polyps: a systematic review. Gastroenterology. (2020) 159:105–18 e25. doi: 10.1053/j.gastro.2020.03.025
47. Rashtak S, Rego R, Sweetser SR, Sinicrope FA. Sessile serrated polyps and colon cancer prevention. Cancer Prev Res (Phila). (2017) 10:270–8. doi: 10.1158/1940-6207.CAPR-16-0264
48. Murakami T, Sakamoto N, Nagahara A. Clinicopathological features, diagnosis, and treatment of sessile serrated adenoma/polyp with dysplasia/carcinoma. J Gastroenterol Hepatol. (2019) 34:1685–95. doi: 10.1111/jgh.14752
Keywords: H. pylori infection, colorectal polyps, adenomatous polyps, hyperplastic polyps, meta-analysis
Citation: Lu D, Wang M, Ke X, Wang Q, Wang J, Li D, Wang M and Wang Q (2022) Association Between H. pylori Infection and Colorectal Polyps: A Meta-Analysis of Observational Studies. Front. Med. 8:706036. doi: 10.3389/fmed.2021.706036
Received: 06 May 2021; Accepted: 20 December 2021;
Published: 18 January 2022.
Edited by:
Ari Fahrial Syam, University of Indonesia, IndonesiaReviewed by:
Amin Talebi Bezmin Abadi, Tarbiat Modares University, IranYaobin Ouyang, First Affiliated Hospital of Nanchang University, China
Copyright © 2022 Lu, Wang, Ke, Wang, Wang, Li, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qizhi Wang, d2FuZ3F6MjAwNEBzaW5hLmNvbQ==
 Depeng Lu1
Depeng Lu1 Qizhi Wang
Qizhi Wang