- 1Woman's Health Sciences Department, Gynecologic Section, Polytechnic University of Marche, Ancona, Italy
- 2Gynecological Oncology Unit, Istituto di Ricovero e Cura a Carattere Scientifico Centro di Riferimento Oncologico (IRCCS CRO) Centro di Riferimento Oncologico – National Cancer Institute, Aviano, Italy
A comprehensive literature review was performed to determine the relationship between HPV infection and infertility and the eventual role of the 9-valent vaccine for infertility prevention. The search was extended from January 1997 through July 2021. Data collected from selected articles focused on three main topics: statistical associations between HPV prevalence and assisted reproductive technology (ART) outcome, association between HPV and characteristics of semen, and associations between HPV and miscarriage. Articles that identified HPV genotypes were selected for this review to study the possible role of the 9-valent vaccine in infertility prevention. To date, there is no agreement on the implication HPV female infection has on the fertility and miscarriage rate. Although it can be stated that HPV prevalence among couples with infertility undergoing ART treatment is consistent, it does not seem to affect the performance of oocytes. Otherwise, HPV infection affects sperm parameters, in particular spermatozoa motility. When an association can be found, most cases of HR-HPV involved are those included in the 9-valent vaccine. The correlation between HPV male infection both with asthenozoospermia and increased risk of pregnancy loss could recommend the extension of anti-HPV vaccination to adolescent males along with cancer prevention. Despite the fact that the relation between 9-valent HPV genotypes involved in female infection and miscarriage/infertility is not clear, the impact of this virus on health reproduction is evident. Considering this, the importance of HPV vaccination in adolescent females is confirmed. A vaccine efficacy study could be useful to confirm the importance of primary prevention for couple reproductive health.
Introduction
Infertility is a complex human health situation that particularly alters the quality of life in couples that face it. It represents a worldwide problem that affects about 10–30% of couples of reproductive ages (1, 2). The American College of Obstetricians and Gynaecologists (ACOG) defines infertility as the failure to achieve pregnancy within 12 months of unprotected intercourse or therapeutic donor insemination in women younger than 35 years or within 6 months in women older than 35 years (3).
Worldwide, the primary cause of infertility is sexually transmitted diseases (STDs) (4, 5). The role of Chlamydia trachomatis and Neisseria gonorrhoeae infection in salpingitis and infertility has been widely studied as well as the possible damage caused by Trichomonas vaginalis, Mycoplasma genitalium, and other microorganisms within the vaginal microbiome. While early diagnosis and treatment for C. trachomatis and N. gonorrhoeae have been developed for the prevention of pelvic inflammatory disease (PID) and subsequent tubal factor infertility (TFI), additional data are needed to determine whether early detection of other potential pathogens can reduce the incidence of TFI (6).
One of those is human papillomavirus (HPV). HPV infections are significantly associated with cancer of the male and female anogenital mucosa (7, 8) and some adverse effects on reproductive functions (9, 10). Most of these consequences are caused by the inability of the immune system to spontaneously clear HPV: in particular, high-risk HPV (HR-HPV) types are more likely to persist than low-risk HPV types (11–13). The infection is often asymptomatic, and most of the time, people are infected without being aware.
Despite genital HPV infection being the most common sexually transmitted viral infection worldwide, with an estimated overall prevalence of 10% in the general female population during reproductive age (14, 15), few studies have investigated the effect of HPV infection on human reproduction.
Research over the last few years has been carried out to understand if HPV could be an etiological agent that determines infertility or miscarriage and if assisted reproductive technology (ART) requires specific management for HPV-positive patients (9).
If this correlation were real, HPV prevention would play a dual role, not only for cancer but also for infertility. However, in order to perform efficacious prevention on infertility and adequate counseling in infertile couples, it is important to assess which HPV type could cause infertility or miscarriage. Identifying the prevalent genotypes could be important also to evaluate the possible role of the vaccine in preventing HPV-related infertility. In fact, it has not been studied yet if one of the three disposable HPV vaccines could significantly provide protection against infertility as well as neoplastic diseases.
The purpose of this comprehensive review is to research the available knowledge regarding the implication of HPV genotypes included in the 9-valent vaccine HR-HPV 16, 18, 31, 33, 45, 52, and 58 and low-risk HPV (LR-HPV) 6 and 11 in couple infertility with the aim to hypothesize whether vaccination could have a protective role for reproductive health.
Methods
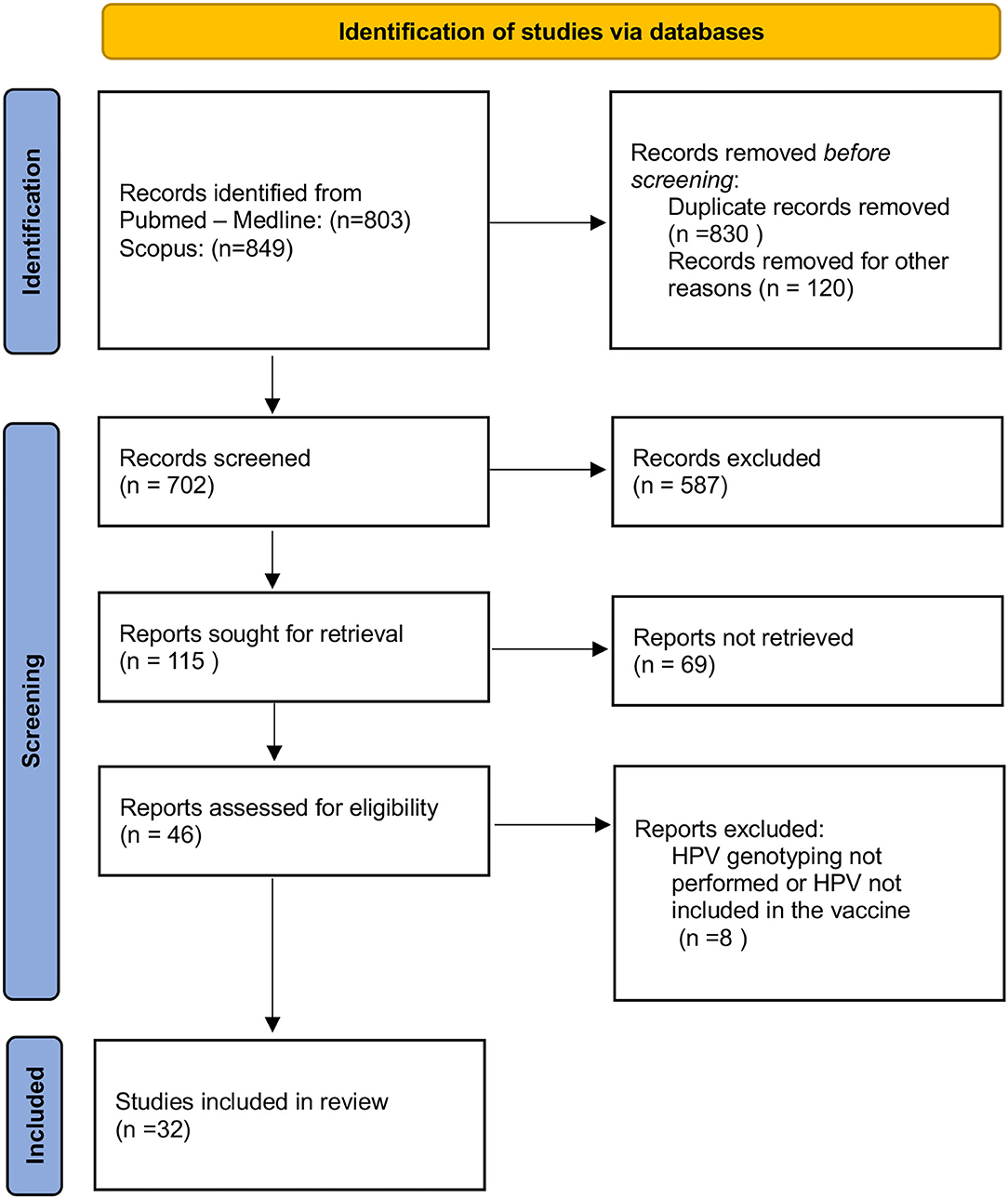
A comprehensive literature search was performed from January 1997 through July 2021 (Figure 1). The following Mesh terms were searched: human papillomavirus, fertility, infertility, miscarriage, in vitro fertilization (IVF), assisted reproductive technology (ART), sperm, and blastocyst in PubMed/Medline (all fields) (last accessed on July 5, 2021) and Scopus (Title/Abstract/Keywords) (last accessed on July 5, 2021) databases. Relevant articles were selected for full-text reading and were restricted to those in the English language. Titles and abstracts were screened for relevance to determine which articles were to undergo full-text review. Articles identified as potentially relevant moved into a full-text review. The references of included studies were reviewed to identify additional publications not found through the database search.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed to systematically review the literature by searching the PubMed/Medline and Scopus database. The present study assessed the following PICOS (Population, Intervention, Comparison, Outcomes) questions:
• Population: couples, men, or women affected by infertility with HPV infection;
• Intervention: observe data on IVF cycles, sperm analysis, ART, and pregnancy (miscarriage) outcomes;
• Comparison: no comparisons are expected;
• Outcomes: (1) prevalence of HPV genotype infection, with particular reference to those included in the 9-valent vaccine; (2) ART, semen parameters, and pregnancy outcomes.
Study Design
Review and meta-analysis, prospective and retrospective observational studies, population-based cohort study, prospective cohort study, case-control study, and cross-sectional clinical study.
Eligibility/Inclusion Criteria
Retrospective as well as prospective studies that present results from studies on population-based correlations between human reproductive health and HPV. The search also retrieved articles that are based on in vitro experimentations on mice embryos to explain the effect of HPV infection on reproductive health (16, 17). Articles refer to almost one HPV genotype included in the 9-valent vaccine.
Exclusion Criteria
Articles referring to HPV genotypes other than those not included in 9-valent HPV vaccines (or unspecified HPV genotypes); cases in a non-English language.
Information Sources and Search Strategy
We searched for (HPV) AND (infertility OR fertility OR miscarriage OR in vitro fertilization—IVF OR assisted reproductive technology—ART OR sperm OR blastocyst) in PubMed (all fields), Medline (all fields) (accessed on July 5, 2021), and Scopus (Title/Abstract/Keywords) (accessed on July 5, 2021) databases. The only filter used was the English language. Relevant articles were obtained in full-text format and screened for additional references.
Study Selection
An independent reviewer (C. M.) selected the studies using a two-step screening method. At first, the screening of titles and abstracts was performed to assess eligibility and inclusion criteria and exclude non-relevant studies. Afterward, the reviewer evaluated full texts of included articles to assess study eligibility and the inclusion criteria and to avoid duplications of the included cases. Then, the same author performed a manual search of reference lists to search for additional relevant publications. J. D. G. and G. D. C. checked the data extracted.
The objective of this review was to determine the relationship between HPV infection and infertility and the eventual role of the 9-valent vaccine for infertility prevention.
Data Collection Process/Data Items
Data collection was study-related (authors and year of study publication) and case-related (HPV genotypes, IVF and pregnancy outcomes, and sperm characteristics).
The data collected from selected articles focused on three main topics:
• statistical associations between HPV genotypes included in the 9-valent vaccine and ART outcome;
• associations between HPV genotypes included in the 9-valent vaccine and characteristics of semen, focusing also in HPV genotype; and
• associations between HPV genotypes included in the 9-valent vaccine and miscarriage.
Statistical Analysis
No statistical analysis was performed.
Results
Many research teams were interested in the possible role of HPV infection in couple infertility. Articles were mainly focused on HPV infection and its relationship with infertility, semen parameters, and IVF outcomes. Only some authors discussed the relationship between HPV and fertility parameters specifying the HPV types or risks.
The selected articles concerning HPV genotypes included in the 9-valent vaccine were as follows:
• 10 of 14 articles focused on the effect of HPV on in vitro fertilization outcomes;
• 9 of 11 articles studied the effect of HPV on semen parameters; and
• 13 of 15 articles studied the effect of HPV infection on the risk of miscarriage.
Prevalence of HPV Genotypes Included in the 9-Valent Vaccine in Infertile Women and ART Success
Studies examining the impact of HPV genotypes on female fertility are limited. Most studies investigate the prevalence of HPV-positive women in ART programs or the connection between HPV infection and ART success. Table 1 summarizes the literature regarding the effect of HPV on infertility and on assisted reproductive outcomes.
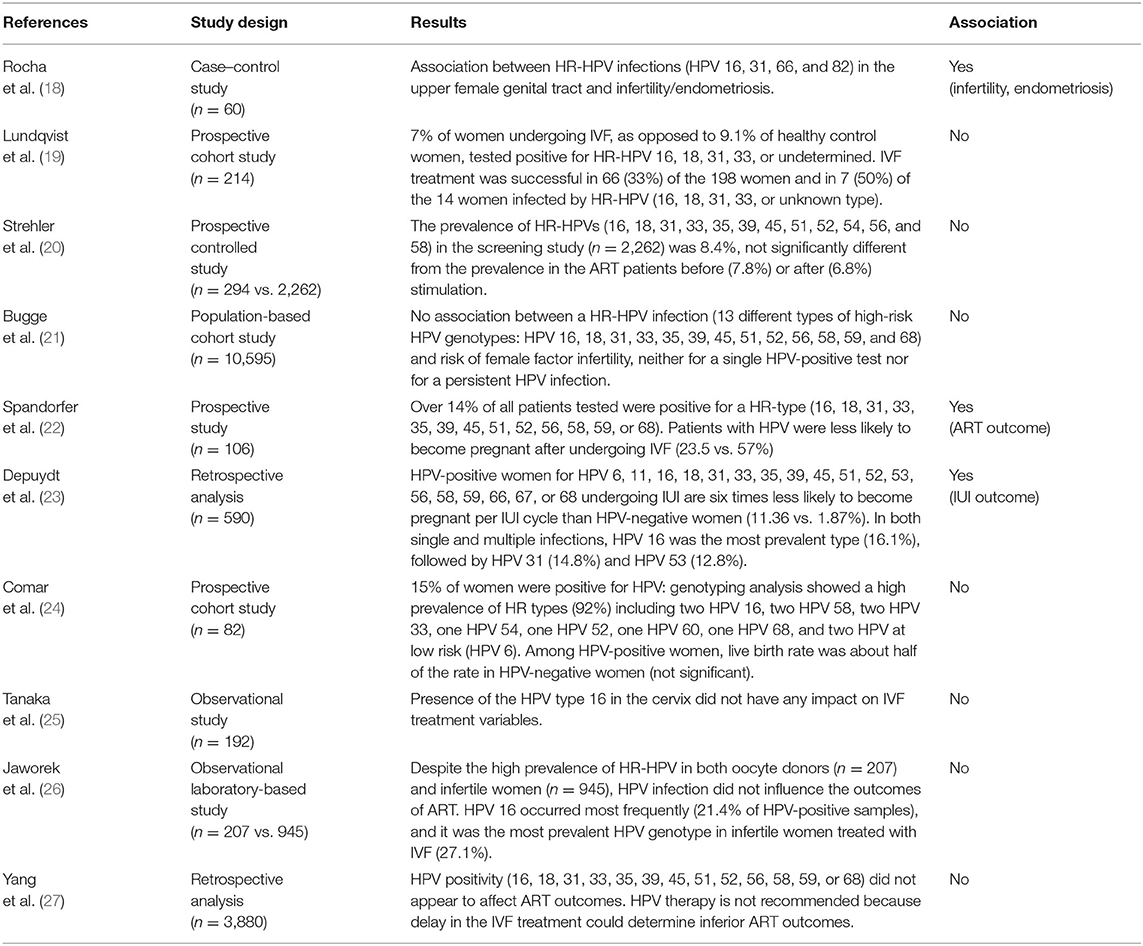
Table 1. Literature regarding the effect of HPV included in the 9-valent vaccine on infertility and assisted reproductive outcome.
Some studies examine the association between HPV infection and infertility. Recently, it has been underlined by Rocha et al. that many high-risk HPV infections included in the 9-valent vaccine are associated with infertility and endometriosis (18). Cobas 4800 HPV testing was used to detect HPV in three separate channels that discriminate HPV 16 individually, HPV 18 individually, and a pool of 12 other HR-HPV types (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68). In addition, it was observed that HR-HPV 16, 31, 66, and 82 had an infection continuum from the lower to the upper genital tract (18).
These results contrast with the observations by Lundqvist et al. (19) that observed that 7% of women undergoing IVF as opposed to 9.1% of healthy control women tested positive for HR-HPV 16, 18, 31, 33, or undetermined. Also, Strehler et al. (20) did not find a higher incidence of HPV infection in the infertile population (n = 294, age range 22–45) compared to the general population (n = 2,262, age-matched). Finally, Bugge et al. (21) did not find an association between HR-HPV infection (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and infertility.
In the prospective study of Spandorfer et al. (22), evaluating the prevalence of HPV in women undergoing IVF in North America, women with a cervical HR-HPV infection (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, or 68) had a significantly lower number of pregnancies after ART than women with negative HPV results (23 vs. 57%). The authors found no association between HPV and atypical responses to ovarian stimulation and between HPV and tubal infertility. Overall, no differences were noted in the etiology of infertility comparing HPV-positive and HPV-negative groups. Concerning the mean age, the number of oocytes retrieved, the number of embryos transferred, and the embryo quality, no differences were found between the two groups. The need for intracytoplasmic sperm injection and the fertilization rate of mature oocytes did not differ between HPV-positive and HPV-negative women. No mechanisms linking HPV infection and negative IVF outcomes were found. However, the pregnancy rate in HPV-positive women was less than half of that observed in patients of similar age with a negative result following HPV screening.
A recent study by Depuydt et al. (23) investigated the impact of HPV on intrauterine insemination (IUI) outcome, reporting that women suffering from an HPV infection undergoing IUI treatments have lower chances to become pregnant. More in detail, among 590 patients undergoing 1,529 IUI cycles, those affected by HPV had a six times lower chance to achieve pregnancy (1.87%) compared to women not infected by HPV (11.4%). HPV 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, and 68 were tested. Low-risk HPV types were not detected. In single-type HPV infection, the most prevalent type was HPV 53 (20.5%), followed by HPV 66 (19.3%) and HPV 16 (13.3%). In infections with multiple types, the more prevalent were HPV 16 and 31 (19.7%). Overall, in both single and multiple infections, the most prevalent type was HPV 16 (16.1%) followed by HPV 31 (14.8%) and HPV 53 (12.8%). Among these, HPV 16 and 31 are included in the 9-valent vaccine. It is interesting that most women (97.3%) who underwent IUI were not vaccinated.
Comar et al. (24) found no significant differences in ovarian response and implantation rate comparing HPV-positive women to HPV-negative ones. Also, the miscarriage rate was not significantly different. However, the live birth rate was about double among HPV-negative women when compared with HPV-positive women, although the difference was not significant probably because of the low number of cases. HPV 16, 58, 33, and 6 found in infertile women are included in the 9-valent vaccine.
An investigation in Japan of 192 women undergoing IVF (25) observed that there was no relationship between HPV 16 detection and any IVF treatment variable, including the cause of infertility or IVF outcome.
Javorek et al. (26) and Yang (27) found no associations between HPV included in the 9-valent vaccine and ART outcomes despite the high prevalence of HR-RPV in both oocyte donors and infertile women. Moreover, Javorek (26) reported that there was no association between HR-HPV-positive status and abortion rate in spontaneously pregnant women.
HPV Included in the 9-Valent Vaccine Genotypes and Semen Alterations
Table 2 summarizes the literature regarding the effect of HPV on seminal liquid.
All studies related to the correlation between male fertility and HPV, including also those excluded because the HPV genotype was not specified, reporting a clear correlation between HPV and asthenospermia and infertility.
Some authors decided to examine the result for specific genotypes. Lai et al. (28) found an association between HPV and oligoasthenozoospermia, but, when considering oligozoospermia, it was found in 33 and 18% of sperm specimens that were positive respectively for HPV 16 and 18. Similarly, they investigated the correlation between genotypes 16 and 18 and asthenozoospermia, finding out that it was present in 83% of men with HPV 16 infection and in 73% of patients with HPV 18 infection.
In addition, Bezold et al. (29) wanted to clarify the percentage of infertile men with HPV 16 infection, finding a prevalence of 37.5%. Moreover, it is interesting to note that the test used to search HPV DNA in that study identified almost all the HR-HPV and all the genotypes included in the 9-valent vaccine.
Similar results have been found by Foresta et al. (30) and Garolla et al. (35): the searched genotypes are present in the 9-valent vaccine. Moreover, Foresta found a relevant percentage of cases (10.2%) positive for HPV 16. In a recent study conducted by Moghimi et al. (36), all the HR-HPV types present in the 9-valent vaccine were detected in the positive population. In another study, Foresta et al. (31) used a more specific test identifying fewer HPV genotypes (6, 16, 18, 53, 58, 59, 61, 62, 66, and 70); however, they wanted to clarify that 4% were HPV 16 and HPV 18 positive, while 4% were HPV 6 positive. In a more recent study (30), the authors specified what genotype was found in order of prevalence: HPV genotypes related to infertility were 6, 53, 18, 16/90, 84, and 61/62. Yang et al. (33) reported that the most common HPV types in decreasing order of prevalence in infertile men were HPV 45, 16, 52, 18/59, and 33; in fertile patients, the most common genotypes significantly differ: HPV-68/81, −33 and −39. A study published in 2013 (34) revealed that 90% of infertile patients were affected by HR-HPV infection. Another study wanted to determine the HR-HPV infection rate among couples undergoing IVF, revealing that HPV 52 was the most commonly found in the case of semen alteration (37), while other studies that specifically targeted oncogenic genotypes found HPV 52 (38) and HPV 16 (39) as the most common genotype overall in semen samples. Other researchers (11, 26) reported that HPV 16, HPV 51, HPV 52, and HPV 45 are often found in semen.
HPV Genotypes Included in the 9-Valent Vaccine Infection and Risk of Miscarriage
Table 3 summarizes the literature regarding the effect of HPV genotypes included in the 9-valent vaccine on the risk of miscarriage: a possible association is reported in a few studies (38, 40, 41) while many articles reported no association with miscarriage (16, 17, 22–24, 26, 42–45).
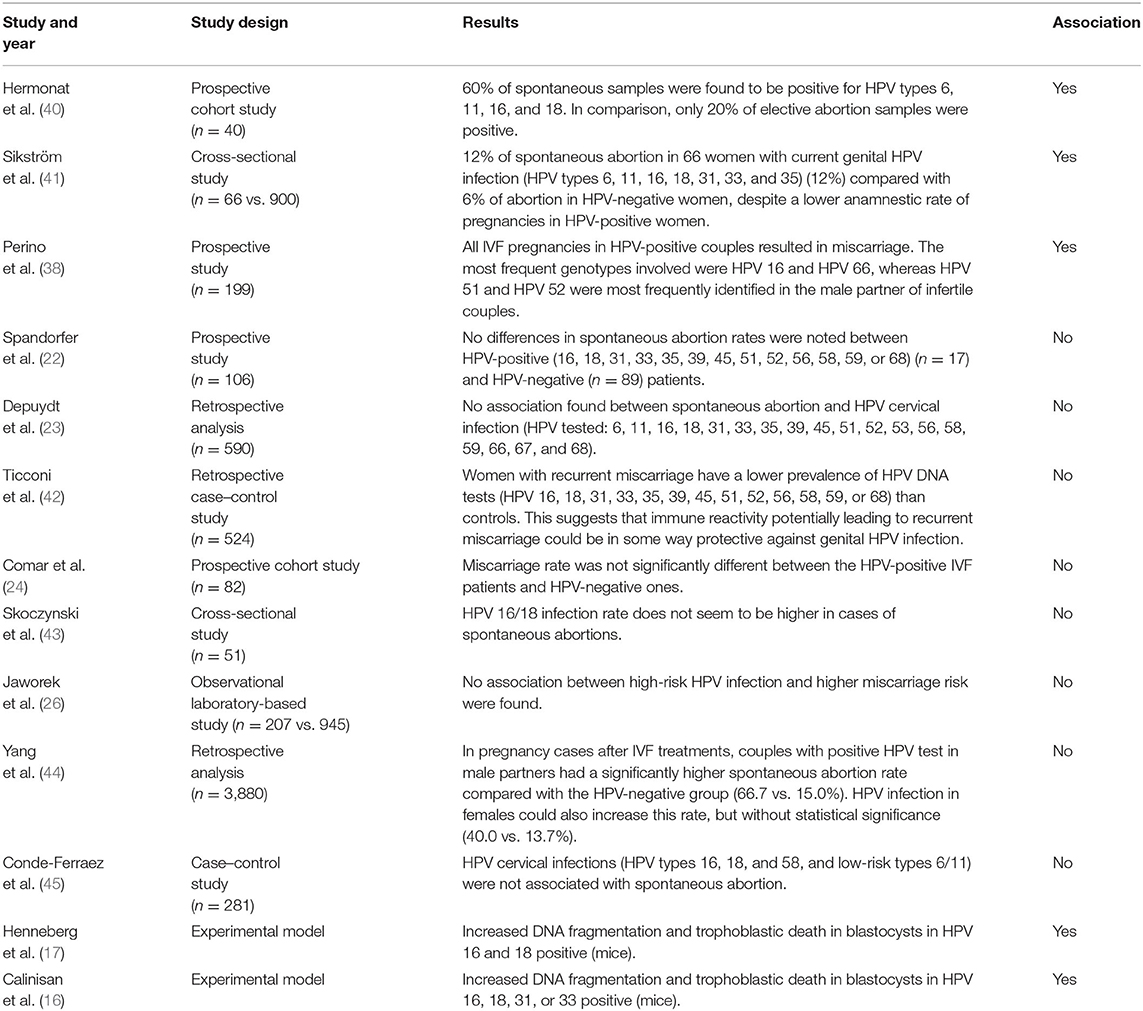
Table 3. Literature regarding the effect of HPV genotypes included in the 9-valent vaccine on the risk of miscarriage.
As early as 1997 (40), it has been shown that HPV infection (types 6, 11, 16, and 18) was three times more prevalent in spontaneous abortion specimens compared with elective ones (60 vs. 20%, respectively) opening the door to the hypothesis that HPV could be an etiologic agent of some miscarriages. Moreover, it was demonstrated that these viruses might be closely linked to fetal pathology.
In 1995, a study by Sikström et al. (41) recorded a two-fold increase in the rate of spontaneous abortion in 66 women with current genital HPV (types 6, 11, 16, 18, 31, 33, and 35) infection (12%) compared with 900 HPV-negative women (6%), despite a lower anamnestic rate of pregnancies in HPV-positive women.
Perino et al. (38) stated that couples who underwent ART cycles experienced an increased risk of pregnancy loss when HPV DNA testing was positive in the male partner, compared with non-infected patients (66.7–15%, p < 0.001). Moreover, all pregnancies in HPV-positive couples resulted in miscarriage, whereas there was a 15.9% overall miscarriage rate in HPV-negative couples (p < 0.001). The most frequent genotypes in women were HPV 16 and HPV 66, whereas HPV 51 and HPV 52 were the most frequently identified in the male partner of infertile couples. HPV 16 and 52 are included in the 9-valent vaccine showing protection for women, male partners, and infertile couples.
A study in 2013 (42) stated that women with recurrent abortion have a lower prevalence of HPV DNA test positivity than controls, suggesting that immune reactivity potentially leading to recurrent miscarriage could be protective against genital HPV infection. However, the study was confined to women with recurrent miscarriages. Other researchers claimed that there is no correlation between women's HPV infection and miscarriage rate at all (24, 26, 27).
Discussion
Infertility affects millions of people, about 10–30% of reproductive-age couples all over the world (1, 2). At the same time, HPV infection is one of the most common STD with high prevalence in some developing and underdeveloped countries, and its consequences cannot be underestimated (4, 5). HPV vaccination remains unavailable in many countries due to economic issues and low public acceptance. The purpose of this comprehensive review is to research the available knowledge regarding the implication of HPV genotypes included in the 9-valent vaccine HR-HPV 16, 18, 31, 33, 45, 52, and 58 and low-risk HPV (LR-HPV) 6 and 11 in couple infertility, with the aim to hypothesize if vaccination could have a protective role for reproductive health, eventually improving vaccine acceptance.
It is interesting to note that many studies reported the involvement of genotypes not included in the 9-valent vaccine. These HPV genotypes may have a role in infertility though they are not yet part of the ones contained in the vaccine. Moreover, the list of known HR-HPVs could change in the next years as the genotypes contained in the vaccine. Arbyn et al. (46, 47) suggested increasing the HR-HPVs in the list from 14 up to 20 due to the growing carcinogenicity of potential/possible risk types. The phenomenon of growing carcinogenicity, including the ability of constant evolution of HPV types, should be considered (48). In IVF patients, a Pap smear is required before the stimulation cycle. In case of abnormal results, further analysis must be conducted, including a high-risk HPV test and/or colposcopy and pathology biopsy. Patients with cervical pathology higher than cervical intraepithelial neoplasia-1 (CIN-1) cannot be enrolled in IVF cycles.
Studies that evaluated the impact of HPV on ART outcomes have shown conflicting results. Most studies did not find a higher incidence of high-risk HPV infections included in the 9-valent vaccine in the infertile population (19–21, 24–27). However, we noticed some limitations of these studies. Lundqvist et al. (19) did not apply age limits: the case and control groups were 20–40 and 25–59 years old, respectively. Moreover, both case and control groups were substantially smaller compared with the previously cited study (18). These criticisms cannot be applied to the study of Strehler et al. (20). However, it is interesting to note that all patients with prior cervical surgery, cervical dysplasia, or a HR-HPV infection were excluded from the study group. Bugge et al. (21) found that the prevalence of high-grade cervical lesions was twice as high in women with infertility compared with the general population, implying a more complex association between HPV and infertility. The study of Tanaka et al. (25) was not conducted on a large population (10 women and 4 men were found to be HPV type 16 DNA positive). Finally, Javorek et al. (26) found that HPV 16 occurred most frequently (21.4% of HPV-positive samples) in infertile women treated with IVF, and it was the most prevalent HPV genotype (27.1%, 13/48).
One of the most quoted articles on the subject is from Spandorfer et al. (22), finding a significantly lower number of pregnancies after ART in women with HPV infection than in women with negative HPV results. The presence of high-risk HPV types probably indicates that these patients are unable to spontaneously clear these viruses. They speculated that immunologic conditions that reduce the likelihood of spontaneous HPV clearance also may limit the likelihood of embryo implantation. In fact, an innate or acquired decrease in the ability to generate the production of high levels of proinflammatory cytokines would explain the linking between HPV infection and lower success of IVF. In addition, Depuydt et al. (23) reported a connection between HPV infection and infertility, but the population study was limited to women who underwent the IUI technique.
Also, Rocha et al. (18) reported an association between HR-HPV infertility and infections, observing an association with endometriosis. Three previous studies have evaluated the possible correlation between endometriosis and HPV (49–51). Two of these (49, 51) highlighted the importance of the high-risk HPV infection from the lower genital tract to the upper genital tract sites. More specifically, the association between endometriosis and viral STD infections has been investigated by Oppelt et al. (49) using PCR-based enzyme-linked immunosorbent assay in 66 tissues, including peritoneum, endometrium, and ovary. HR-HPV included in the 9-valent vaccine (16 or 18, and possibly one among HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, or 68) were detected in 11.3 and 27.5% of lesions in the case and control groups, respectively. Heidarpour et al. (51) found an association between the presence of HR-HPV types (16, 18, 31, 33, 35, 39, 45, 52, 56, 58, and 59) in the upper genital tract and infertility, primarily caused by endometriosis. Conversely, Vestergaard et al. (50) searched the presence of HPV in endometriotic samples using sensitive PCR tests, finding a low prevalence of HPV 35, 68, 70, and 90 in endometriotic lesions. They concluded that HPV could not be the cause of endometriosis. However, no genotypes are included in the 9-valent vaccine.
In conclusion, the effect of HPV infection in women on ART outcome remains undefined also due to the methodological limitations of the studies performed. However, for years now, it has been known to clinicians studying reproductive medicine that the patient must always be the couple and not the individual. It is precisely in this consideration that we could attempt to explain the conflicting data. Most studies analyze the connection between HPV and infertility or ART outcome searching women HPV infection. However, taking the assumption that most couples suffering from infertility are stable couples, we should consider both partners positive for HPV regardless of whether the detection of the virus is done on a sperm or cervical swab. We should take into consideration HPV positivity not only in women but also in men. If we correlate infertility only with cervical positivity for HPV, we could not consider a large part of HPV-positive couples. On the other hand, any molecular techniques for the detection of HPV including PCR do not have a 100% sensibility. Not by chance, if we analyze the connection between ART and HPV by only studying sperm infections, the results will agree. Therefore, we should analyze the impact of HPV in infertility and ART outcomes by studying the presence of HPV not only in the female genital tract but also in the male counterpart.
In the past years, some viral diseases such as hepatitis B and HIV were widely studied in men attempting ART to reduce the risk of transmission of STDs via the seminal fluid. Nevertheless, HPV infection was not considered as well.
In the last years, there has been an increasing interest in the prevalence of HPV among men. Many studies have provided evidence of possible HPV-related subfertility in men, suggesting that HPV infection could be a risk factor (36). Indeed, HPV has been isolated in seminal samples, and its presence has been correlated with idiopathic asthenospermia (28, 31, 52, 53), sperm cell numbers (29), and semen pH (54). All studies related to the correlation between male fertility and HPV, including also those excluded because the HPV genotype was not specified, reporting a clear correlation between HPV and asthenospermia and infertility.
However, not only HPV detection but also HPV genotyping could be of great value in infertility diagnosis at least in idiopathic infertility cases. As for the risk of carcinogenesis, another classification of HPV regarding the risk of fertility alteration may be considered after in-depth investigations (10). That is why some authors decided to examine the result for specific genotypes.
It is interesting to note that many HR-HPV genotypes included in the 9-valent vaccine have a close relationship with semen alterations. Nevertheless, the role of HPV in determining couple infertility cannot be relegated to the mere damage to sperm production. Indeed, infected seminal liquid could be a carrier of HPV DNA in the reproductive tract, with the possibility of detrimental effects on oocytes during fertilization (55, 56). Men with infected sperm should be adequately counseled before an IVF treatment (32).
The results of the study conducted by Perino et al. (38) showed, for the first time, a significant increase in the risk of abortion if sperm cells of the male partner are infected by HPV. In addition, all the pregnancies achieved in couples where both partners were HPV positive resulted in a pregnancy loss.
A recent study by Garolla et al. (57) investigated 226 infertile couples, finding out that HPV-positive couples with infertility problems had a significant reduction of cumulative pregnancy rate by ART in both IUI (from 20% in HPV negative to 9.5% in HPV positive) and ICSI (from 40.8% in HPV negative to 18.2 HPV positive). However, the prevalence of HPV in couples was determined by semen analysis instead of cervical analysis, and HPV genotypes were not identified.
While it is widely recognized that spontaneous abortion can be linked to fetal genetic abnormalities or viral infections involving cytomegalovirus (58), Epstein–Barr virus (59), Herpes Simplex (60), mumps (61), and rubella (62), little is known about HPV and early embryos.
The association between HPV infection and failed placental invasion has already been widely demonstrated in studies that correlate HPV infection with spontaneous preterm delivery (63). The HPV-transfected blastocysts could have a progressive loss of invasiveness leading to spontaneous abortion, before pregnancy diagnosis. Studying the connection between HPV infection and miscarriages after pregnancy has been difficult.
Conde-Ferráez et al. (45) did not detect an association between HPV 16, 18, 58, 6, and 11 and spontaneous abortion, but they found out that HPV prevalence tends to increase during the trimesters of pregnancy. They speculated that it could be caused by elevated estrogen levels that may affect viral replication or alter immunity during pregnancy. This suggests the difficulty in finding the connection between miscarriage and the real prevalence of infection in the couple if we analyze only cervical samples. The immune capacity of resolving the HPV infection and the lack of some HPV positivity in those women who contracted the infection from their partner after the cervical withdrawal make it difficult to determine the real HPV prevalence and consequently the miscarriage correlation. In addition, Ticconi et al. (42) and Spandorfer et al. (22) suggested that immune reactivity, potentially leading to recurrent miscarriage, could be, in some way, protective against genital HPV infection.
Some studies (22, 23) did not find a correlation between HPV infection and miscarriage but noticed a higher failure of ART. Although it is very difficult to discriminate between an initial pregnancy loss and a reduced ART pregnancy rate, we could hypothesize that mechanisms involved in ART failure could be the same ones causing miscarriage in other studies.
In 1997, Hermonat et al. (40) noticed a high prevalence of HPV infection in spontaneous abortion samples; however, he did not hypothesize any etiopathogenetic cause. In the latest studies, both Sikström et al. (41) and Hermonat (40) explained the cause of a higher rate of abortion with the possible transmission of the virus to oocytes during fertilization, which affects or induces the immune system response.
A recent review of Isaguliants et al. (64) assumed that infertility may be associated with an anti-HPV immune response. Immune system activation to eliminate HPV-infected cells could cause an immune rejection of the HPV-infected embryo as a maternal graft-vs.-host disease against HPV-infected fetuses.
The same authors underline that HPV infection could arrest the aquaporin AQP8 function involved in the cleaning of excessive reactive oxygen species, causing additional oxidative stress in sperm and oocyte, resulting in additional DNA damage and apoptosis (65, 66). Moreover, HPV infection can reduce the cells' ability to repair DNA, amplifying its damage on gonadal cells (67).
In vitro experiments (17) have shown that trophoblast cells in women with HPV 16 or 18 infection have a higher chance of stage-specific maturation arrest and apoptosis and a reduced placental invasion into the uterine wall compared with control cells. The same studies showed that HPV exposure and two-cell embryo demise were associated. Furthermore, the exposure of HPV until later embryo stages causes some deleterious effects on embryos. HPV 16 decreases blastocyst formation, while HPV 18 inhibits the blastocyst hatching process (17).
In a study by Foresta et al. (68), the oocytes penetrated by transfected sperm (hamster egg penetration test) with human HPV 16 expressed the viral gene, suggesting an active transcription mechanism. To confirm these data, in vivo studies should be performed.
Although the precise pathway that the virus uses to infect sperm cells has not been identified, Perez-Andino et al. (69) showed that HPV (type 16, 18, 31, or 33) capsid binds to two distinct sites at the equatorial region of the sperm head surface. This suggests that sperm cells promote virus dispersal and mucosal penetration within the female genital tract. Many studies demonstrated that the relationship between HPV and miscarriage could be explained by the transmission of virus-destabilized genes to oocytes during fertilization, determining apoptosis of the embryo cells through DNA fragmentation (16, 17, 56). When the HPV virus infects sperm liquid, it could affect the acrosomal reaction, leading to a reduction of the acrosome capacity and functionality. Fujita et al. (70) suggested that this could be related to the presence of E6–E7-derived HPV DNA genes associated with cell transformation. In particular, the expressed oncoprotein of the HPV E6 gene degrades p53 protein through the cellular ubiquitin-protein ligase E6–AP pathway (71, 72). At the same time, E7 oncoprotein binds to the retinoblastoma gene products, pRb, p107, cyclin A, AP-1 transcription factor, and the TATA box binding protein, TBP 58-60. This leads to cellular instability. The sites for HPV DNA integration in the host genome seem to be near oncogenes (73). This would determine the transformation of cells at the chromosomal fragile site (74, 75), at 3p14 (76, 77), and the FRA8C site or at 8q24 (78), causing a gene interruption and a loss of chromosome heterozygosity (79).
This topic is relevant because HPV has a very high prevalence (65%) among men aged 18–40 years (80). It is estimated that 10% of male subjects may have a subclinical infection during an extended period of their life (29, 31). Moreover, sperm washing in preparing and purification of seminal liquid used in ART is not able to remove the risk of transmission of viral infection (81, 82), thus allowing viral transmission through artificial insemination procedures (54, 82, 83).
The literature on the association between HPV infection and abortion is still conflicting. Few older prospective or cross-sectional studies and “in vitro” studies have shown a possible relation, especially with high-risk viral strains. However, most recent clinical studies did not show a possible association. Considering the results of the experimental studies and the strong association between HPV infection and male infertility, we could hypothesize that infected spermatozoa may play a role as carriers of HPV DNA both in the reproductive tract and within the oocyte, with the possibility of detrimental effects during fertilization. This suggests to us the need for further studies concerning the association between HPV infection and miscarriage, testing contemporary HPV DNA in female and male counterparts.
In conclusion, while numerous reviews analyzing the correlation between HPV and male infertility seem to find agreement (84–87), there are few analyses that speculate about the correlation between HPV and female or couple infertility (64, 88). The few reviews available do not seem to find agreement with each other and with many other published studies. The conflicting results of some of these can be explained by the disjointed analysis. Our review focused in particular on the viral genotypes included in the 9-valent vaccine. Few studies consider the impact of viral infection on both partners and the product of conception, and none have focused on the genotypes included in the vaccine. We believe that it is necessary to search HPV infection in both female and male partners and to genotype HPV to discover the real impact of HPV infection on the risk of infertility or ART failure.
The relevance of the problem raised in this paper is undeniable, given how many HPV-infected couples, many of whom are also infertile as a couple, try to solve the problem of childbirth every day. In general, the value of vaccination against cervical cancer has been well-proven globally. Now, researchers should shed light on the issues of additional benefits that vaccination might be able or not able to provide.
Conclusions
To date, there is no agreement in the literature on the implication that HPV infection has on the fertility and miscarriage rate. Although it can be stated that HPV prevalence among couples with infertility problems undergoing IVF treatment is consistent, it does not seem to affect the performance of oocytes. Otherwise, HPV infection affects sperm parameters, in particular spermatozoa motility.
The proven correlation between HPV male infection both with infertility and abortion should implement new gynecological researches: it appears always clearer that it is necessary to consider contemporary female and male infection, particularly for infertile couples.
The HPV DNA testing in male partners of infertile couples could be useful to follow up individuals with infected sperm. In these cases, despite the absence of treatment for HPV infection, the possibility of delaying IVF procedures until the viral infection has been cleared or eliminating HPV from sperm through a specific washing procedure of semen (89) could be considered. HPV genotype could be useful for counseling and the decision-making process.
The correlation between HPV male infection both with asthenozoospermia and increased risk of pregnancy loss could recommend the extension of anti-HPV vaccination to adolescent males, along with anogenital and oral cancer prevention. In fact, when an association can be found, most HR-HPV involved are those included in the 9-valent vaccine.
Despite the fact that the relation between 9-valent HPV genotypes involved in female infection and miscarriage/infertility is not clear, the impact of this virus on health reproduction is evident. Considering this, the importance of HPV vaccination in adolescent females is confirmed for not only preventing cancer but also couple infertility.
A vaccine efficacy study could be useful to confirm the importance of primary prevention for the reproductive health of couples.
Key Questions
➢ Can HPV infection have a role in female infertility? To date, most studies did not find an association between female infertility and HPV infection.
➢ Can HPV have a role in ART outcomes? To date, it is not in accordance with the literature. Most included studies did not find a possible association between HPV women infection and ART outcomes.
➢ Can HPV infection have a role in male infertility? All included studies showed an association between asthenozoospermia and HPV male infection.
➢ Can HPV infection modify the risk of miscarriage? To date, there is no agreement in the literature. Further prospective studies are necessary to confirm this association. We believe that it is necessary to search HPV infection in both females and males and to genotype HPV to discover the real impact of HPV infection on the risk of miscarriage.
➢ Can immunological status modify the risk of infertility in couples affected by HPV? Immune system, pregnancy, and HPV infection appear to interact by modifying the host's immune response. The capacity of resolving the HPV infection in those women that contracted the infection from their partner makes it difficult to determine the real HPV prevalence and consequently the miscarriage correlation.
➢ Can HPV the 9-valent vaccine have a role in preventing infertility? To date, we could hypothesize that the 9-valent vaccine could have an important role in preventing male infertility because current literature shows a correlation with HPV genotypes included in the 9-valent vaccine.
Author Contributions
AC and JDG contributed to conception and design of the study. LG and GDC organized the database. CM, JDG, and FS wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACOG, American College of Obstetricians and Gynaecologists; STDs, sexually transmitted diseases; PID, pelvic inflammatory disease; TFI, tubal factor infertility; HPV, human papilloma virus; HR-HPV, high-risk HPV; ART, assisted reproductive technology; LR-HPV, low-risk HPV; CIN-1, cervical intraepithelial neoplasia-1; IVF, in vitro fertilization.
References
1. Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum Reprod. (2016) 31:2108–18. doi: 10.1093/humrep/dew123
2. Polis CB, Cox CM, Tunçalp Ö, McLain AC, Thoma ME. Estimating infertility prevalence in low-to-middle-income countries: an application of a current duration approach to demographic and health survey data. Hum Reprod. (2017) 32:1064–74. doi: 10.1093/humrep/dex025
3. Infertility Workup for the Women's Health Specialist: ACOG Committee Opinion Summary Number 781. Obstet Gynecol. (2019) 133:1294–5. doi: 10.1097/AOG.0000000000003272
4. Paavonen J, Eggert-Kruse W. Chlamydia trachomatis: impact on human reproduction. Hum Reprod Update. (1999) 5:433–47. doi: 10.1093/humupd/5.5.433
5. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. (2006) 18:503–10. doi: 10.1097/01.gco.0000242952.87125.69
6. Tsevat DG, Wiesenfeld HC, Parks C, Peipert JF. Sexually transmitted diseases and infertility. Am J Obstet Gynecol. (2017) 216:1–9. doi: 10.1016/j.ajog.2016.08.008
7. Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. (2005) 32 (Suppl. 1):S16–24. doi: 10.1016/j.jcv.2004.12.008
8. Zur Hausen H. Papillomaviruses in the causation of human cancers-a brief historical account. Virology. (2009) 384:260–5 doi: 10.1016/j.virol.2008.11.046
9. Souho T, Benlemlih M, Bennani B. Human papillomavirus infection and fertility alteration: a systematic review. PLoS ONE. (2015) 10:e0126936 doi: 10.1371/journal.pone.0126936
10. Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. (2012) 10:681–92 doi: 10.1038/nrmicro2872
11. Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. (1994) 169:235–40. doi: 10.1093/infdis/169.2.235
12. Park TW, Fujiwara H, Wright TC. Molecular biology of cervical cancer. Cancer. (1995) 76:1902–8. doi: 10.1002/1097-0142(19951115)76:10+<1902::AID-CNCR2820761306>3.0.CO;2-0
13. Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. (2002) 2:59–64. doi: 10.1038/nrc700
14. Ault KA. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect Dis Obstet Gynecol. (2006) 40470:1–5. doi: 10.1155/IDOG/2006/40470
15. De Sanjose S, Diaz M, Castellsagu X, Clifford G, Bruni L, Muoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet. (2007) 7:453–9. doi: 10.1016/S1473-3099(07)70158-5
16. Calinisan JH, Chan SR, King A, Chan PJ. Human papillomavirus and blastocyst apoptosis. J Assist Reprod Genet. (2002) 19:132–6. doi: 10.1023/A:1014736805127
17. Henneberg AA, Patton WC, Jacobson JD, Chan PJ. Human papilloma virus DNA exposure and embryo survival is stage-specific. J Assist Reprod Genet. (2006) 23:255–9. doi: 10.1007/s10815-006-9030-8
18. Rocha RM, Souza RP, Gimenes F, Consolaro MEL. The high-risk human papillomavirus continuum along the female reproductive tract and its relationship to infertility and endometriosis. Reprod Biomed Online. (2019) 38:926–37. doi: 10.1016/j.rbmo.2018.11.032
19. Lundqvust M, Westin C, Lundkvist O, Simberg N, Strand A, Andersson S, et al. Cytologic screening and human papilloma virus test in women undergoing artificial fertilization. Acta Obstet Gynecol Scand. (2002) 81: 949–53. doi: 10.1034/j.1600-0412.2002.811009.x
20. Strehler E, Sterzik K, Malthaner D, Hoyer H, Nindl I, Schneider A. Influence of ovarian stimulation on the detection of human papilloma virus DNA in cervical scrapes obtained from patients undergoing ART. Fertil Steril. (1999) 71:815–20. doi: 10.1016/S0015-0282(99)00012-6
21. Bugge N, Krüger Kjaer S, Soylu L, Jensen A. High-risk human papillomavirus infection in female and subsequent risk of infertility: a population-based cohort study. Fertil Steril. (2019) 111:1236–42. doi: 10.1016/j.fertnstert.2019.02.001
22. Spandorfer SD, Bongiovanni AM, Fasioulotis S, Rosenwaks Z, Ledger WJ, Witkin SS. Prevalence of cervical human papillomavirus in women undergoing in vitro fertilization and association with outcome. Fertil Steril. (2006) 86:765–7. doi: 10.1016/j.fertnstert.2006.01.051
23. Depuydt EC, Vestraete L, Berth M, Beert J, Bogers JP, Salembier G, et al. Human papillomavirus positivity in women undergoing intrauterine insemination has a negative effect on pregnancy rates. Gynecol Obstet Invest. (2015) 81:41–6. doi: 10.1159/000434749
24. Comar M, Monasta L, Zanotta N, Vecchi Brumatti L, Ricci G, Zauli G. Human papillomavirus infection is associated with decreased levels of GM-CSF in cervico-vaginal fluid of infected women. J Clin Virol. (2013) 58:479–81. doi: 10.1016/j.jcv.2013.07.001
25. Tanaka H, Karube A, Kodama H, Fukuda J, Tanaka T. Mass screening for human papilloma virus type 16 infection in infertile couples. J Reprod Med. (2000) 45:907–11.
26. Jaworek H, Zborilova B, Koudelakova V, Brezinova J, Vrbkova J, Oborna I, et al. Prevalence of human papillomavirus infection in oocyte donors and women treated for infertility: an observational laboratory-based study. Eur J Obstet Gynecol Reprod Biol X. (2019) 4:100068. doi: 10.1016/j.eurox.2019.100068
27. Yang R, Wang Y, Qiao J, Liu P, Geng L, Guo YL. Does human papillomavirus infection do harm to in-vitro fertilization outcomes and subsequent pregnancy outcomes? Chin Med J. (2013) 126:683–7. doi: 10.3760/cma.j.issn.0366-6999.20121625
28. Lai YM, Lee JF, Huang HY, Soong YK, Yang FP, Pao CC. The effect of human papillomavirus infection on sperm cell motility. Fertil Steril. (1997) 67:1152–5. doi: 10.1016/S0015-0282(97)81454-9
29. Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. (2007) 87:1087–97. doi: 10.1016/j.fertnstert.2006.08.109
30. Foresta C, Pizzol D, Moretti A, Barzon L, Palù G, Garolla A. Clinical and prognostic significance of human papillomavirus DNA in the sperm or exfoliated cells of infertile patients and subjects with risk factors. Fertil Steril. (2010) 94:1723–7. doi: 10.1016/j.fertnstert.2009.11.012
31. Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, et al. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril. (2010) 93:802–6. doi: 10.1016/j.fertnstert.2008.10.050
32. Foresta C, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Garolla A. Semen washing procedures do not eliminate human papilloma virus sperm infection in infertile patients. Fertil Steril. (2011) 96:1077–82. doi: 10.1016/j.fertnstert.2011.04.009
33. Yang Y, Jia CW, Ma YM, Zhou LY, Wang SY. Correlation between HPV sperm infection and male infertility. Asian J Androl. (2013) 15:529–32. doi: 10.1038/aja.2013.36
34. Schillaci R, Capra G, Bellavia C, Ruvolo G, Scazzone C, Venezia R, et al. Detection of oncogenic human papillomavirus genotypes on spermatozoa from male partners of infertile couples. Fertil Steril. (2013) 100:1236–40. doi: 10.1016/j.fertnstert.2013.06.042
35. Garolla A, Pizzol D, Bertoldo A, De Toni L, Barzon L, Foresta C. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil Steril. (2013) 99:125–31. doi: 10.1016/j.fertnstert.2012.09.006
36. Moghimi M, Zabihi-Mahmoodabadi S, Kheirkhah-Vakilabad A, Kargar Z. Significant correlation between high-risk HPV DNA in semen and impairment of sperm quality in infertile men. Int J Fertil Steri. (2019) 12:306–9.
37. Jeršoviene V, Gudlevičiene Ž, Rimiene J, Butkauskas D. Human papillomavirus and infertility. Medicina. (2019) 55:377. doi: 10.3390/medicina55070377
38. Perino A, Giovannelli L, Schillaci R, Ruvolo G, Fiorentino FP, Alimondi P, et al. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril. (2011) 95:1845–8. doi: 10.1016/j.fertnstert.2010.11.047
39. Laprise C, Trottier H, Monnier P, Coutlée F, Mayrand MH. Prevalence of human papillomaviruses in semen: a systematic review and meta-analysis. Hum Reprod. (2014) 29:640–51. doi: 10.1093/humrep/det453
40. Hermonat PL, Han L, Wendel PJ, Quirk JG, Stern S, Lowery CL, et al. Human papillomavirus is more prevalent in first trimester spontaneously aborted products of conception compared to elective specimens. Virus Genes. (1997) 14:13–7. doi: 10.1023/A:1007975005433
41. Sikström B, Hellberg D, Nilsson S, Brihmer C, Mårdh PA. Contraceptive use and reproductive history in women with cervical human papillomavirus infection. Adv Contracept. (1995) 11:273–84. doi: 10.1007/BF01983286
42. Ticconi C, Pietropolli A, Fabbri G, Capogna MV, Perno CF, Piccione E. Recurrent miscarriage and cervical human papillomavirus infection. Am J Reprod Immunol. (2013) 70:343–6. doi: 10.1111/aji.12156
43. Skoczynski M, Godzicka-Jozefiak A, Kwasniewska A. Prevalence of human papillomavirus in spontaneously aborted products of conception. Acta Obstet Gynecol Scand. (2011) 90:1402–5. doi: 10.1111/j.1600-0412.2011.01189.x
44. Abdullgaffar B, O Kamal M, Hasoub A. The prevalence of abnormal cervical cytology in women with infertility. Diagn Cytopathol. (2010) 38:791–4. doi: 10.1002/dc.21288
45. Conde-Ferráez L, Chan May Ade A, Carrillo-Martínez JR, Ayora-Talavera G, González-Losa Mdel R. Human papillomavirus infection and spontaneous abortion: a case-control study performed in Mexico. Eur J Obstet Gynecol Reprod Biol. (2013) 170:468–73. doi: 10.1016/j.ejogrb.2013.07.002
46. Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol. (2014) 234:431–5. doi: 10.1002/path.4424
47. Arbyn M, Simon M, Peeters E, Xu L, Meijer CJLM, Berkhof J, et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin Microbiol Infect. (2021) 8:S1198-743X(21)00219-6. doi: 10.1016/j.cmi.2021.04.031
48. Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. (2016) 2:16086. doi: 10.1038/nrdp.2016.86
49. Oppelt P, Renner SP, Strick R, Valletta D, Mehlhorn G, Fasching PA, et al. Correlation of high-risk human papilloma viruses but not of herpes viruses or Chlamydia trachomatis with endometriosis lesions. Fertil Steril. (2010) 93:1778–86. doi: 10.1016/j.fertnstert.2008.12.061
50. Vestergaard AL, Knudsen UB, Munk T, Rosbach H, Bialasiewicz S, Sloots TP, et al. Low prevalence of DNA viruses in the human endometrium and endometriosis. Arch Virol. (2010) 155:695–703. doi: 10.1007/s00705-010-0643-y
51. Heidarpour M, Derakhshan M, Derakhshan-Horeh M, Kheirollahi M, Dashti S. Prevalence of high-risk human papillomavirus infection in women with ovarian endometriosis. J Obstet Gynaecol Res. (2017) 43:135–9. doi: 10.1111/jog.13188
52. Çaglar GS, Garrido N. The implications of male human papilloma virus infection in couples seeking assisted reproduction technologies. J Turk Ger Gynecol Assoc. (2018) 19:48–52. doi: 10.4274/jtgga.2017.0031
53. Foresta C, Ferlin A, Bertoldo A, Patassini C, Zuccarello D, Garolla A. Human papilloma virus in the sperm cryobank: an emerging problem? Int J Androl. (2011) 34:242–6. doi: 10.1111/j.1365-2605.2010.01075.x
54. Rintala MA, Grnman SE, Pllnen PP, Suominen JJ, Syrjnen SM. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Intern J STD AIDS. (2004) 15:740–3. doi: 10.1258/0956462042395122
55. Chan PJ, Seraj IM, Kalugdan TH, King A. Evidence for ease of transmission of human papillomavirus DNA from sperm to cells of the uterus and embryo. J Assist Reprod Genet. (1996) 13:516–9. doi: 10.1007/BF02066536
56. Lee CA, Huang CT, King A, Chan PJ. Differential effects of human papillomavirus DNA types on p53 tumor-suppressor gene apoptosis in sperm. Gynecol Oncol. (2002) 85:511–6. doi: 10.1006/gyno.2002.6662
57. Garolla A, Engl B, Pizzol D, Ghezzi M, Bertoldo A, Bottacin A, et al. Spontaneous fertility and in vitro fertilization outcome: new evidence of human papillomavirus sperm infection. Fertil Steril. (2016) 105:65–72. doi: 10.1016/j.fertnstert.2015.09.018
58. Stagno S, Pass RF, Dworsky ME, Henderson RE, Moore EG, Walton PD, et al. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N Engl J Med. (1982) 306:945–9. doi: 10.1056/NEJM198204223061601
59. Icart J, Didier J, Dalens M, Chabanon G, Boucays A. Prospective study of Epstein Barr virus (EBV) infection during pregnancy. Biomedicine. (1981) 34:160–3.
60. Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, et al. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. (1987) 317:1246–51. doi: 10.1056/NEJM198711123172002
61. Monif GR. Maternal mumps infections during gestation: observations in the progeny. Am J Obstet Gynecol. (1974) 119:549–51. doi: 10.1016/0002-9378(74)90218-X
62. Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet. (1982) 2:781–4. doi: 10.1016/S0140-6736(82)92677-0
63. Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. (2008) 23:709–15. doi: 10.1093/humrep/dem404
64. Isaguliants M, Krasnyak S, Smirnova O, Colonna V, Apolikhin O, Buonaguro FM. Genetic instability and anti-HPV immune response as drivers of infertility associated with HPV infection. Infect Agent Cancer. (2021) 16:29. doi: 10.1186/s13027-021-00368-1
65. Pellavio G, Todaro F, Alberizzi P, Scotti C, Gastaldi G, Lolicato M, et al. HPV infection affects human sperm functionality by inhibition of aquaporin-8. Cells. (2020) 9:1241. doi: 10.3390/cells9051241
66. Laforenza U, Pellavio G, Marchetti AL, Omes C, Todaro F, Gastaldi G. Aquaporin-mediated water and hydrogen peroxide transport is involved in normal human spermatozoa functioning. Int J Mol Sci. (2016) 18:66. doi: 10.3390/ijms18010066
67. Cruz-Gregorio A, Manzo-Merino J, Lizano M. Cellular redox, cancer and human papillomavirus. Virus Res. (2018) 246:35–45. doi: 10.1016/j.virusres.2018.01.003
68. Foresta C, Patassini C, Bertoldo A, Menegazzo M, Francavilla F, Barzon L, et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infecting spermatozoa. PLoS ONE. (2011) 6:e15036. doi: 10.1371/journal.pone.0015036
69. Perez-Andino J, Buck CB, Ribbeck K. Adsorption of human papillomavirus 16 to live human sperm. PLoS ONE. (2009) 4:e5847. doi: 10.1371/journal.pone.0005847
70. Fujita M, Inoue M, Tanizawa O, Iwamoto S, Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. (1992) 52:5323–8.
71. Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitinprotein ligase in the ubiquitination of p53. Cell. (1993) 75:495–505. doi: 10.1016/0092-8674(93)90384-3
72. Hengstermann A, Linares LK, Ciechanover A, Whitaker NJ, Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc Natl Acad Sci USA. (2001) 98:1218–23. doi: 10.1073/pnas.98.3.1218
73. Durst M, Croce CM, Gissmann L, Schwarz E, Huebner K. Papillomavirus sequences integrate nearcellular oncogenes in some cervical carcinomas. Proc Natl Acad Sci USA. (1987) 84:1070–4. doi: 10.1073/pnas.84.4.1070
74. Yunis JJ, Soreng AL, Bowe AE. Fragile sites are targets of diverse mutagens and carcinogens. Oncogene. (1987) 1:59–69.
75. Smith PP, Friedman CL, Bryant EM, McDougall JK. Viral integration and fragile sites in human papillomavirus-immortalized human keratinocyte cell lines. Genes Chromosomes Cancer. (1992) 5:150–7. doi: 10.1002/gcc.2870050209
76. Rassool FV, McKeithan TW, Neilly ME, van Melle E, Espinosa R-III, Le Beau MM. Preferential integration of marker DNA into the chromosomal fragile site at 3p14: an approach to cloning fragile sites. Proc Natl Acad Sci USA. (1991) 88:6657–61. doi: 10.1073/pnas.88.15.6657
77. Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV 16 E6 or E7 increases integration of foreign DNA. Oncogene. (1996) 13:427–31.
78. Ferber MJ, Eilers P, Schuuring E, Fenton JA, Fleuren GJ, Kenter G, et al. Positioning of cervical carcinoma and Burkitt lymphoma translocation breakpoints with respect to the human papillomavirus integration cluster in FRA8C at 8q24.13. Cancer Genet Cytogenet. (2004) 154:1–9. doi: 10.1016/j.cancergencyto.2004.01.028
79. Gallego MI, Lazo PA. Deletion in human chromosome region 12q13–15 by integration of human papillomvirus DNA in a cervical carcinoma cell line. J Biol Chem. (1995) 270:24321–24326. doi: 10.1074/jbc.270.41.24321
80. Nielson CM, Flores R, Harris RB, Abrahamsen M, Papenfuss MR, Dunne EF, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev. (2007) 16:1107–14. doi: 10.1158/1055-9965.EPI-06-0997
81. Brossfield JE, Chan PJ, Patton WC, King A. Tenacity of exogenous human papillomavirus DNA in sperm washing. J Assist Reprod Genet. (1999) 16:325–8. doi: 10.1023/A:1020458100382
82. Olatunbosun O, Deneer H, Pierson R. Human papillomavirus DNA detection in sperm using polymerase chain reaction. Obstet Gynecol. (2001) 97:357–60. doi: 10.1097/00006250-200103000-00006
83. Chan PJ, Su BC, Kalugdan T, Seraj IM, Tredway DR, King A. Human papilloma virus gene sequences in washed human sperm deoxyribonucleic acid. Fertil Steril. (1994) 61:982–5. doi: 10.1016/S0015-0282(16)56719-3
84. Muscianisi F, De Toni L, Giorato G, Carosso A, Foresta C, Garolla A. Is HPV the novel target in male idiopathic infertility? A systematic review of the literature. Front Endocrinol. (2021) 12:643539. doi: 10.3389/fendo.2021.643539
85. Moreno-Sepulveda J, Rajmil O. Seminal human papillomavirus infection and reproduction: a systematic review and meta-analysis. Andrology. (2021) 9:478– 502. doi: 10.1111/andr.12948
86. Wang S, Liu L, Zhang A, Song Y, Kang J, Liu X. Association between human papillomavirus infection and sperm quality: a systematic review and a meta-analysis. Andrologia. (2021) 53:e14034. doi: 10.1111/and.14034
87. Lyu Z, Feng X, Li N, Zhao W, Wei L, Chen Y, et al. Human papillomavirus in semen and the risk for male infertility: a systematic review and meta-analysis. BMC Infect Dis. (2017) 17:714. doi: 10.1186/s12879-017-2812-z
88. Siristatidis C, Vaidakis D, Sertedaki E, Martins WP. Effect of human papilloma virus infection on in-vitro fertilization outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2018) 51:87–93. doi: 10.1002/uog.17550
Keywords: infertility, human papillomavirus, vaccine, assisted reproduction (ART), miscarriage
Citation: Ciavattini A, Marconi C, Giannella L, Delli Carpini G, Sopracordevole F and Di Giuseppe J (2021) The Impact of 9-Valent HPV Vaccination on Couple Infertility Prevention: A Comprehensive Review. Front. Med. 8:700792. doi: 10.3389/fmed.2021.700792
Received: 26 April 2021; Accepted: 22 July 2021;
Published: 17 August 2021.
Edited by:
Andrea Tinelli, Moscow Institute of Physics and Technology, RussiaReviewed by:
Gulzhanat Aimagambetova, Nazarbayev University, KazakhstanSaule Balmagambetova, West Kazakhstan Marat Ospanov State Medical University, Kazakhstan
Copyright © 2021 Ciavattini, Marconi, Giannella, Delli Carpini, Sopracordevole and Di Giuseppe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Ciavattini, Y2lhdmF0dGluaS5hQGxpYmVyby5pdA==
 Andrea Ciavattini
Andrea Ciavattini Chiara Marconi
Chiara Marconi Luca Giannella
Luca Giannella Giovanni Delli Carpini1
Giovanni Delli Carpini1 Francesco Sopracordevole
Francesco Sopracordevole