
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 27 April 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.693915
 Zhili Hu1,2,3,4†‡
Zhili Hu1,2,3,4†‡ Yingjie Liu1,2,3,4†‡
Yingjie Liu1,2,3,4†‡ Jibao Wang5‡
Jibao Wang5‡ Zhefeng Meng6†‡
Zhefeng Meng6†‡ Sequoia I. Leuba7†
Sequoia I. Leuba7† Jie Wei1,2,3,4
Jie Wei1,2,3,4 Xing Duan5
Xing Duan5 Zhenxing Chu1,2,3,4†
Zhenxing Chu1,2,3,4† Min Chen8*†§
Min Chen8*†§ Hong Shang1,2,3,4*†§
Hong Shang1,2,3,4*†§ Junjie Xu1,2,3,4*†§
Junjie Xu1,2,3,4*†§
Background: Accurate identification of molecular transmission clusters (MTCs) and understanding the dynamics of human immunodeficiency virus (HIV) transmission are necessary to develop targeted interventions to prevent HIV transmission. We evaluated the characteristics of antiretroviral therapy-naïve individuals who belonged to HIV-1 MTCs in the China–Myanmar border region to inform targeted effective HIV intervention.
Methods: Phylogenetic analyses were undertaken on HIV-1 pol sequences to characterize subtypes or circulating recombinant forms and identify MTCs. MTCs were defined as those with 2 or more sequences having bootstrap support > 80% and a pairwise gene distance less than or equal to 0.03. Factors correlated with MTCs were evaluated using logistic regression analysis. The chi-square test was used to compare differences between Chinese and Burmese participants belonging to MTCs.
Results: A total of 900 people had their pol gene successfully sequenced. Twenty-one MTCs were identified and included 110 individuals (12.2%). Individuals in MTCs were more likely to be Burmese [aOR = 2.24 (95% CI: 1.33, 3.79), P = 0.003], be younger [aOR = 0.34 (95% CI: 0.20, 0.58), P < 0.001 for age 26–50 vs. 25 years or younger], have a lower CD4 T cell count [aOR = 2.86 (95% CI: 1.34, 6.11), P = 0.007 for < 200 vs. 350 or greater], and have subtypes CRF07_BC or C [CRF07_BC: aOR = 7.88 (95% CI: 3.55, 17.52), P < 0.001; C: aOR = 2.38 (95% CI: 1.23, 4.62), P = 0.010 compared to CRF01_AE]. In MTCs, Burmese were younger (89.7 vs. 57.7% for age 25 years or younger), had a lower education level (41.0 vs. 8.5% for illiterate), were more likely to be infected through injection drug use (35.9 vs. 12.7%), and had a higher proportion of subtype BC (33.3 vs. 15.5%) and CRF01_AE (20.5 vs. 8.5%) compared to Chinese (P < 0.05 for all).
Conclusion: Burmese participants were more likely to belong to MTCs, and most MTCs had both Burmese and Chinese participants. These data highlight the bidirectional transmission of HIV-1 frequently transmission and close relationship among immigrants in the China–Myanmar border region. Local health departments should pay more attention to HIV screening and intervention to immigrants Burmese with the characteristics of younger age, having lower CD4 T cell count and infected with HIV subtypes CRF07_ BC or C.
Human immunodeficiency virus (HIV) infection is a major global public health disease and can be spread through population mobility (1). Researchers can trace these migrating HIV strains to specific geographical regions and modes of transmission (2, 3). In border areas, the population mobility of transnational floating populations, including high-risk HIV key populations (e.g., injection drug users and sex workers), can drive HIV transmission between countries (4). Thus, multiple studies have focused on preventing HIV transmission in these border regions. A study on the US-Mexico border region has shown that there was bi-directional and cross-border HIV transmission (5). In addition, clustering with neighboring countries was found between 10.1% of people newly diagnosed with HIV-1 in Europe (6). As China shares borders with multiple countries, and previous research has shown that the HIV prevalence rate among transnational floating populations at Chinese frontier ports is significantly higher than the general population, prevention of HIV transmission across borders in China is critical (4).
Yunnan Province is the one of the most affected provinces by HIV/AIDS in China (7). More than 93,000 people were living with HIV/AIDS (PLWHA) in Yunnan in 2016, accounting for 14.3% of the total number in China (8). Located in southern China, Yunnan Province borders Myanmar in the west and Laos and Vietnam in the south and is near the “Golden Triangle,” a large narcotic production area. Due to its location and high HIV prevalence, Yunnan serves as a gateway for HIV transmission between Southeast Asia and China (9–11). In recent years, China and Myanmar have focused on developing strong trade and personnel exchanges, and many Burmese live, work, and study in Yunnan (12); almost 16 million Burmese annually enter Yunnan (13). In 2010, the Chinese State Council ended mandatory HIV/AIDS testing of returning Chinese and foreign visitors (14). Thus, the HIV infection status of this transnational population in Yunnan is mainly unknown (14). While incident cases of HIV/AIDS in Yunnan are decreasing, the proportion of newly diagnosed with HIV/AIDS in Yunnan province who are Burmese is almost 60% and increasing annually in Yunnan (4). Therefore, it is essential to identify and address migrating HIV strains spread among transnational floating populations to address the HIV epidemic in Yunnan.
One way to describe and monitor local HIV strains is through phylogenetic analysis, i.e., determining associations between genetic sequences and information on nationality, infection route, and drug resistance to determine evolutionary changes over time (15, 16). Phylogenetic cluster analysis can identify potential factors promoting the epidemic spread and likely transmission networks (17–19). Researchers in Beijing (20) have previously shown that targeting individuals for antiretroviral therapy (ART) using phylogenetic cluster analysis had a much higher prevention efficiency of future infections (42%) compared to providing all participants ART (24%). However, most studies conducted on phylogenetic analysis in Yunnan focus on subtype distribution and identifying drug resistance or new subtypes instead of assessing the associations between demographic and clinical factors and molecular transmission clusters (MTCs) (21–24).
We conducted a molecular phylogenetic analysis of an HIV-1 sequence dataset from the China-Myanmar border region to identify and characterize MTCs in the Yunnan. We also compared the differences between Chinese and Burmese clusters of ART-naive individuals to help target HIV strategies to prevent further transmission.
In Dehong Prefecture in Yunnan Province, 900 individuals newly reported with HIV had their pol sequences successfully amplified from 2009 to 2017 (Figure 1). Participants were recruited by advertising in local HIV/AIDS clinics and peer referral. Participants were eligible for this study if they were (1) infected with HIV-1 by western blot confirmatory test, and (2) had not started antiretroviral therapy (ART) and were consent to attend this study. Participants were asked about their epidemiological history through face-to-face interviews, and were collected whole blood samples (10 mL).

Figure 1. Study site location at the China-Myanmar border. The black dotted line with a gray shadow shows the border between China and Myanmar, and the light purple area indicates the region of Yunnan Province. The red box indicates our study site. Magnified images of boxed areas are shown in the upper right corners.
HIV-1 RNA was extracted from plasma using the QIAamp Viral RNA Mini kit (Qiagen, Valencia, CA, United States) according to the manufacturer’s instructions. The One-Step RNA polymerase chain reaction (PCR) Kit (TaKaRa Biotechnology, Shiga, Japan) was used for reverse transcription and the first round of amplification of pol. The nested PCR was performed using 2 × Taq PCR Master Mix (Tiangen, Beijing, China). The primers, conditions and procedures for reverse transcription PCR were described in a previous study (23). The PCR product was identified by electrophoresis using 1% agarose gels and amplified PCR products were sent to Sino Geno Max (Beijing, China) for sequencing.
Sequences were assembled using Sequencer 5.1 (Gene Codes, Ann Arbor, MI, United States). The assembled sequences were aligned with BioEdit 7.0.5.3 and then edited manually. Reference sequences for HIV-1 subtyping were selected and downloaded from the HIV databases of the Los Alamos National Laboratory.1 Neighbor-joining phylogenetic trees were constructed using MEGA 7.0,2 with the Kimura 2-parameter model with 1,000 bootstrap replicates to determining the HIV-1 genotype. The possible inter-subtype recombination was analyzed with the Recombination Identification Program (RIP).3 Molecular transmission clusters (MTCs) were defined as clusters with 2 or more sequences having bootstrap support greater than 80% (the criterion for phylogenetic confidence) and a pairwise gene distance less than or equal to 0.03 (the pairwise gene distance was calculated using the Tamura–Nei parameter model and setting the bootstrap to 1,000 with MEGA 7.0) (25–27).
Demographic and clinical data are summarized using median and interquartile ranges (IQR) for continuous variables or absolute and relative frequencies for categorical variables. Differences in characteristics of Chinese or Burmese who were included in MTCs were compared using the Chi-square test. Univariable and multivariable logistic regression models were used to determine correlations between potential demographic and clinical factors (e.g., sex, age, ethnicity, nationality, occupation, marital status, infection routes, CD4 cell count, and subtype) and inclusion in a molecular transmission cluster (MTC). The multivariable analysis was conducted using forward stepwise regression (entry criteria: P < 0.05; exit criteria: P > 0.1) to select the variables. Continuous variables such as age and CD4 cell count were categorized for statistical analysis. All statistical analyses were conducted using SPSS 25.0 (IBM, Armonk, NY, United States). P < 0.05 (two-sided) was considered significant.
The Institutional Review Board of the First Affiliated Hospital of China Medical University (Shenyang, China) approved the study protocol (2019-152-3). Adults provided written informed consent before collection of blood samples; guardians signed the consent forms of adolescents.
The study enrolled 900 individuals who had an HIV-1 pol region successfully sequenced. Most participants were male (59.9%, 539/900) and non-Han ethnicity (56.2%, 506/900), about 16.6% (149/900) were Burmese living in Yunnan. The median age was 28 years (IQR: 23, 39). Less than half had graduated from junior middle school or above (42.9%, 386/900), most identified their occupation as peasant (70.6%, 635/900), and about half were married (51.6%, 464/900). The median CD4 cell count was 440 (IQR: 324, 617). Most participants were infected with HIV through heterosexual contact (76.1%, 685/900), followed by injection drug use (IDU) (17.3%, 156/900) and homosexual contact (4.7%, 42/900) (Table 1).
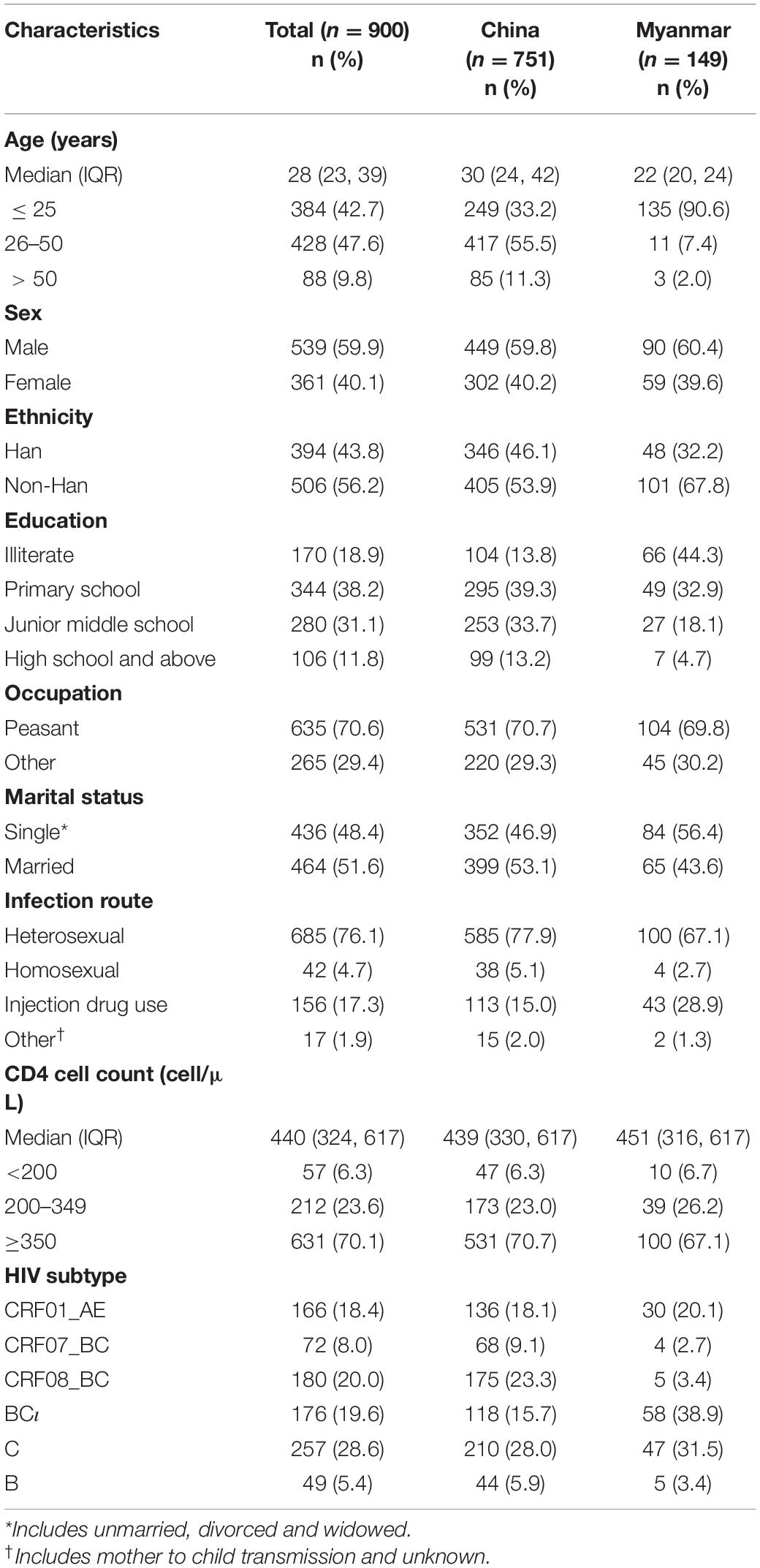
Table 1. Demographic and clinical characteristics on participants, stratified by nationality (N = 900).
Phylogenetic analysis of blood samples found that individuals (28.6%; 257/900) were frequently infected with subtype C, followed by CRF08_BC (20.0%; 180/900), BC (19.6%; 176/900), CRF01_AE (18.4%; 166/900), CRF07_BC (8.0%; 72/900), and B (5.4%; 49/900) (Figure 2 and Table 1), and that the distribution of subtypes differed by country. Chinese participants were frequently infected with HIV subtype C (28.0%; 210/751), followed by subtype CRF08_BC (23.3%; 175/751), CRF01_AE (18.1%; 136/751) and BC (15.7%; 118/751). In contrast, Burmese participants were frequently infected with HIV subtype BC (38.9%; 58/149), followed by subtype C (31.5%; 47/149) and CRF01_AE (20.1%; 30/149).
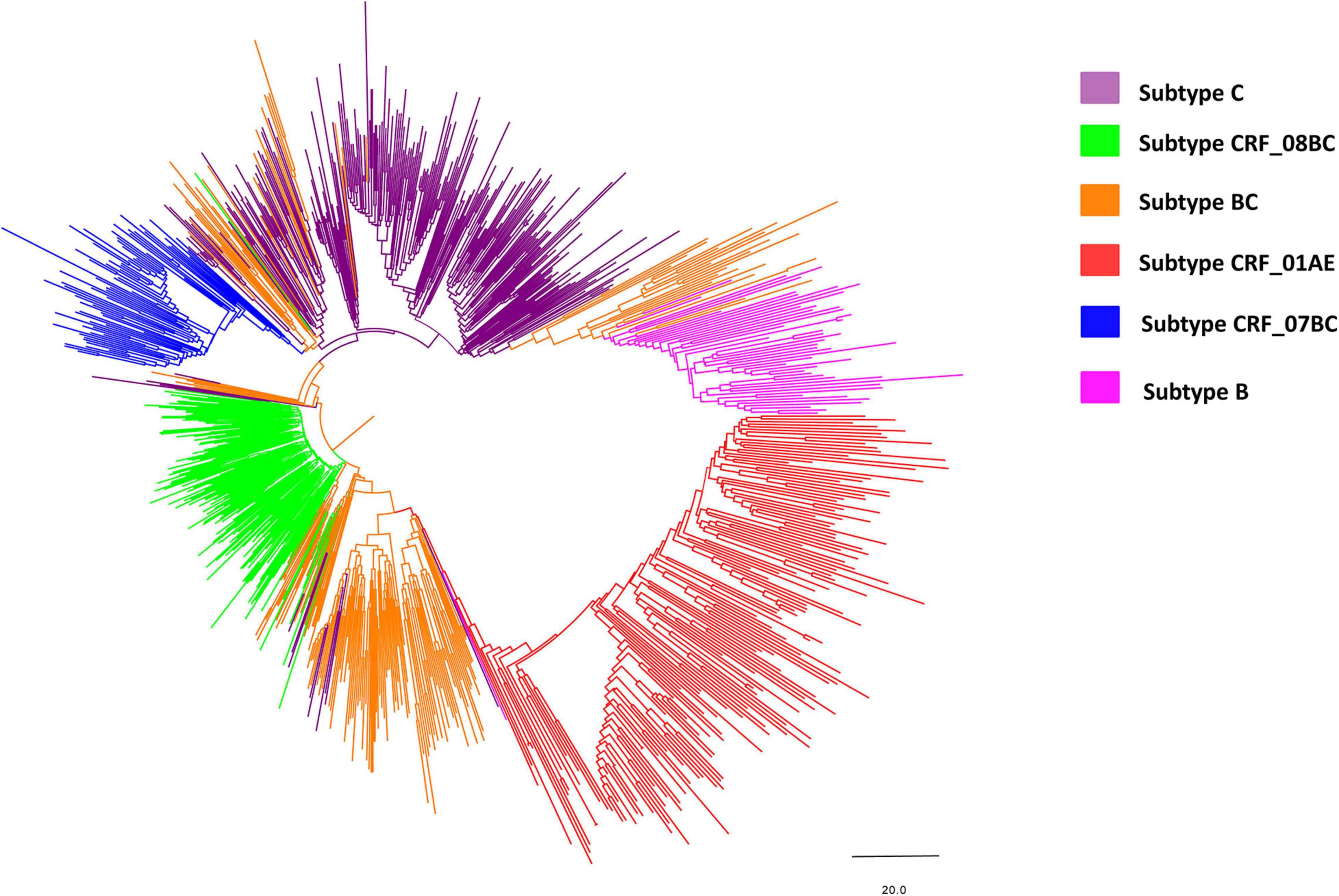
Figure 2. Neighbor-joining phylogenetic tree of the whole pol gene. The neighbor-joining phylogenetic tree was constructed using MEGA 7.0 with 1,000 bootstrap replicates. The nucleotide substitution model was the Kimura 2-parameter model. The line with different colors indicates different subtypes/recombinant forms of HIV-1. Purple, green, orange, red, blue and magenta lines denote subtype C, CRF_08BC, BC, CRF_01AE, CRF_07BC, and subtype B, respectively.
Phylogenetic analyses identified 21 MTCs involving 12.2% (110/900) of participants. The clustering rate among Chinese participants was 9.5% (71/751), and among Burmese participants was 26.2% (39/149). Most MTCs (95.2%, 20/21) consisted of sequences from China and Myanmar, whereas only one MTC (4.8%) consisted exclusively of sequences from Myanmar. The highest proportion of MTC sequences belonged to subtypes C (42/110; 38.2%), BC (24/110; 21.8%), and CRF07_BC (22/110; 20.0%) (Table 2 and Figures 3, 4).
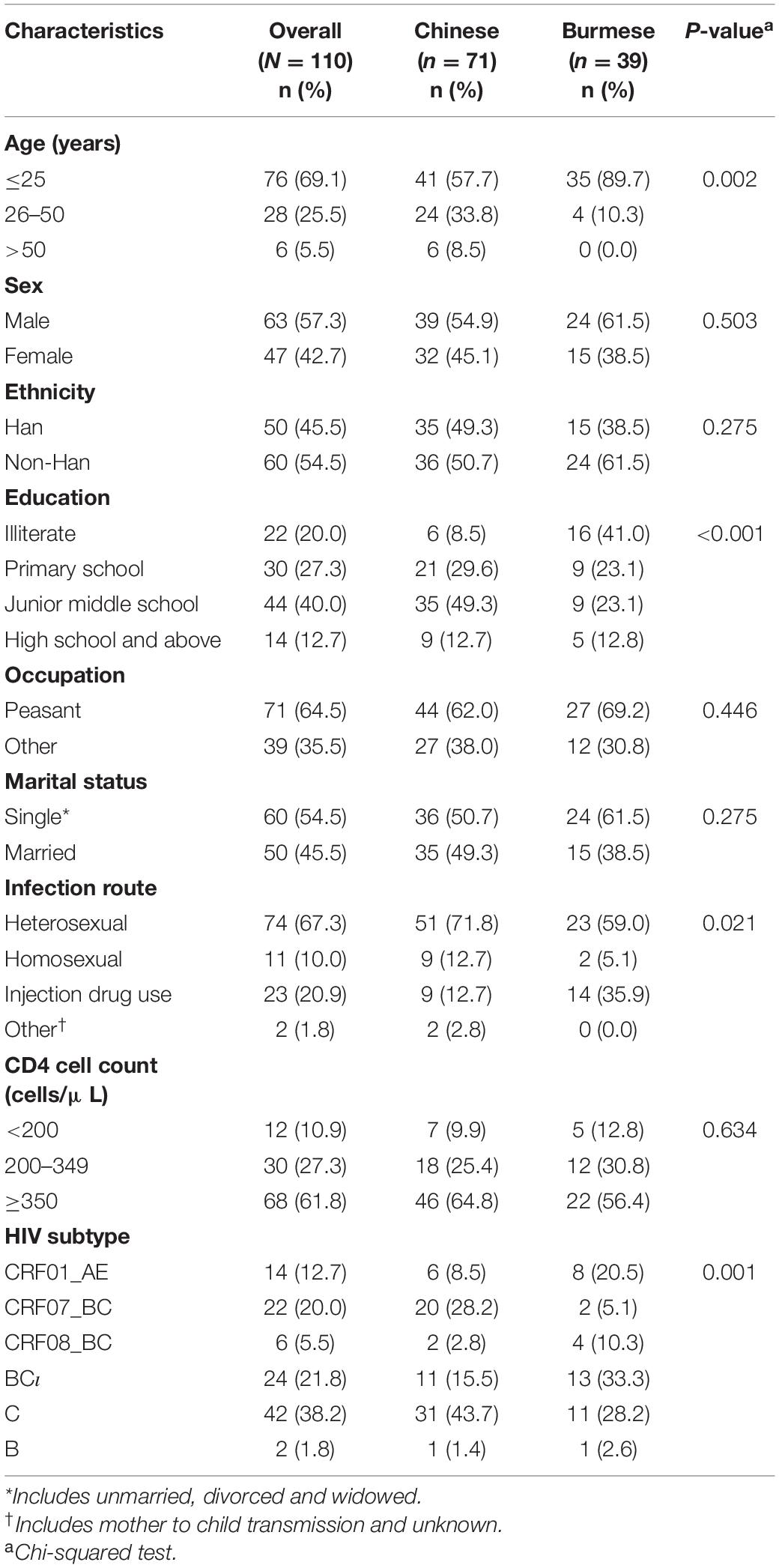
Table 2. Demographic and clinical characteristics of individuals included in the MTCs stratified by nationality (N = 110).
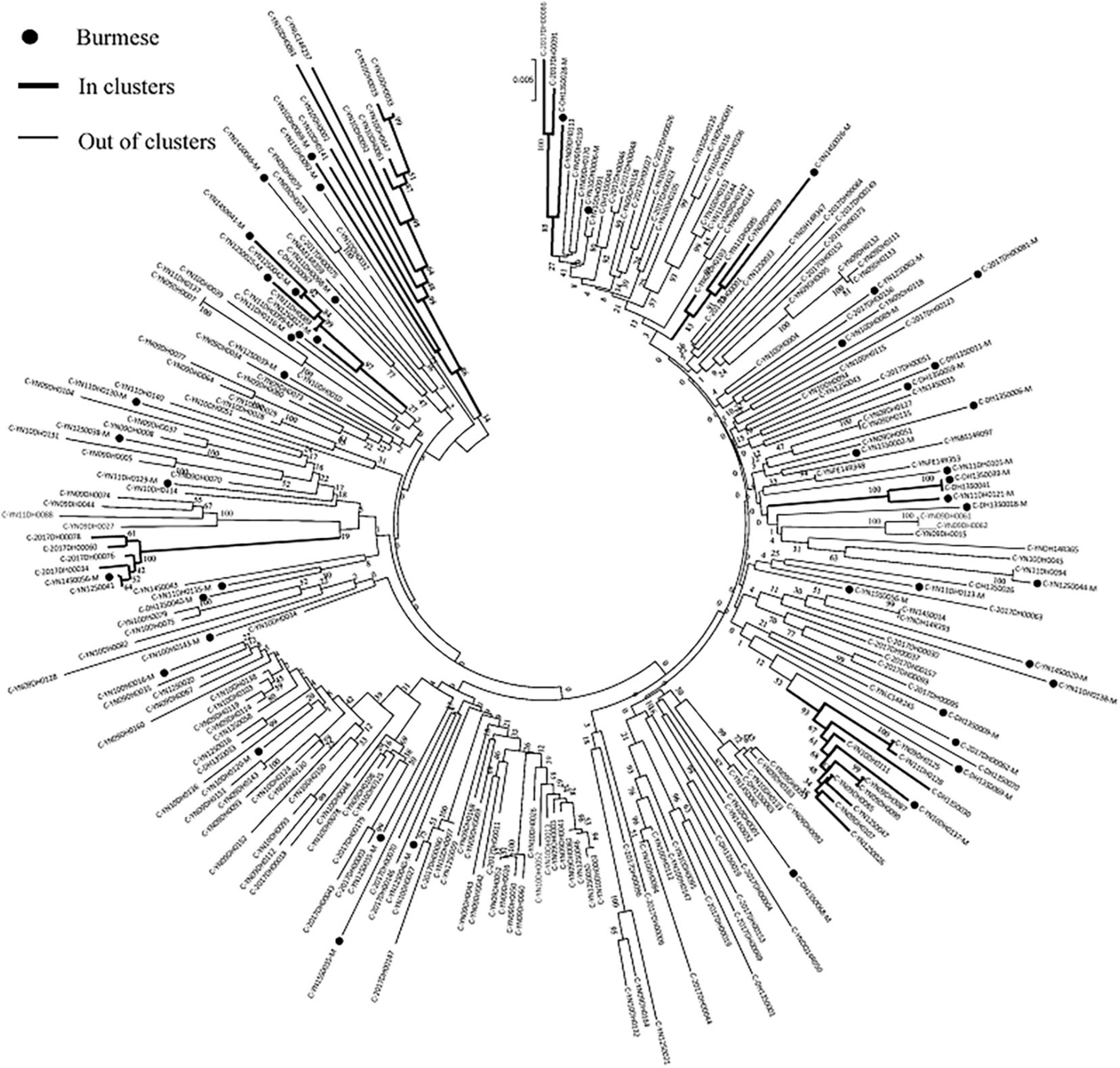
Figure 3. Clustering of HIV-1 sequences within the biggest molecular transmission clusters for subtypes C. The tree was constructed using MEGA 7.0 with 1,000 bootstrap replicates. Molecular transmission clusters (MTC) were defined as clusters with two or more sequences and the pairwise gene distance less than or equal to 0.03 with bootstrap values ≥ 80%. The scale bar indicates 0.5% nucleotide sequence divergence. The black nodes represent Burmese. The thick line indicates individuals in clusters. The thin line indicates individuals out of clusters.
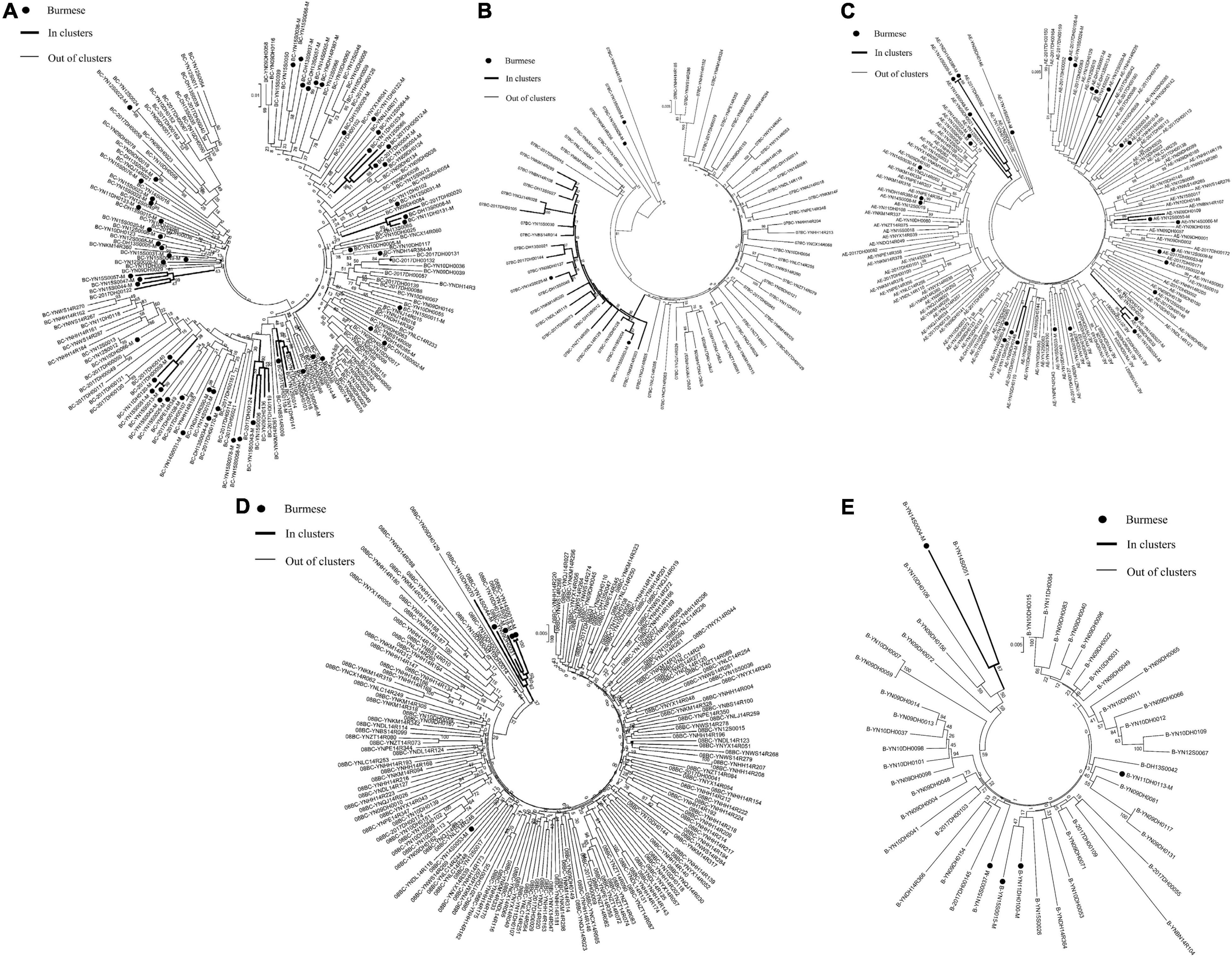
Figure 4. Clustering of HIV-1 sequences for other subtypes. The tree was constructed using MEGA 7.0 with 1,000 bootstrap replicates. Molecular transmission clusters (MTC) were defined as clusters with two or more sequences and the pairwise gene distance less than or equal to 0.03 with bootstrap values ≥ 80%. The scale bar indicates 0.5% nucleotide sequence divergence. The black nodes represent Burmese. The thick line indicates individuals in clusters. The thin line indicates individuals out of clusters. (A), subtype BC; (B), subtype CRF_07BC; (C), subtype CRF_01AE; (D), subtype CRF_08BC; (E), subtype B.
Individuals included in the MTCs were stratified based on nationality: 71 Chinese and 39 Burmese. Burmese were more likely to be younger (25 years or younger: 89.7 vs. 57.7%), have a lower levels of education (illiterate: 41.0 vs. 8.5%), have been infected through IDU (35.9 vs. 12.7%), and have HIV subtype BC (33.3 vs. 15.5%) or subtype CRF01_AE (20.5 vs. 8.5%) compared to Chinese participants (P < 0.05 for all) (Table 2).
Based on the results from the univariable logistic regression, older participants were less likely to be in MTCs [age 26–50 years: OR = 0.28 (95% CI: 0.18, 0.45); age > 50 years: OR = 0.30 [95% CI: 0.12, 0.71] compared to age 25 years or younger]. Participants with HIV subtype CRF07_BC [OR = 4.78 (95% CI: 2.27, 10.04)], CRF08_BC [OR = 0.37 (95% CI: 0.14, 1.00)] or HIV subtype C [OR = 2.12 (95% CI: 1.12, 4.02)] were more likely to be grouped into an MTC when compared to individuals with subtype CRF01_AE. Being Burmese compared to being Chinese [OR = 3.40 (95% CI: 2.19, 5.27)], being infected through homosexual sex compared to through heterosexual sex [OR = 2.93 (95% CI: 1.41, 6.07)], and having a CD4 cell count below 200 compared to 350 or greater [OR = 2.21 (95% CI: 1.13, 4.38)] were associated with increased likelihood of being grouped into an MTC (Table 3).
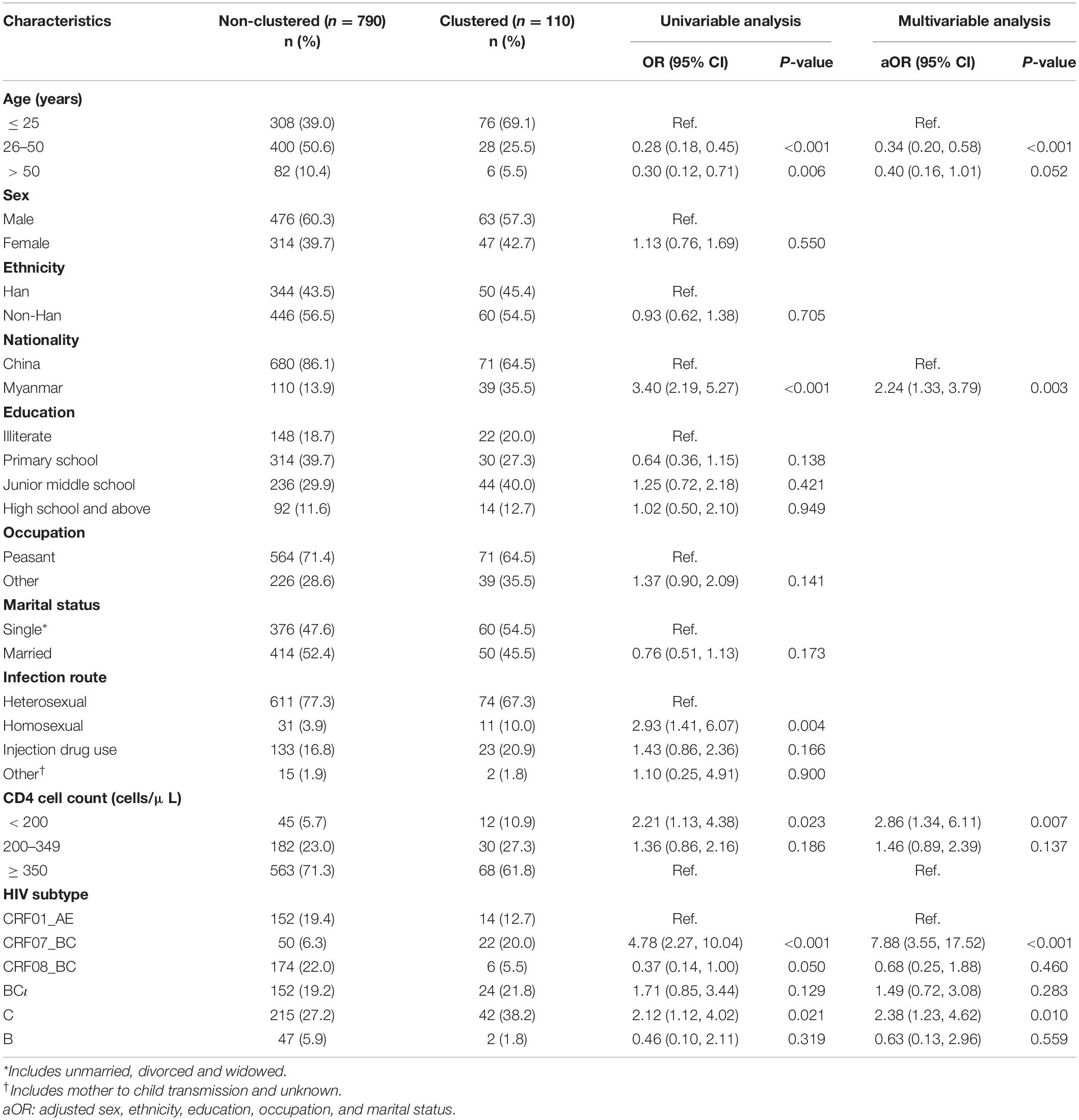
Table 3. Univariable and multivariable logistic regression of factors associated with being grouped with a molecular transmission cluster (MTC).
In the multivariable logistic regression using forward stepwise regression, we adjusted for sex, occupation, education, and marital status variables. Participants were more likely to be grouped into an MTC if they were Burmese compared to Chinese [aOR = 2.24 (95% CI: 1.33, 3.79)], have a CD4 cell count below 200 compared to 350 or greater [aOR = 2.86 (95% CI: 1.34, 6.11)], or have HIV subtype CRF07_BC [aOR = 7.88 (95% CI: 3.55, 17.52)] or subtype C [aOR = 2.38 (95% CI: 1.23, 4.62)] compared to subtype CRF01_AE. Participants were less likely to be grouped into an MTC if they were between 26 and 50 years old compared to 25 years old or younger [aOR = 0.34 (95% CI: 0.20, 0.58)] (Table 3).
We evaluated 900 ART-naïve individuals to determine the characteristics and distribution of HIV-1 molecular transmission clusters (MTCs) in the China–Myanmar border region using phylogenetic analyses. We found that 12.2% of the HIV-1 sequences belonged in MTCs, and that the factors associated with belonging to MTCs were younger age, Burmese nationality, lower CD4 cell count, and HIV-1 subtype of CRF07_BC or subtype C compared to subtype CRF01_AE. Among those included in MTCs, Burmese were younger, had a lower education level, and more were infected through IDU compared to Chinese. These results provide evidence of the molecular epidemiology of HIV-1 in the southwest border region needed for public-health policy and targeted interventions to prevent HIV-1 transmission in the China–Myanmar border region.
We found that 12.2% of HIV-1 pol sequences included in this study belonged in MTCs, which is lower than that reported locally (33% in 2015–2016) (28). We may have found a lower percentage belonging to MTCs by having an earlier and longer study period (2009–2017 vs. 2015–2016). Similar to previous published results, we found that younger age was correlated with belonging to MTCs, suggesting that local government should target HIV prevention strategies toward younger people living near the China–Myanmar border (29, 30). While only 6.3% (57/900) of participants had a CD4 cell count below 200, 21.1% (12/57) of these participants with low CD4 cell counts belonged to MTCs [aOR = 2.86 (95% CI: 1.34, 6.11)]. In contrast, a previous study found that high, not low, CD4 cell count was more likely to belong to MTCs (30). Our conflicting result may be explained that due to low HIV testing, many people who are diagnosed with HIV in Yunnan may be diagnosed at a later stage and have lower CD4 cell counts at diagnosis (31). Thus, local HIV testing must be expanded to reach these delayed diagnosed individuals to reduce transmission of HIV.
We also found that specific HIV subtypes were correlated with belonging to MTCs. Subtype CRF07_BC and subtype C were more likely to belong to MTCs than CRF01_AE (20.0 and 38.2 vs. 12.7%), suggesting that the two subtypes were more likely to have genetically close infections in Yunnan. Subtype C was the most prevalent subtype in this dataset (28.6%), followed by subtypes CRF08_BC (20.0%), BC (19.6%), and CRF01_AE (18.4%), consistent with previous reports of the main local HIV subtypes (2, 32–35). Similar to previous studies, we also found differences in primary subtype among Chinese and Burmese (21, 36, 37). Subtypes C, CRF08_BC, and CRF01_AE were the most prevalent HIV subtypes present among Chinese, while subtypes BC, C, and CRF01_AE were the most prevalent among Burmese. These different distributions of subtype based on nationality may be correlated with the high prevalence of HIV-1 recombinants among northern Burmese (38–40). We found more CRFs among Chinese not Burmese and the high prevalence of CRFs among Burmese. This result might suggest the frequently transmission of HIV-1 in the China–Myanmar border region.
While Burmese comprised only 16.6% of the study cohort, 35.5% (39/110) were in MTCs and Burmese had more than twice the odds of belonging to MTCs compared to Chinese, suggesting that Burmese participants from Yunnan had higher odds of transmitting HIV. Most MTCs included both Burmese and Chinese participants, and only one MTC was comprised only of Burmese, suggesting cross-border HIV transmission. Among participants belonging to MTCs, Burmese participants were younger (25 years old or younger: 89.7 vs. 57.7%), had more illiterate education (41.0 vs. 8.5%), and more HIV infections through IDU (35.9 vs. 12.7%) and fewer HIV infections through homosexual behavior (5.1 vs. 12.7%) compared to Chinese participants. These demographic differences among participants belonging to MTCs based on nationality were also found among all participants. These findings suggest that local governments should take different targeted approaches depending on nationality to prevent and control HIV transmission in the China–Myanmar region such as providing treatment and support for reducing injection drug use among Burmese and increasing condom use or pre-exposure prophylaxis among Chinese in Yunnan in order to address the main infection routes for HIV acquisition.
To the best of our knowledge, we are the first to determining clinical and demographic factors correlated with MTCs between antiretroviral therapy-naïve Chinese and Burmese in the China–Myanmar border region. We found that younger age, Burmese nationality, lower CD4 cell count, and having subtype CRF07_BC or subtype C (compared to subtype CRF01_AE) were significantly correlated with belonging to MTCs. Our findings support local CDC conducting targeted interventions addressing cross-border HIV transmission in the China-Myanmar border, in particular to local young Burmese. Lower CD4 cell count was significantly correlated with belonging to MTCs, which indicates that some delayed diagnosed PLWHA is still transmitting HIV, hence continue expanding local HIV testing is also necessary to curb the local HIV transmission. Meanwhile, we identified 21 MTCs totally, but only one MTC was comprised only of Burmese and we found popular HIV subtypes were similar between Chinese and Burmese in the China–Myanmar border region. This result might suggest the frequently transmission and close relationship among immigrants. Hence we need to further strengthen the surveillance of HIV clustering characteristics in China-Myanmar border region.
However, although seven other prefectures in Yunnan bordered other countries, participants were only recruited from one prefecture: Dehong prefecture. However, Dehong prefecture has the highest prevalence of HIV-1 infections compared to the other prefectures and thus may suggest a pattern found in other prefectures in Yunnan (41). Second, we could not infer the direction of transmission through phylogenetic analyses and the cross-sectional study design has limited causal inferences and the representativeness and generalizability of our study are uncertain owing to the small number of samples. Finally, despite the extensive use of pol as a main target to reconstruct transmission clusters, the more genes and the longer the alignment, the better the phylogenetic signal and the better the tree resolution. Despite these limitations, we characterized HIV-1 transmission in the China-Myanmar border region and provided evidence for targeted interventions to address cross-border transmission.
We were able to identify 21 MTCs of which 20 included both Burmese and Chinese participants. Burmese were more likely to belong to MTCs compared to Chinese. Among those clustered, Burmese were more likely to be younger, have lower education, and be infected through injection drug use compared to Chinese participants. In contrast, among those who belonged to MTCs, Chinese participants were more likely to be infected through homosexual behavior. These data highlight the frequently transmission and close relationship among immigrants in the China–Myanmar border region, and suggest that the local government develop targeted interventions stratified by nationality to reduce cross-border HIV transmission.
The data analyzed in this study is subject to the following licenses/restrictions: data not publicly available but may be obtained upon request and approval from a third party, NHC Key Laboratory of AIDS Immunology (China Medical University). Requests to access these datasets should be directed to JX, eGpqY211QDE2My5jb20=.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of China Medical University (2019-152-3). Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
ZH, YL, JBW, ZM, MC, HS, and JX conceived and designed the study. JBW, XD, and ZC performed the study. JBW and XD collected the data. ZH, YL, JW, and ZM analyzed the data. ZH, ZM, and YL drew the figures and tables. ZH wrote the first draft of the manuscript. ZH, YL, JBW, ZM, SL, HS, MC, and JX revised the manuscript. All authors revised the manuscript, and approved the final draft.
This work was supported by the National Science and Technology Major Project (2018ZX10101-001-001-003) and the National Natural Science Foundation of China (81872674).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with one of the author ZM at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the staff at the local Centers for Disease Control and Prevention (Yunnan, China) for their assistance in sample collection.
MTC, molecular transmission clusters; aOR, adjust odd ratio; PLWHA, people living with HIV/AIDS.
1. Cassels S. Time, population mobility, and HIV transmission. Lancet HIV. (2020) 7:e151–2. doi: 10.1016/s2352-3018(19)30413-8
2. Hué S, Clewley JP, Cane PA, Pillay D. HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. Aids. (2004) 18:719–28. doi: 10.1097/00002030-200403260-00002
3. Walter BL, Armitage AE, Graham SC, De Oliveira T, Iversen AK. Functional characteristics of HIV-1 subtype C compatible with increased heterosexual transmissibility. Aids. (2009) 23:1047–57. doi: 10.1097/QAD.0b013e32832a1806
4. Wang H, Yang Z, Zhu H, Mo Q, Tan H. High HIV-1 prevalence and viral diversity among entry-exit populations at Frontier ports of China, 2012–2016: a cross-sectional molecular epidemiology study. Infect Genet Evol. (2018) 65:231–7. doi: 10.1016/j.meegid.2018.08.003
5. Vrancken B, Mehta SR, Avila-Rios S, Garcia-Morales C, Tapia-Trejo D, Reyes-Teran G, et al. Dynamics and dispersal of local HIV epidemics within San Diego and across the San Diego-Tijuana border. Clin Infect Dis. (2020) 73:e2018–25. doi: 10.1093/cid/ciaa1588
6. Frentz D, Wensing AMJ, Albert J, Paraskevis D, Abecasis AB, Hamouda O, et al. Limited cross-border infections in patients newly diagnosed with HIV in Europe. Retrovirology. (2013) 10:36. doi: 10.1186/1742-4690-10-36
7. Lu L, Jia M, Ma Y, Yang L, Chen Z, Ho DD, et al. The changing face of HIV in China. Nature. (2008) 455:609–11. doi: 10.1038/455609a
8. Ren L, Wang B, Miao Z, Liu P, Zhou S, Feng Y, et al. A correlation analysis of HHV infection and its predictive factors in an HIV-seropositive population in Yunnan, China. J Med Virol. (2020) 92:295–301. doi: 10.1002/jmv.25609
9. Tee KK, Pybus OG, Li XJ, Han X, Shang H, Kamarulzaman A, et al. Temporal and spatial dynamics of human immunodeficiency virus type 1 circulating recombinant forms 08_BC and 07_BC in Asia. J Virol. (2008) 82:9206–15. doi: 10.1128/jvi.00399-08
10. Takebe Y, Liao H, Hase S, Uenishi R, Li Y, Li XJ, et al. Reconstructing the epidemic history of HIV-1 circulating recombinant forms CRF07_BC and CRF08_BC in East Asia: the relevance of genetic diversity and phylodynamics for vaccine strategies. Vaccine. (2010) 28(Suppl. 2):B39–44. doi: 10.1016/j.vaccine.2009.07.101
11. Li Z, He X, Wang Z, Xing H, Li F, Yang Y, et al. Tracing the origin and history of HIV-1 subtype B’ epidemic by near full-length genome analyses. Aids. (2012) 26:877–84. doi: 10.1097/QAD.0b013e328351430d
12. Li L, Assanangkornchai S, Duo L, McNeil E, Li J. Cross-border activities and association with current methamphetamine use among Chinese injection drug users (IDUs) in a China-Myanmar border region. Drug Alcohol Depend. (2014) 138:48–53. doi: 10.1016/j.drugalcdep.2014.01.021
13. Zhang L, Wang B, Liang Y, Feng Y, Dong S, Wang Y, et al. Phylogenetic characteristics of HIV-1 among travelers entering China from Myanmar: a retrospective study. J Med Virol. (2017) 89:1404–11. doi: 10.1002/jmv.24786
14. Lai D, Hwang LY, Beasley RP. HIV/AIDS testing at ports of entry in China. J Public Health Policy. (2011) 32:251–62. doi: 10.1057/jphp.2011.9
15. Drummond AJ, Nicholls GK, Rodrigo AG, Solomon W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics. (2002) 161:1307–20. doi: 10.1093/genetics/161.3.1307
16. Brenner BG, Roger M, Stephens D, Moisi D, Hardy I, Weinberg J, et al. Transmission clustering drives the onward spread of the HIV epidemic among men who have sex with men in Quebec. J Infect Dis. (2011) 204:1115–9. doi: 10.1093/infdis/jir468
17. Leigh Brown AJ, Lycett SJ, Weinert L, Hughes GJ, Fearnhill E, Dunn DT. Transmission network parameters estimated from HIV sequences for a nationwide epidemic. J Infect Dis. (2011) 204:1463–9. doi: 10.1093/infdis/jir550
18. Brenner BG, Michel R, Jean-Pierre R, Daniela M, Michel N, Claudine M, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. (2007) 195:951–9. doi: 10.1086/512088
19. Kouyos RD, Viktor VW, Sabine Y, Jürg B, Patrick T, Cyril S, et al. Molecular epidemiology reveals long-term changes in HIV type 1 subtype B transmission in Switzerland. J Infect Dis. (2010) 201:1488–97. doi: 10.1086/651951
20. Wang X, Wu Y, Mao L, Xia W, Zhang W, Dai L, et al. Targeting HIV prevention based on molecular epidemiology among deeply sampled subnetworks of men who have sex with men. Clin Infect Dis. (2015) 61:1462–8. doi: 10.1093/cid/civ526
21. Chen M, Ma Y, Chen H, Dai J, Jia M. Spatial clusters of HIV-1 genotypes in a recently infected population in Yunnan, China. BMC Infect Dis. (2019) 19:669. doi: 10.1186/s12879-019-4276-9
22. Chen M, Jia MH, Ma YL, Luo HB, Chen HC, Yang CJ, et al. The changing HIV-1 genetic characteristics and transmitted drug resistance among recently infected population in Yunnan, China. Epidemiol Infect. (2018) 146:775–81. doi: 10.1017/s0950268818000389
23. Xuan Q, Liang S, Qin W, Yang S, Zhang AM, Zhao T, et al. High prevalence of HIV-1 transmitted drug resistance among therapy-naive Burmese entering travelers at Dehong ports in Yunnan, China. BMC Infect Dis. (2018) 18:211. doi: 10.1186/s12879-018-3130-9
24. Ye M, Chen X, Wang Y, Zhou YH, Pang W, Zhang C, et al. HIV-1 drug resistance in ART-Naïve individuals in Myanmar. Infect Drug Resist. (2020) 13:1123–32. doi: 10.2147/idr.S246462
25. Akrim M, Lemrabet S, Elharti E, Gray RR, Tardy JC, Cook RL, et al. HIV-1 Subtype distribution in morocco based on national sentinel surveillance data 2004-2005. AIDS Res Ther. (2012) 9:5. doi: 10.1186/1742-6405-9-5
26. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. (1993) 10:512–26. doi: 10.1093/oxfordjournals.molbev.a040023
27. Eshleman SH, Hudelson SE, Redd AD, Wang L, Debes R, Chen YQ, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV prevention trials network 052 trial. J Infect Dis. (2011) 204:1918–26. doi: 10.1093/infdis/jir651
28. Duan X, Chen X, Wang J, Zhou S, Runhua YE, Wang Y, et al. Molecular transmission networks of HIV major subtypes strains in Dehong prefecture in Yunnan province during 2015-2016. Chin J Aids STD. (2019) 25:119–22. doi: 10.13419/j.cnki.aids.2019.02.03
29. Paraskevis D, Beloukas A, Stasinos K, Pantazis N, de Mendoza C, Bannert N, et al. HIV-1 molecular transmission clusters in nine European countries and Canada: association with demographic and clinical factors. BMC Med. (2019) 17:4. doi: 10.1186/s12916-018-1241-1
30. Fabeni L, Alteri C, Berno G, Scutari R, Orchi N, De Carli G, et al. Characterisation of HIV-1 molecular transmission clusters among newly diagnosed individuals infected with non-B subtypes in Italy. Sex Transm Infect. (2019) 95:619–25. doi: 10.1136/sextrans-2019-054017
31. Xin C, Lin D, Mei Y, Zhang C, Zheng YT. Non-Chinese immigrants: challenge faced by Yunnan of China to achieve the 90–90–90 Goals. Virol Sin. (2018) 33:291–3. doi: 10.1007/s12250-018-0038-x
32. Hao M, Wang J, Xin R, Li X, Hao Y, Chen J, et al. Low rates of transmitted drug resistances among treatment-Naive HIV-1-infected students in Beijing, China. AIDS Res Hum Retroviruses. (2017) 33:970–6. doi: 10.1089/aid.2017.0053
33. Graf M, Shao Y, Zhao Q, Seidl T, Köstler J, Wolf H, et al. Cloning and characterization of a virtually full-length HIV type 1 genome from a subtype B’-Thai strain representing the most prevalent B-clade isolate in China. AIDS Res Hum Retroviruses. (1998) 14:285–8. doi: 10.1089/aid.1998.14.285
34. Li W, Zhu Z, Chu J, Ge Y, Wei P. Multiple HIV-1 genotypes circulating among college students in Nanjing, China. AIDS Res Hum Retroviruses. (2020) 36:616–24. doi: 10.1089/AID.2019.0288
35. Feng Y, Takebe Y, Wei H, He X, Hsi JH, Li Z, et al. Geographic origin and evolutionary history of China’s two predominant HIV-1 circulating recombinant forms, CRF07_BC and CRF08_BC. Sci Rep. (2016) 6:19279. doi: 10.1038/srep19279
36. Yuan R, Cheng H, Chen LS, Zhang X, Wang B. Prevalence of different HIV-1 subtypes in sexual transmission in China: a systematic review and meta-analysis. Epidemiol Infect. (2016) 144:2144–53. doi: 10.1017/s0950268816000212
37. Kusagawa S, Sato H, Watanabe S, Nohtomi K, Takebe Y. Genetic and serologic characterization of HIV type 1 prevailing in Myanmar (Burma)*. Aids Res Hum Retroviruses. (1998) 14:1379–85. doi: 10.1089/aid.1998.14.1379
38. Pang W, Zhang C, Duo L, Zhou YH, Yao ZH, Liu FL, et al. Extensive and complex HIV-1 recombination between B’, C and CRF01_AE among IDUs in south-east Asia. Aids. (2012) 26:1121. doi: 10.1097/QAD.0b013e3283522c97
39. Zhou YH, Liang YB, Pang W, Qin WH, Yao ZH, Chen X, et al. Diverse forms of HIV-1 among Burmese long-distance truck drivers imply their contribution to HIV-1 cross-border transmission. BMC Infect Dis. (2014) 14:463. doi: 10.1186/1471-2334-14-463
40. Wan Z, Chen Q, Xin C, Lin D, Zhang C. HCV diversity among Chinese and Burmese IDUs in Dehong, Yunnan, China. PLoS One. (2016) 11:e0163062. doi: 10.1371/journal.pone.0163062
Keywords: HIV, molecular epidemiology, phylogenetic analysis, cross-border, subtype
Citation: Hu Z, Liu Y, Wang J, Meng Z, Leuba SI, Wei J, Duan X, Chu Z, Chen M, Shang H and Xu J (2022) Frequently Transmission and Close Relationship Among Immigrants in the China–Myanmar Border Region Indicated by Molecular Transmission Analysis From a Cross-Sectional Data. Front. Med. 8:693915. doi: 10.3389/fmed.2021.693915
Received: 12 April 2021; Accepted: 20 December 2021;
Published: 27 April 2022.
Edited by:
Jun Chen, Fudan University, ChinaReviewed by:
Yuhua Ruan, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, ChinaCopyright © 2022 Hu, Liu, Wang, Meng, Leuba, Wei, Duan, Chu, Chen, Shang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Chen, Y2hlbm1pbnl4QGhvdG1haWwuY29t; Hong Shang, aG9uZ3NoYW5nMTAwQGhvdG1haWwuY29t; Junjie Xu, eGpqY211QDE2My5jb20=
†ORCID: Zhili Hu, orcid.org/0000-0002-9574-6440; Yingjie Liu, orcid.org/0000-0002-6645-7359; Zhefeng Meng, orcid.org/0000-0001-9396-0673; Sequoia I. Leuba, orcid.org/0000-0002-6585-2824; Zhenxing Chu, orcid.org/0000-0002-6398-2107; Min Chen, orcid.org/0000-0002-9699-7524; Hong Shang, orcid.org/0000-0001-5333-8943; Junjie Xu, orcid.org/0000-0003-4303-7295
‡These authors have contributed equally to this work and share first authorship
§These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.