- 1Department of Endocrinology, Second Medical Center, Chinese People's Liberation Army General Hospital, National Clinical Research Center for Geriatric Diseases, Beijing, China
- 2School of Medicine, Dentistry and Biomedical Sciences, Queen's University Belfast, Belfast, United Kingdom
- 3Department of Gastroenterology, Second Medical Center, Chinese People's Liberation Army General Hospital, National Clinical Research Center for Geriatric Diseases, Beijing, China
- 4Department of Statistics and Epidemiology, Graduate School of Chinese People's Liberation Army General Hospital, Beijing, China
- 5Office of Information Management, Second Medical Center, Chinese People's Liberation Army General Hospital, Beijing, China
Objective: It is currently unclear whether the Helicobacter pylori (H. pylori) infection leads to associated alterations in thyroid functions and thyroidal illnesses. This study aims to analyse this relationship in an elderly male cohort over a five-year period.
Design: A case retrospective study.
Methods: A longitudinal study was designed to collect subjects (≥65 years old) receiving both a thyroid examination and H. pylori infection status determined by 13C-urea breath test in 2013 at our unit. Subjects were followed every 1 to 2 years until December 2017 for laboratory results, visits to outpatient clinics/emergency departments etc. Blood tests and thyroid ultrasonography were performed to determine thyroid function and morphology.
Results: 356 male subjects with mean age 78.5 ± 9.8 years were included. Active H. pylori infection was positive in 88 subjects (24.7%). Thyroid function tests and ultrasonography showed similar patterns between H. pylori positive and negative groups. Non-thyroidal-illness syndrome (NTIS) was diagnosed in 30/210 (14%) patients who experienced acute illnesses and hospitalization over five-year follow-up. Notably, NTIS demonstrated significantly higher prevalence in the H. pylori positive group compared to the negative group (17.1 vs. 5.6%, P = 0.001). Multivariate analysis showed that when age, APACHE II score and hemoglobin levels were adjusted, H. pylori status still has significant interrelationship with NTIS (OR = 3.497, P = 0.003).
Conclusions: There is a positive association between chronic active H. pylori infection and NTIS prevalence in this elderly male cohort. Further studies are needed to elucidate the role of H. pylori infection on NTIS in elderly male patients.
Introduction
Helicobacter pylori (H. pylori) infection is a common disease, affecting nearly half the world population, with more than an 80% prevalence in some areas of Asia (1). Besides its harmful effects on the gastrointestinal system, H. pylori's impact on other organ systems has been studied, and can lead to coronary artery disease, metabolic syndrome and insulin resistance, type 2 diabetes mellitus, among many other illnesses (2). Out of these manifestations, the link between H. pylori infection and thyroid abnormalities [including autoimmune thyroid diseases (ATD)] has been researched (3), however, all of these have applied no restrictions on the age of participants.
As age increases, the possibility of thyroid function disturbances rises due to morphological and physiological changes of the thyroid gland, which may ultimately lead to both the hospitalization and death of the patient (4), demonstrating the importance of thyroid function in elderly patients' health status. Transcriptome analyses have identified changes in the “aging thyroid's” gene expression, such as mitochondrial dysfunction and the loss of proteostasis (5). International guidelines have specified treatment considerations for clinical and subclinical thyroid dysfunction in elderly people (6).
Similar to their thyroid status, elderly people are likely to be colonized for longer with H. pylori compared to younger patients, and they also are more likely to experience prominent and severe immune-sequelae from its infection. Persistent active inflammation induced by H. pylori infection can lead to autoimmune immunopathological reactivity in affected patients, involving almost all body compartments (7). Systemic immune and inflammatory responses to H. pylori have been found to be related to extra-gastrointestinal system diseases, including thyroid abnormalities (8), such as lower free thyroxine level, thyroid nodules, high thyroid volume, and ATD (9–11).
This paper is a retrospective longitudinal study analyzing the relationship between active H. pylori infection and the patients' thyroid function over a five-year period, with an exclusive focus on elderly patients. Thyroid status was examined by thyroid function test, thyroid antibody testing, and ultrasonography of the gland. We presented significant associations between H. pylori infection and non-thyroidal-illness syndrome (NTIS) in our cohort. NTIS is often present in the critically ill patient, affecting the patient's recovery and prognosis (12, 13); therefore it is worthwhile to investigate its clinical significance in an elderly cohort.
Subjects and Methods
Study Design
We identified subjects who had received both thyroid examination and 13C-urea breath test (13C-UBT) (which was used for active H. pylori detection), between January 2013 and December 2017 from the database of Second Medical Center, Chinese PLA General Hospital, Beijing. Participants aged 65 years or older were enrolled during their visits to outpatient clinics in 2013, with all essential clinical information being recorded, including patient demographics, diagnoses, their main underlying diseases, laboratory results, procedures, and prescriptions. The subjects were then followed up every 1 to 2 years until 2017, for laboratory results; hospitalization; visits to outpatient clinics and emergency departments; diagnoses; procedures; prescriptions; and death. Blood test and thyroid ultrasonography were performed on the same day or no longer than 1 week before or after the 13C-UBT. At least three 13C-UBT tests were performed for each subject, with one test at the beginning and one at the end of the follow-up. Patient comorbidities were collected and analyzed by the Charlson Comorbidity Index (CCI) scoring system (14). Clinical information from the patients who were hospitalized and/or visited the emergency department during the study period was additionally collected. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score, widely used by Intensive Care Units to predict mortality (15), and Mini Nutritional Assessment Short-Form (MNA-SF) score, the screening tool for older patients' nutritional status (16), were recorded and analyzed in the first 24 h after admission. With regards to APACHE II score, it was sub-divided into groups scoring < 10 and ≥ 10, whereas for MNA-SF it was sub-categorized into scores < 12 and ≥ 12 groups.
At the start of study, we excluded subjects who had a diagnosis of hyperthyroidism or hypothyroidism, abnormal thyroid function, a prior history of thyroidal, hypophyseal or hypothalamic diseases, and those who were taking anti-thyroid or thyroid replacement medication and/or iodine-containing drugs. Subjects who had a conversion of 13C-UBT results (positive/negative alteration) and who became deceased during follow-up were also excluded. Furthermore, subjects taking drugs that might influence the results of 13C-UBT examination up to 4 weeks prior to screening (17) were additionally excluded from this study.
The study protocol was approved by the Institutional Review Board of Chinese PLA General Hospital (reference no: S2020-330-01). Since this is a retrospective statistical analysis on clinical data without disclosing the patients' identity, the need of consent was waived by the institutional ethical committee of the hospital.
Laboratory Assessments
Thyroid function tests were assessed via measurements of thyroid stimulating hormone (TSH) and total T3 (TT3), free T3 (FT3), total T4 (TT4), free T4 (FT4), along with anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-Tg) using immunochemiluminescent assays (Cobas e601, Roche Diagnostics Ltd., Switzerland) with the following normal reference ranges: TSH (0.35–5.5 mU/ml); TT3 (1.01–2.95 nmol/L); FT3 (2.76–6.3 pmol/L); TT4 (55.34–160.88 nmol/L); FT4 (10.42–24.32 pmol/L); anti-TPO (<60 IU/ml); anti-Tg (<60 IU/ml). Blood counts and blood chemistry panels were determined using an automated electronic counter (Sysmex XN3000, Sysmex Corporation, Kobe, Japan) and chemiluminescence on an autoanalyzer (Cobas c501, Roche Diagnostics Ltd., Switzerland), respectively.
Measurements
H. pylori infection was detected via a 13C-breath test instrument (Fischer Analysen Instrumente GmbH, Germany). The results of 13C-UBT were reported as H. pylori positive (delta over baseline value ≥ 4) or negative (delta over baseline value < 4). Thyroid nodules were determined by ultrasonography, and both the longest diameter and maximum cross-sectional area were calculated according to patient medical records. Enlargement of thyroid nodules was defined as the length and width of nodules increasing by more than 20%. The criteria for NTIS (18) applied in our study were as follows: (1) TT3 level <1.01 nmol/L and/or FT3 level <6.3 pmol/L; (2) TSH at the upper normal limit of 5.5 mU/L; (3) TT3 and FT3 level returned to normal range when the patients' condition improved or when they were discharged from hospital. Anemia was defined as hemoglobin values lower than 130.0 g/L (19).
Statistical Analysis
The Kolmogorov–Smirnov test was used to assess normal distribution variables. Student's t-test and analysis of variance (ANOVA) were used to compare continuous variables. One-way ANOVA and simple linear correlation were used to assess the relationship between continuous variables. Logistic regression was used to assess the relative influence of independent variables on H. pylori infection and calculate the odds ratios (ORs) and 95% confidence intervals (CIs). Continuous variables are presented as mean ± standard deviation (SD) and median (interquartile range) for variables of skewness distribution. Categorical variables were expressed as n (%). A P value of <0.05 was considered significant. Statistical analysis was performed using Statistical Package for SPSS (Windows version 19.0).
Results
Participants' General Information and Investigations
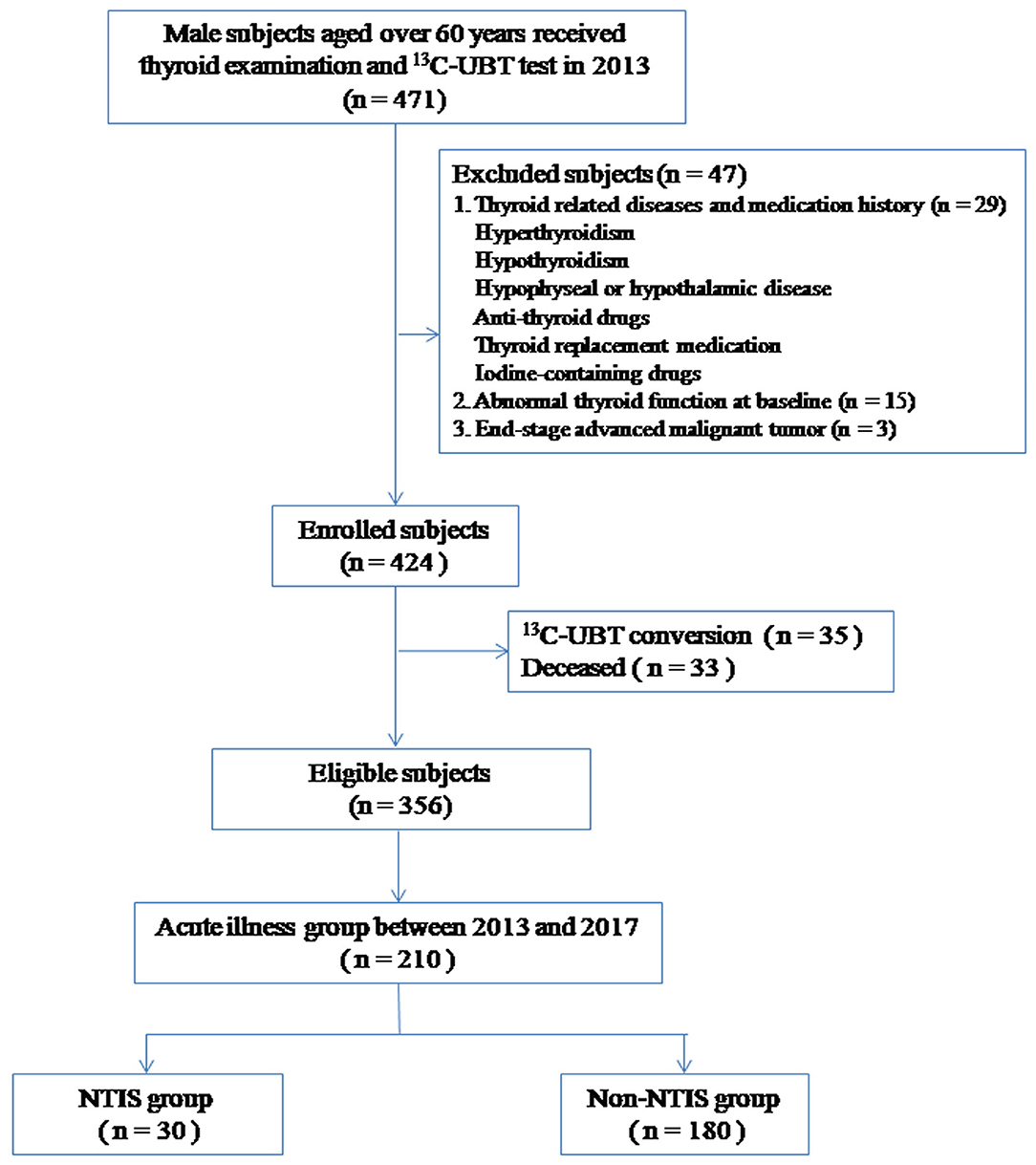
At the beginning of the study, 471 male subjects aged over 65 years were grouped. 47 were excluded according to the exclusion criteria, 33 were deceased and 35 showed 13C-UBT conversion during the follow-up. Finally, a total of 356 subjects were enrolled in the analysis (Figure 1). The mean age was 78.5 ± 9.8 years.
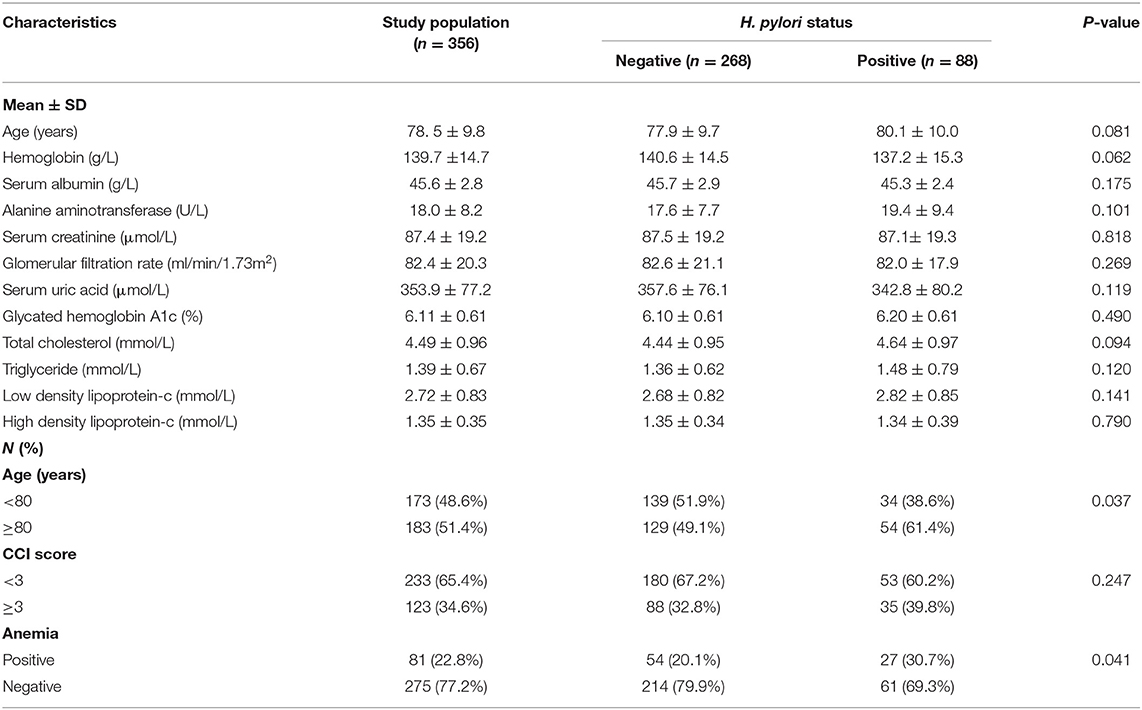
H. pylori positive results were present in 88 subjects (24.7%). A significantly higher portion of H. pylori positive patients were allocated in the ≥80 year group (61.4%) which demonstrated a correlation with patient age (P = 0.037). Patient comorbidities were measured via the CCI score, in which 65.4% of the participants (233 patients) scored < 3 points and 34.6% (123 patients) scored ≥ 3 points, but no interrelationship was found between H. pylori and particular CCI ranges.
The hemoglobin level was relatively lower in the H. pylori positive group compared to the negative group (137.3 ± 15.2 vs. 140.6 ± 14.5 g/L, P = 0.062). Eighty-one cases with hemoglobin <130 g/L were observed in this cohort. In general, the prevalence of anemia in the H. pylori positive group was higher than that in the negative group (30.7 vs. 20.1%, P = 0.041). There were no differences in the liver function tests, renal function tests, blood lipids, and chemical elements among the two groups (Table 1).
Thyroid Investigations at the Beginning of the Study
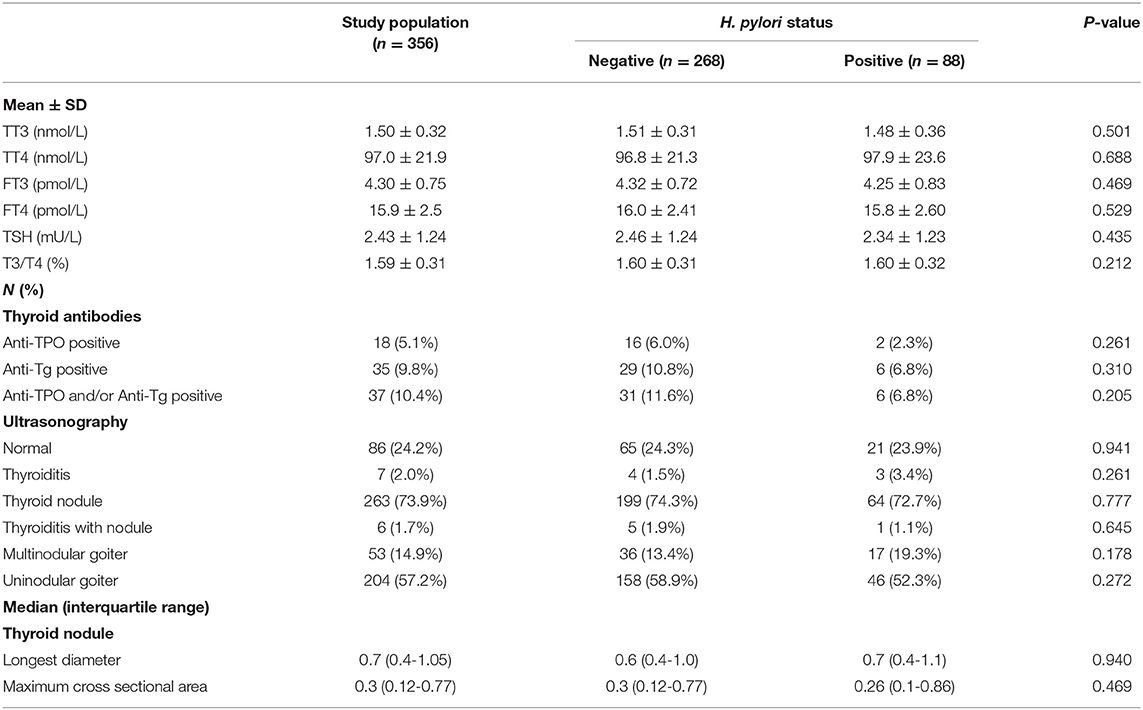
TT3, TT4, FT3, and FT4 levels did not differ significantly between the H. pylori positive and negative groups upon enrollment in this study. TSH levels were similar between these groups, as was the T3/T4 ratio and the presence of thyroid antibodies (anti-TPO and anti-Tg). The features of thyroid ultrasonography results (as shown in Table 2) were similarly distributed between these two groups.
Thyroid Investigations After 5 Years of Follow-Up
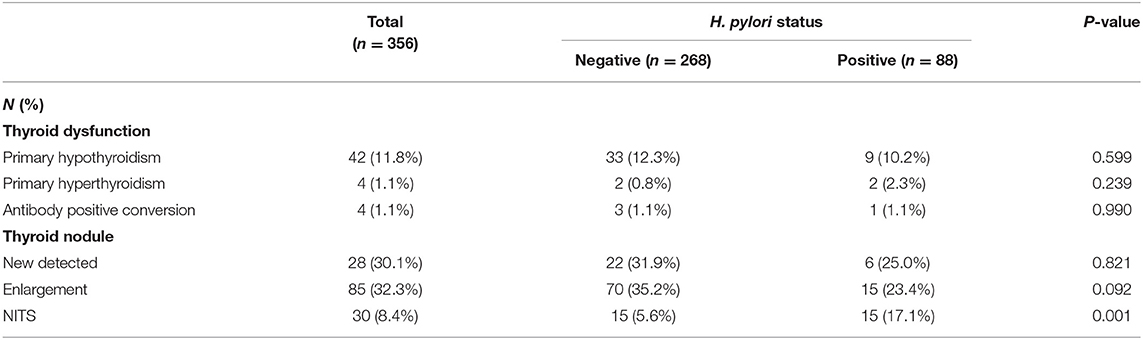
All 356 patients were followed up during the 5-year period, and they were still categorized based on their H. pylori status when they went through thyroid investigations. Overall, thyroid antibodies turned positive in 1.1% of all subjects, new thyroid nodules appeared in 30.1%, whereas nodule enlargement occurred in 32.3% of participants, primary hypothyroidism arose in 11.8% and primary hyperthyroidism emerged in 1.1% of the participants within the study group. Yet, none of these conditions' prevalence varies greatly between H. pylori positive and negative groups (Table 3 and Supplementary Table 1).
NTIS was newly diagnosed in 30 out of 210 patients who experienced acute illnesses and hospitalization during the 5-year follow-up. Among the 30 NTIS patients, the most diagnosis of acute illness upon hospitalization was pneumonia, followed by acute cerebral infarction and acute coronary syndrome. Detailed information was listed in Supplementary Table 2. No patient was hospitalized due to gastrointestinal bleeding or acute anemization due to peptic ulcer or gastric cancer. NTIS has demonstrated a significantly higher prevalence in the H. pylori positive group, for which the figure was approximately three times greater in comparison to the H. pylori negative group (17.1 vs. 5.6%, P = 0.001) (Table 3).
Factors Correlate With NTIS
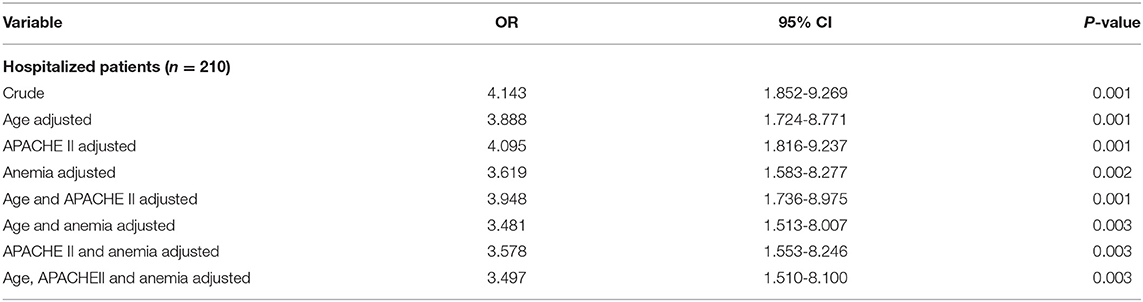
Our results have illustrated the positive correlation between H. pylori and NTIS (OR = 4.143, P = 0.001), however, multiple additional factors could also affect the prevalence of NTIS. Patients with NTIS have displayed a higher mean age compared to their non-NTIS counterparts, which were 85.0 ± 6.8 years and 81.9 ± 8.9 years accordingly (P = 0.033). Hemoglobin levels were significantly lower in the NTIS group which was 125.8 ± 16.7 g/L compared to 137.8 ± 13.9 g/L in the non-NTIS group (p = 0.001). The NTIS prevalence in anemia group was higher than that in the non-anemia group (25.8 vs. 9.0%, P = 0.001). In contrast, patients' CCI score, liver function, renal function, blood lipid, and thyroid function upon enrollment showed no correlation with the presence of NTIS. Neither the APACHE II nor the MNA-SF sub-groups demonstrated significant difference in their prevalence of NTIS. However, the NTIS proportion displayed higher values in the APACHE II score ≥ 10 group with a borderline p-value (20.0 vs. 10.8%, P = 0.063) (Supplementary Table 3). Multivariate analysis showed that when age, APACHE II score and anemia were adjusted, the H. pylori status still had significant correlation with NTIS (OR = 3.497, P = 0.003) (Table 4). We also studied the relationship between the occurrence of NTIS and patients' acute presenting diseases, including cardiovascular diseases, pulmonary diseases, stroke, trauma, etc. None of these diseases have demonstrated any significant relationship with the development of NTIS (P = 0.915, Supplementary Table 4).
Discussion
The most important finding from this study is that H. pylori infection over a chronic time frame exhibits significant correlation to NTIS prevalence in an elderly male cohort, and to the best of our knowledge this is the first study reporting this association.
NTIS is a term used to encompass a syndrome whereby there is an alteration in thyroid hormone function during non-thyroidal illness (20); characteristically low T3 and/or T4 levels and increased reverse T3 levels (and in some cases reduced or normal TSH) on thyroid hormone profile, despite the patient being clinically euthyroid (21, 22). It has been well-documented in critically-ill patients (23), but it is also generally accepted that it can occur as an adaptive response secondary to nearly any type of acute or chronic illness (21). This is thought to result from an allostatic response from the thyroid in response to conditions of strain or stress, possibly to conserve energy (24). Clinically, there may be no symptoms or signs of NTIS in an affected patient, and indeed there are no findings specific to the condition (25). Some debates exist as to whether the illness requires medical management, in the form of hormone replacement (25, 26), but definitive management lies in treating the underlying disease contributing to systemic inflammation. NTIS is very common in critically-ill, hospitalized elderly patients, emerging as the most sensitive independent predictor of short-term survival. Research indicates that the NTIS prevalence (specifically low T3 syndrome) was 31.9% in hospitalized elderly patients. The mortality rate was significantly higher among patients with low T3 syndrome, which emerged as the sole predictive factor of death (27).
It is plausible that the inflammatory processes related to chronic H. pylori infection may have systemic manifestations, and this could lead to an adaptive response such as that seen in NTIS. Indeed, there are studies demonstrating that chronic H. pylori infection may extend to inflame and compromise extra-intestinal organs; in particular, endocrine organs (28). Since NTIS may develop on a background of any chronic inflammatory illness, it seems plausible that H. pylori may also be contributing to NTIS in our patient cohort through similar means.
Although there has been no direct evidence suggesting the relationship between H. pylori and NTIS to date, a study investigating the link between NTIS and chronic obstructive pulmonary disease (COPD) found that tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were significantly higher in NTIS patients (29). Meanwhile, H. pylori-mediated chronic inflammation has also been shown to increase the expression of TNF-α and IL-6 (30, 31). A causal role for IL-6 in the development of NTIS in mice has been shown since IL-6 knock out mice show a less pronounced drop in serum T3 during illness (32). Infusions of rTNF in men appears to decrease serum T3 and TSH and increase reverse-T3 (rT3) (33), leading to the thyroid hormone profile characteristically seen in NTIS.
Under acute illnesses, patients experience the same NTIS hormonal pattern via the manipulation type 2 deiodinase (D2) and type 1 deiodinase (D1), with the subsequent activation of cytokines TNF, IL-1, IL-6, and NF-kB (12), which may exhibit some common molecular pathways irrespective of the underlying disease in question. In our study, a similar likelihood of NTIS was found in different disease types; this demonstrates that NTIS prevalence was independent to the disease category. Our findings are further backed up by Plikat et al. (34), who has stated that the frequency of NTIS is only influenced by disease severity and prognosis, rather than disease type.
However, aforementioned in our results, there were additional factors identified possibly correlating with the development of NTIS other than H. pylori, and in our study we have demonstrated a potential link between APACHE II score and NTIS (P = 0.063, borderline significant). APACHE II score is used for disease severity classification typically for Intensive Care Unit patients (15), and a higher score is indicative of greater severity of systemic illness and a subsequently higher mortality rate. Multiple pieces of evidence have shown that NTIS emergence is closely related to the severity of illness of the host (12); this is due to the upregulation of type 3 deiodinase (D3) in severely-ill patients, which inactivates both T4 and T3 molecules and creates rT3, as well as increasing D2 expression, which activates macrophages and cytokines, resulting in NTIS patterns via hypothalamic pituitary axis interference and thyroid hormones binding inhibition (25). Therefore, a potential link can be established between high APACHE II score and the development of NTIS.
We have also demonstrated a significant association between NTIS and baseline serum hemoglobin (P < 0.05) suggesting anemia itself may contribute to the development of NTIS. Anemia due to chronic illness is often referred to as anemia of chronic disease (also known as anemia of inflammation) (35). In this context, anemia is evidence of an activated immune system and has been proposed to be the result of a protective defense strategy (36); this parallels to NTIS which is thought to be an allostatic response (24). A significant association (P = 0.041) between active H. pylori infection and anemia was identified at the beginning of the study, which corroborated our previous findings (37). While there is an inverse relationship between TNF-α and hemoglobin (38), this also adds consideration to the hypothesis that inflammation in H. pylori may cause the initial rise of TNF-α, which itself may potentially lead to anemia. As anemia is a marker of inflammation that may result as part of an adaptive response as is the case in NTIS, it is possible to establish a plausible link between H. pylori, anemia and NTIS. Our study worked within the hypothesis that anemia was secondary to inflammation, but it should also be kept in mind that individuals experienced anemia secondary to acute or chronic gastrointestinal bleeding. In the clinical practice, acute gastrointestinal bleeding is a common entity in the elderly due to peptic ulcer or gastric cancer which is closely related to H. pylori infection. Furthermore, it is common for elderly patients to take long term aspirin and other anticoagulants, and we are also aware of the effects of these drugs on gastric bleeding, when the incidence was even higher with the coexistence of H. pylori (39). However, no gastrointestinal bleeding case was exhibited in the NTIS group in this study, we did not focus on drugs such as aspirin and anticoagulants in this group. The only cancerous patient in the NTIS group (Supplementary Table 2) was hospitalized due to obstruction of the gastric cardia, and his hemoglobin was normal and fecal occult blood test was negative when the NTIS occured. All in all, it could be speculated that eradication of H. pylori would help to reduce the prevalence of NTIS subsequent to acute gastrointestinal bleeding in the elderly.
In our cohort, we additionally showed no association between H. pylori infection and ATD. The relationship between H. pylori and ATD is an area of some uncertainty, with some studies finding positive associations whereas others find no correlation (28). However, a lack of information regarding this association in Asian populations has been previously identified (3) and our results add to the literature in this area. Furthermore, even within the continent, H. pylori prevalence is shown to differ widely by geography (40), as does genotypic variation (41), and our results at least suggest no correlation between H. pylori infection and ATD in our study cohort. Although NTIS is a disturbance in thyroid function, the causative agent is the underlying chronic inflammation secondary to another illness (21). Owing to the high prevalence of chronic H. pylori infection in elderly people, it could be speculated that H. pylori infection in combination with physiological changes of aging process could lower the NTIS threshold and increase its occurrence in this specific population, although no causal relationship could be found between H. pylori infection and NTIS yet. Therefore, clinically ensuring that H. pylori is identified and eradicated in individuals colonized with the bacteria may help to reduce subsequent development of NTIS, which in turn could improve patient prognosis, especially in elderly patients.
Our study has several limitations. Although 13C-UBT has the greatest combination of sensitivity and specificity (41) for H. pylori infection diagnosis and the guideline recommends it as the first choice of non-invasive test in the clinical practice, especially in the elderly (42), false positive or negative 13C-breath test results should be noticed since they are correlated with gastric atrophy, common in the elderly (43). Therefore, using additional confirmatory testing (i.e., biopsy for histological diagnosis) has the potential to verify the validity of H. pylori diagnosis. As a retrospective study from a single center, rT3 data was not available to be recorded from our data collection. Nevertheless, serum concentrations of rT3 can be increased or remain unchanged in both acute and chronically critically ill patients with NTIS (24). Furthermore, H. pylori strain and genotypic markers are not reported, which may relate to disease risk and clinical outcome (44). We noticed that all our samples are males and cannot be representative of the whole population, however, we have not excluded any patients base on their sex and gender. Unfortunately, we could enroll only male subjects due to the nature of our institution. We believe this elderly-male sample has also helped to exclude the impact of gender on our results. Conversely, a relatively large study population with equivalent gender distribution investigating multiple clinical outcomes over a long period of time, with multivariate analysis to identify potentially confounding factors, has the potential to add both validity and weight to our study. Additionally, our study only established indefinite correlations between variables due to its observational nature and would be more reliable if the underlying molecular mechanisms were researched further (i.e., detection of IL-6, TNF etc.), which could indicate the direction of future study. As the first study of its kind, further research is necessary in order to make clinical recommendations and conclusions on the association.
Conclusion
There is a positive association between chronic active H. pylori infection and the prevalence of NTIS in this elderly male cohort over a 5-year period. The prevalence of NTIS tends to increase with age, low hemoglobin and high APACHE II score, but not with underlying illnesses. We found no correlation between H. pylori infection and ATD or thyroid morphological (ultrasonographic) investigation. Further studies are needed to delineate the role and underlying mechanisms of chronic H. pylori infection on NTIS in the elderly, then to provide valuable suggestions to geriatric practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethical committee of the Chinese PLA General Hospital, Beijing, China had permitted to use the data for this project. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
BS collected data, performed the data analysis, statistical analysis, and manuscript preparation. XW and MM searched literature, conducted research, and wrote the initial manuscript. YD and WD collected data and conducted research. ML performed statistical analysis. GW conceived and designed research, revised the manuscript, and had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This study was supported by Healthcare Research Projects, China PLA (grant number: 18BJZ22) and National Clinical Research Center for Geriatric Diseases (grant number: NCRCG-PLAGH-2019015). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank National Clinical Research Center for Geriatric Diseases, China for their support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.682116/full#supplementary-material
Abbreviations
13C-UBT, 13C-urea breath test; anti-Tg, anti-thyroglobulin; anti-TPO, anti-thyroid peroxidase; APACHE II, Acute Physiology and Chronic Health Evaluation II, ATD, autoimmune thyroid diseases; CCI, Charlson comorbidity index; CIs, Confidence intervals; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; D1, Type 1 deiodinase; D2, Type 2 deiodinase; D3, Type 3 deiodinase; DOB, Delta over baseline value of 13C-UBT; FT3, Free triiodothyronine; FT4, Free thyroxine; H. pylori, Helicobacter pylori; IL-6, Interleukin-6; MNA-SF, Mini Nutritional Assessment Short-Form; NF-κB, Nuclear factor-κ-gene binding; NTIS, Non-thyroidal-illness syndrome; OR, Odds ratio; rT3, Reverse-triiodothyronine; SD, Standard deviation; TFT, Thyroid function test; TNF-α, Tumor necrosis factor-α; TSH, Thyroid-stimulating hormone; TT3, Total triiodothyronine; TT4, Total thyroxine.
References
1. Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. (2010) 25:479-86. doi: 10.1111/j.1440-1746.2009.06188.x
2. Wong F, Rayner-Hartley E, Byrne MF. Extraintestinal manifestations of Helicobacter pylori: a concise review. World J Gastroenterol. (2014) 20:11950-61. doi: 10.3748/wjg.v20.i34.11950
3. Choi YM, Kim TY, Kim EY, Jang EK, Jeon MJ, Kim WG, et al. Association between thyroid autoimmunity and Helicobacter pylori infection. Korean J Intern Med. (2017) 32:309-13. doi: 10.3904/kjim.2014.369
4. Gesing A, Lewinski A, Karbownik-Lewinska M. The thyroid gland and the process of aging; what is new? Thyroid Res. (2012) 5:16. doi: 10.1186/1756-6614-5-16
5. Cho BA, Yoo SK, Song YS, Kim SJ, Lee KE, Shong M, et al. Transcriptome network analysis reveals aging-related mitochondrial and proteasomal dysfunction and immune activation in human thyroid. Thyroid. (2018) 28:656-66. doi: 10.1089/thy.2017.0359
6. Chaker L, Cappola AR, Mooijaart SP, Peeters RP. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. (2018) 6:733-42. doi: 10.1016/S2213-8587(18)30028-7
7. Sorrentino D, Ferraccioli GF, De Vita S, Labombarda A, Boiocchi M, Bartoli E. Helicobacter pylori infection and autoimmune processes: an emerging field of study. Ital J Gastroenterol Hepatol. (1998) 30(Suppl 3):S310-2.
8. Banic M, Franceschi F, Babic Z, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. (2012) 17(Suppl 1):49-55. doi: 10.1111/j.1523-5378.2012.00983.x
9. Shen Z, Qin Y, Liu Y, Lu Y, Munker S, Chen L, et al. Helicobacter pylori infection is associated with the presence of thyroid nodules in the euthyroid population. PLoS ONE. (2013) 8:e80042. doi: 10.1371/journal.pone.0080042
10. Arslan MS, Ekiz F, Deveci M, Sahin M, Topaloglu O, Karbek B, et al. The relationship between cytotoxin-associated gene A positive Helicobacter pylori infection and autoimmune thyroid disease. Endocr Res. (2015) 40:211-4. doi: 10.3109/07435800.2015.1015727
11. Triantafillidis JK, Georgakopoulos D, Gikas A, Merikas E, Peros G, Sofroniadou K, et al. Relation between Helicobacter pylori infection, thyroid hormone levels and cardiovascular risk factors on blood donors. Hepatogastroenterology. (2003) 50(Suppl 2):cccxviii-cccxx.
12. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. (2015) 3:816-25. doi: 10.1016/S2213-8587(15)00225-9
13. Xiong H, Yan P, Huang Q, Shuai T, Liu J, Zhu L, et al. A prognostic role for non-thyroidal illness syndrome in chronic renal failure:a systematic review and meta-analysis. Int J Surg. (2019) 70:44-52. doi: 10.1016/j.ijsu.2019.08.019
14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373-83. doi: 10.1016/0021-9681(87)90171-8
15. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818-29. doi: 10.1097/00003246-198510000-00009
16. Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. (2001) 56:7. doi: 10.1093/gerona/56.6.M366
17. Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. (2015) 21:1305-14. doi: 10.3748/wjg.v21.i4.1305
18. Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol. (1993) 39499-518. doi: 10.1111/j.1365-2265.1993.tb02401.x
19. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. (1968) 405:5-37.
20. Papanicolaou DA. Euthyroid sick syndrome and the role of cytokines. Rev Endocr Metab Disord. (2000) 1:43-8. doi: 10.1023/a:1010060303031
21. Pappa TA, Vagenakis AG, Alevizaki M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. (2011) 41:212-20. doi: 10.1111/j.1365-2362.2010.02395.x
22. Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocr Rev. (1982) 3:164-217. doi: 10.1210/edrv-3-2-164
23. Golombek SG. Nonthyroidal illness syndrome and euthyroid sick syndrome in intensive care patients. Semin Perinatol. (2008) 32:413-8. doi: 10.1053/j.semperi.2008.09.010
24. Chatzitomaris A, Hoermann R, Midgley JE, Hering S, Urban A, Dietrich B, et al. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front Endocrinol. (2017) 8:163. doi: 10.3389/fendo.2017.00163
26. DeGroot LJ. The Non-Thyroidal Illness Syndrome. Edited by Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland J, Kaltsas G et al. South Dartmouth (MA): Endotext (2000).
27. Tognini S, Marchini F, Dardano A, Polini A, Ferdeghini M, Castiglioni M, et al. Non-thyroidal illness syndrome and short-term survival in a hospitalised older population. Age Ageing. (2010) 39:46-50. doi: 10.1093/ageing/afp197
28. Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ. Helicobacter pylori infection and endocrine disorders: is there a link? World J Gastroenterol. (2009) 15:2701-7. doi: 10.3748/wjg.15.2701
29. Karadag F, Ozcan H, Karul AB, Yilmaz M, Cildag O. Correlates of non-thyroidal illness syndrome in chronic obstructive pulmonary disease. Respir Med. (2007) 101:1439-46. doi: 10.1016/j.rmed.2007.01.016
30. Tsai S, Clemente-Casares X, Revelo XS, Winer S, Winer DA. Are obesity-related insulin resistance and type 2 diabetes autoimmune diseases? Diabetes. (2015) 64:1886-97. doi: 10.2337/db14-1488
31. Yildirim Z, Bozkurt B, Ozol D, Armutcu F, Akgedik R, Karamanli H, et al. Increased exhaled 8-isoprostane and interleukin-6 in patients with Helicobacter pylori infection. Helicobacter. (2016) 21:389-94. doi: 10.1111/hel.12302
32. Boelen A, Maas MA, Lowik CW, Platvoet MC, Wiersinga WM. Induced illness in interleukin-6 (IL-6) knock-out mice: a causal role of IL-6 in the development of the low 3,5,3'-triiodothyronine syndrome. Endocrinology. (1996) 137:5250-4. doi: 10.1210/endo.137.12.8940342
33. van der Poll T, Romijn JA, Wiersinga WM, Sauerwein HP. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab. (1990) 71:1567-72. doi: 10.1210/jcem-71-6-1567
34. Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Scholmerich J, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. (2007) 56:239-44. doi: 10.1016/j.metabol.2006.09.020
35. Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. (2014) 28:671-81, vi. doi: 10.1016/j.hoc.2014.04.005
36. Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. (2002) 16:87-96. doi: 10.1054/blre.2002.0193
37. Hou B, Zhang M, Liu M, Dai W, Lin Y, Li Y, et al. Association of active Helicobacter pylori infection and anemia in elderly males. BMC Infect Dis. (2019) 19:228. doi: 10.1186/s12879-019-3849-y
38. Afifi MT, Abd El-Aziz HK, Hamed NA, Barghash NA, Abdo A, Gamal M. Role of Helicobacter pylori in refractory iron deficiency anaemia. Br J Biomed Sci. (2009) 66:133-6. doi: 10.1080/09674845.2009.11730259
39. Fashner J, Gitu AC. Diagnosis and treatment of peptic ulcer disease and H. pylori infection. Am Fam Physician. (2015) 91:236-42.
40. Lu C, Yu Y, Li L, Yu C, Xu P. Systematic review of the relationship of Helicobacter pylori infection with geographical latitude, average annual temperature and average daily sunshine. BMC Gastroenterol. (2018) 18:50. doi: 10.1186/s12876-018-0779-x
41. Blaser MJ. Heterogeneity of Helicobacter pylori. Eur J Gastroenterol Hepatol. (2012) 9(Suppl 1):S3-6; discussion S6-7. doi: 10.1097/00042737-201204001-00002
42. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. (2017) 66:6-30. doi: 10.1136/gutjnl-2016-312288
43. Gomollon F, Ducons JA, Santolaria S, Lera Omiste I, Guirao R, Ferrero M, et al. Breath test is very reliable for diagnosis of Helicobacter pylori infection in real clinical practice. Dig Liver Dis. (2003) 35:612-8. doi: 10.1016/S1590-8658(03)00373-6
Keywords: Helicobacter pylori, non-thyroidal-illness syndrome, thyroid function, thyroid morphology, elderly, male
Citation: Sun B, Wang X, McLarnon MED, Ding Y, Liu M, Dai W and Wang G (2021) Higher Prevalence of Non-thyroidal-Illness Syndrome in Elderly Male Patients With Active Helicobacter pylori Infection. Front. Med. 8:682116. doi: 10.3389/fmed.2021.682116
Received: 23 March 2021; Accepted: 11 June 2021;
Published: 08 July 2021.
Edited by:
Giuseppe Losurdo, University of Bari Medical School, ItalyReviewed by:
Edith Lahner, Sapienza University of Rome, ItalyFabrizio Bossa, Casa Sollievo della Sofferenza (IRCCS), Italy
Copyright © 2021 Sun, Wang, McLarnon, Ding, Liu, Dai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gangshi Wang, d2FuZ2dhbmdzaGkmI3gwMDA0MDtob3RtYWlsLmNvbQ==; orcid.org/0000-0003-3831-6871
 Banruo Sun
Banruo Sun Xuanping Wang
Xuanping Wang Michael Edmund David McLarnon
Michael Edmund David McLarnon Yu Ding
Yu Ding Miao Liu
Miao Liu Wei Dai
Wei Dai Gangshi Wang
Gangshi Wang