
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 28 May 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.675207
 Jong-In Chang1†
Jong-In Chang1† Keol Lee1†
Keol Lee1† Dongwuk Kim1†
Dongwuk Kim1† Ju-II Yang2
Ju-II Yang2 Jae Keun Park3
Jae Keun Park3 Kyu Choi1
Kyu Choi1 Soo Hoon Kang1
Soo Hoon Kang1 Kwang Hyuck Lee1
Kwang Hyuck Lee1 Kyu Taek Lee1
Kyu Taek Lee1 Jong Kyun Lee1
Jong Kyun Lee1 Seon Mee Park4*
Seon Mee Park4* Joo Kyung Park1*
Joo Kyung Park1*Background: Clonorchis sinensis (CS) infection is considered a group 1 carcinogen of cholangiocarcinoma (CCA). There were very few studies regarding clinical characteristics of CS-associated CCA (CACC). This study aimed to investigate clinical characteristics of patients with CCA with or without CS infection.
Methods: A total of 367 patients diagnosed with CCA who underwent diagnostic tests for CS infection were enrolled. CS infection was defined as follows: at least one positive serum ELISA test, skin test, stool microscopy, or bile microscopy.
Results: There were 95 (26%) patients with CS infections. The median follow-up duration was 14.9 months (range, 6.07–36.17). The following significant differences were noted among patients with CACC compared to non-CACC; diagnosis at younger age (median 62 years vs. 65 years, p = 0.018), higher male to female ratio (83.2 vs. 61.8%, p < 0.001), and residence in CS-endemic area (46.3 vs. 25.4%, p = 0.014). Univariate analysis of prognostic factors indicated that tumor location, curative resection, tumor stage, and laboratory tests including CA 19-9, CEA, and bilirubin were significantly associated with overall survival, but CS infection was not. In multivariate analysis, tumor location, CEA, curative resection and tumor stage were identified as independent prognostic factors. Among patients under age 64, CACC group had lower survival rate than non-CACC group (p = 0.022).
Conclusions: CACC had the following significant characteristics compared to non-CACC; diagnosis at younger age, higher male to female ratio, higher prevalence in CS endemic areas and poorer overall survival in patients under age 64.
Clonorchiasis is a parasitic infection by Clonorchis sinensis (CS), which people contract by ingestion of metacercariae in raw or undercooked freshwater fish (1). CS infestation can persist in bile duct for at least 26 years and causes various complications in liver and biliary systems, mainly cholelithiasis, cholangitis, and even cholangiocarcinoma (CCA) (2–4). CS infection was considered as a group 1 carcinogen by the International Agency for Research on Cancer (5). In particular, East Asia including Southeast China, Vietnam, and Korea is an endemic area for clonorchiasis, and the estimated CCA incidence rate among the population infected with CS was extremely high, reaching 35 per 100,000 men and 25 per 100,000 women (6).
Recently, the incidence of intrahepatic CCA has increased worldwide, while the incidence of extrahepatic CCA has decreased in Western countries and remained unchanged in Asian countries (3). In East Asia, clonorchiasis is a powerful risk factor along with choledochal cysts, primary sclerosing cholangitis, and hepatolithiasis (7). The proportion of CS-associated CCA (CACC) among entire CCA has ranged widely from 2.2 to 41.4% in previous studies (8–10). Although there is a strong relationship between clonorchiasis and CCA, there have been few studies regarding clinical characteristics and prognosis in patients with CACC.
In this study, we investigated the clinical characteristics and prognosis of CACC compared to CCA without chronic CS infection (non-CACC) among a large number of patients over long-term follow-up.
This is a retrospective study at Samsung Medical Center, between December 1994 and March 2015. We screened adult patients (over 18 years of age) who met the following inclusion criteria: (1) diagnosed with CCA histologically confirmed by surgical specimen, biopsy or cytology with endoscopic retrograde cholangiopancreatography or endoscopic ultrasound, liver biopsy, bile cytology from percutaneous transhepatic biliary drainage, or core needle biopsy or fine-needle aspiration of lymph nodes (2) underwent diagnostic tests for CS infection before the diagnosis of CCA. A total of 439 patients met the inclusion criteria. Among them, 72 patients who met any of the following criteria were excluded: (1) hepatocellular carcinoma combined with CCA on pathology, (2) a follow-up duration <30 days, or (3) other hepatobiliary malignancies. Therefore, a total of 367 patients were analyzed.
Enrolled patients were divided into two groups according to CS infection: the CACC group and the non-CACC group. Evidence of CS infection was defined as a positive result on serologic ELISA or skin test, or microscopic confirmation of CS eggs or adult worms on stool or bile specimens.
We also collected demographic, biochemical, and clinical data including cancer stage, treatment intent, and survival time. The endemic area in this study consisted of the basin of four major rivers (Gum, Seomjin, Youngsan, and Nagdong rivers) located in the southern part of Korea (11). CCA was staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis staging system. Treatment intent was classified as curative or palliative intent. Curative intent treatment included R0 or R1 resection by segmental common bile duct resection, hepatectomy, or Whipple operation. Palliative intent treatment included R2 resection, repetitive biliary drainage, local radiotherapy, or chemotherapy. Overall survival (OS) was defined as the period between the date of diagnosis of CCA and the date of death or the last day of hospital visitation until November 30, 2015. The data of death were obtained from the national death register.
We evaluated the methodological quality of our study using the Newcastle-Ottawa Scale (NOS) for cohort study (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp), which is the most frequently used tool nowadays (12, 13).
The study protocol was approved by the Institutional Review Board of Samsung Medical Center. Because this study involved a retrospective analysis of existing clinical data, the need for informed consent from patients was waived (IRB No. 2015-10-129-002).
SPSS 21.0 (IBM, Armonk, NY) was used for statistical analysis. Student's t-test, Pearson's chi-square test, Fisher's exact test, and Mann-Whitney U-test were used to compare groups with or without CS infection. The Kaplan–Meier method with Log-rank test was performed to compare OS between groups with different clinical parameters. The Cox proportional hazards model was used to identify independent prognostic factors. All tests of significance were two-tailed and a P < 0.05 was considered statistically significant.
The clinical characteristics of study patients are summarized in Table 1. The median age was 64 years and 67.3% of patients were male. The median follow-up duration was 14.9 months (range: 6.07–36.17). Tumor location was classified as intrahepatic, perihilar, and distal bile duct, which was 125 (34.1%), 128 (34.9%), and 114 (31.1%), respectively. Eighty-seven patients (23.7%) were stage I, 50 (13.6%) were stage II, 85 (22.9%) were stage III, and 146 (39.8%) were stage IV. Among them, 170 (46.3%) patients were treated with curative intent, while 196 (53.6%) were treated with palliative intent.
The CACC group and the non-CACC group included 95 (25.9%) and 272 (74.1%) patients, respectively. The proportion of CACC and non-CACC did not change over 20 years, with a proportion of CACC during the two periods, 1995–2004 and 2005–2015, of 25.6% and 25.7% (p = 0.021).
Comparison of clinical characteristics of the two groups is summarized in Table 2. The CACC group was significantly younger at diagnosis of CCA (median, 65 years vs. 62 years, p = 0.018), had higher male to female ratio (83.2 vs. 61.8%, p < 0.001), more frequent elevated serum CEA levels (>5 U/ml), and included more patients living in CS-endemic areas (46.3 vs. 25.4%, p = 0.014) than the non-CACC group. The non-CACC group had more patients with a history of hepatolithiasis than the CACC group (12.9 vs. 3.2%, p = 0.005).
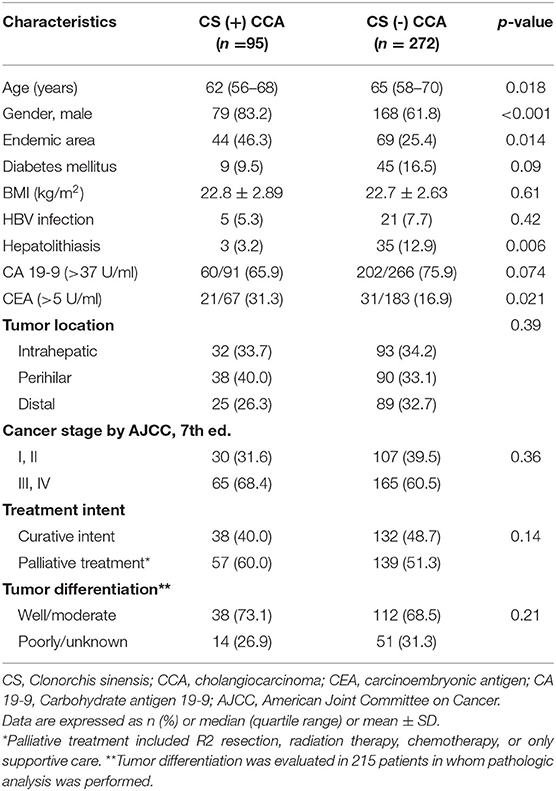
Table 2. Comparison of clinical characteristics of cholangiocarcinoma patients according to Clonorchis sinensis infection.
Among the 367 CCA patients, 308 died during the follow-up period. 1-, 3-, and 5-year survival was 57.9, 27.2, 18.2%, respectively, with a median OS of 15.3 months (95% CI, 13.3–17.3) (Figure 1). The survival rate of the CACC group was not significantly different from that of the non-CACC group, with a median OS of 12.7 months (95% CI, 7.56–17.84) and 15.7 months (95% CI, 13.5–17.9), respectively (p = 0.141) (Figure 2A). Subgroup analysis divided according to median age of 64 years indicated that the CACC group had a significantly lower survival rate than the non-CACC group, with median OS of 8.2 months (95% CI 5.2–11.2) and 17.1 months (95% CI 11.8–22.4), respectively, in patients aged under 64 years (p = 0.022) (Figure 2B); however, OS was similar among those aged 64 or over regardless of CS infection (Figure 2C).
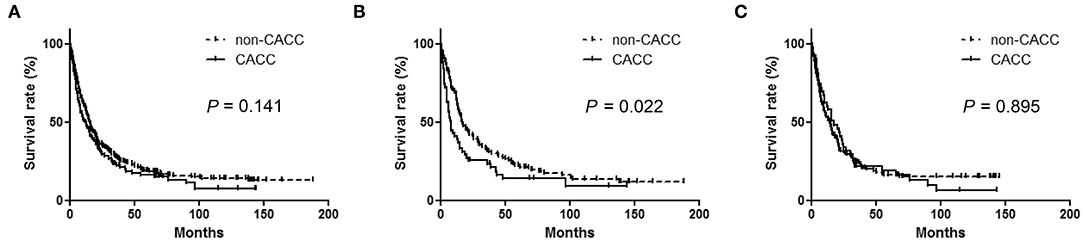
Figure 2. Kaplan-Meier curves according to Clonorchis sinensis infection in analysis: (A) The entire cohort (B) Age <64 years (C) Age ≥ 64 years. Comparison of median survival time in patients younger than 64 years old between Clonorchis sinensis-associated cholangiocarcinoma (CACC) and non-Clonorchis sinensis-associated cholangiocarcinoma (non-CACC).
Among patients aged under 64 years, elevated CEA levels (>5 U/ml) (34.3 vs. 13.0%, p = 0.019) and smoking history (55.6 vs. 27.1%, p = 0.004) were more frequent in the CACC group compared to the non-CACC group. Among patients aged 64 years or over, the characteristics of the CACC group and non-CACC group were similar. Subgroup analysis according to sex and the endemic area did not show survival differences between younger and older groups.
In univariate analysis, tumor location, cancer stage, treatment intent, CA 19-9, CEA, and total bilirubin were significantly associated with overall survival in CCA patients (Table 3). To identify independent prognostic factors for survival, all parameters under a P-value of 0.2 in univariate analysis were included in multivariable Cox proportional hazards regression analysis. Tumor location, CEA levels, cancer stage, and treatment intent were independent prognostic factors in CCA patients (Table 4). We had a subgroup analysis for the patients under 64 years old. Univariate analysis showed CS infection, tumor location, cancer stage, tumor differentiation, treatment intent, CA 19-9, CEA, and total bilirubin are associated with overall survival in CCA patients under 64 years old. In multivariate analysis, treatment intent and cancer stage were independent prognostic factors in CCA patients under 64 years old.
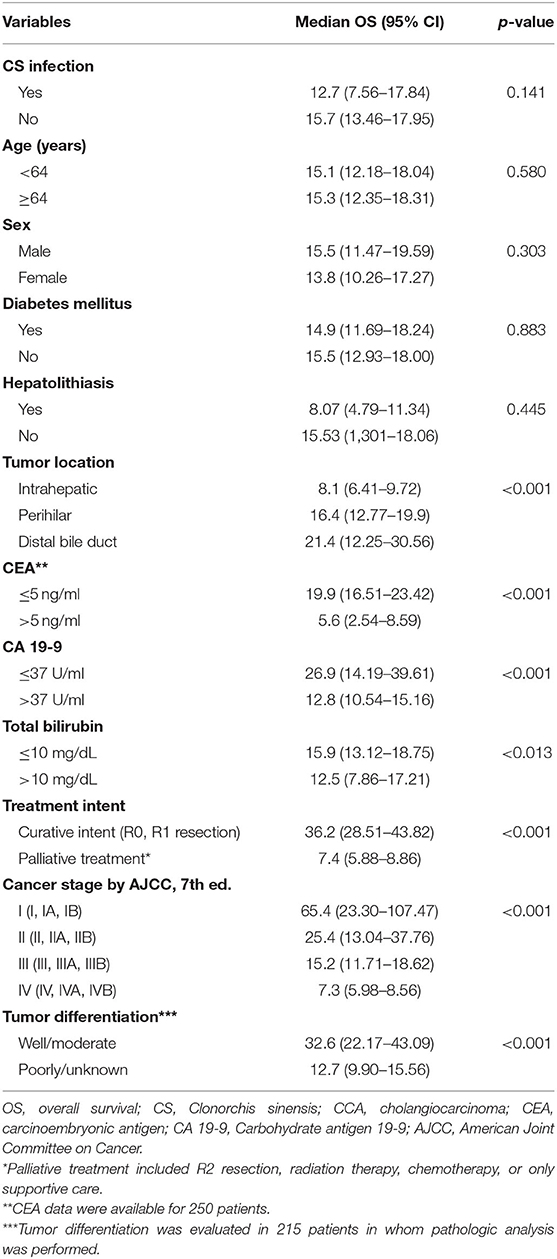
Table 3. Univariate analyses of prognostic factors associated with overall survival in 367 cholangiocarcinoma patients.
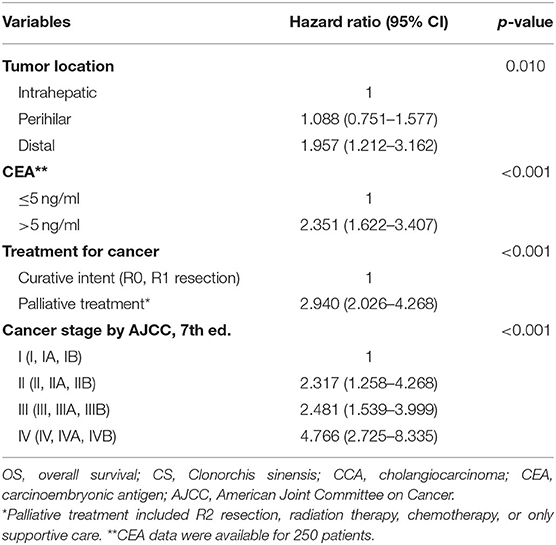
Table 4. Multivariate analysis using the Cox proportional hazards M = model in cholangiocarcinoma patients.
This hospital-based retrospective cohort study demonstrated that clonorchiasis is still a main cause of CCA. The total proportion of CACC was 25.9% but waSubgroup analysis divided accordings 46.3% in the endemic area. Patients with CACC had different characteristics compared to those with non-CACC: younger age at diagnosis of CCA, higher male to female ratio, higher prevalence in CS-endemic areas, and poorer overall survival in patients aged under 64 years.
The prevalence of clonorchiasis, the most common parasite in Korea, decreased from 2.42% in 2004 to 1.86% in 2012 among the general population (8, 14). Infection rates of CS have also decreased in endemic areas along major river basins (11). Despite the decline in clonorchiasis prevalence, the proportion of CACC was still high and not decreased in the recent 10 years compared to the previous 10 years in this study. The reason for this difference in CS infection rate and CACC proportion remains unclear. We suggest that CCA developed due to irreversible pathogenic pathway despite CS eradication. In a recent study of China, the duration of raw fish consumption more than 28 years was related to tumorigenesis in patients with clonorchiasis (15). We expect that CS-associated disease will continue to be a major public health concern in Korea for a while even if raw fish consumption decreases.
The proportions of CACC in this study were higher than those of previous studies in Korea. A prior study reported that CS infestation causes one-fourth of CCA in the endemic area and approximately 10% of CCA among the general population (3). The differences might have been caused by variation in the diagnostic criteria of CACC. The proportion of CACC varies on the study population and diagnostic criteria. It was from 2.2 to 4.2% when diagnosed by direct stool microscopy and 41.4% using any positive test among stool exam, ELISA test, skin test, bile or surgical specimens, or imaging criteria (8–10). Microscopic examination of the feces for eggs is the gold standard for the diagnosis of CS infection. However, stool exam cannot diagnose past infections and is less sensitive to mild infections (16). Compared to stool exam, ELISA test is an accurate diagnostic method with sensitivity up to 92.5% and specificity up to 93.1% (17). However, ELISA test showed low sensitivity for past CS infection because of low serologic titers (18). Imaging diagnosis by ultrasonography and CT are auxiliary methods for the diagnosis of CS infection (19). Imaging tests are important for the diagnosis of past infections with identifying bile duct changes. Until accurate diagnostic methods are developed, we recommend performing all tests because these tests are specific but less sensitive.
The median age of the patients at the time of diagnosis with CACC was younger than non-CACC. This finding is compatible with previous results that CCA develops earlier in patients with risk factors such as choledochal cysts at a median age of 42 years or primary sclerosing cholangitis in their 40 s (20, 21). Persistent chronic inflammatory responses may lead to malignant transformation at earlier time points.
Seventy percentage of CCAs are sporadic and 30% are associated with more than one risk factor, such as primary sclerosing cholangitis, hepatolithiasis, Caroli's disease, hepatitis B or C infection, liver flukes, cirrhosis, diabetes, obesity, or alcohol (22). Risk factors related to CCA vary by location. In East Asia, CS infection is the most common risk factor, especially in CS-endemic areas. The causative role of CS in intrahepatic CCA has been established (15). However, the role of CS in hilar or extrahepatic CCA has been defined in only a few studies (8). In this study, the proportions of hilar and distal CACC were similar to intrahepatic CCA. CS infection is related to extrahepatic CCA as well as intrahepatic CCA.
In this cohort, hepatitis B or C, and hepatolithiasis were identified in 7.1, 0.8, and 10.4% of patients. Hepatitis B and hepatolithiasis were more prevalent in intrahepatic CCA, which indicated an etiologic role in intrahepatic CCA. HCV infection was very rare, consistent with previous studies in Koreans and other Asian populations (9, 10, 23). Clonorchiasis is a risk factor of hepatolithiasis and silent CCA develops in 10% of patients with hepatolithiasis even after removal of stones (24). CS causes recurrent pyogenic cholangitis as a nidus for stone formation or by damaging the bile ducts, resulting in stricture and stone formation (25). However, a positive immunodiagnosis of clonorchiasis in hepatolithiasis patients was only seen in 6.9% of patients in a Taiwanese study, although this was higher compared to that in controls (0.8%) (26). This study revealed that 10% of CCA patients had hepatolithiasis and 7.9% had clonorchiasis. However, the rates of hepatolithiasis in CACC and non-CACC were 3.2 and 12.9%, respectively. We suggest that clonorchiasis and hepatolithiasis are independent risk factors of CCA.
CACC had a poor prognosis compared to non-CACC in the group <64 years old. However, multivariate analysis revealed prognostic factors are not CS infection but stages, curative resection, tumor location, and CEA levels. Tumor location has been reported as an important prognostic factor of CCA. In this study, distal CCA had a survival advantage over hilar or intrahepatic CCA. The previous studies showed the difference in the prognosis of CCA with three locations and survival advantage in distal CCA (27). Also, high CEA levels were related to poor prognosis, as shown in previous studies. Preoperative CEA levels were related to tumor stage survival rate and tumor recurrence in hilar and intrahepatic CCA (28–30). Earlier stage, curative resection, and low serum CEA levels were favorable prognostic factors in a previous study (15). High CA19-9 levels (>37 U/ml) and hyperbilirubinemia (>10 mg/dL) were poor prognostic factors in CCA on univariate analysis but not on multivariate analysis. The prognostic roles of these factors have been reported in previous studies. Pre-treatment low CA19-9 levels (≤ 1,000 U/ml) and a decline of more than 50% after treatment were significant for predicting a favorable prognosis (9).
Outcomes were poor in CACC patients under 64 years of age at diagnosis in univariate analysis. The reason for poor prognosis in CACC compared with non-CACC in younger patients is not clear. Younger CACC patients had a more frequent smoking history and higher CEA levels. Smoking is a risk factor for intrahepatic and hilar CCA (9). Because high serum CEA level is a poor prognostic factor (31), CACC patients showed a poorer prognosis than non-CACC patients.
This study has some potential limitations. First, all patients did not undergo all tests for CS infection. Therefore, it is possible to underestimate CS infection. Secondly, we compared regional differences in the CS infection rate between endemic areas and non-endemic areas using living area data. Because CS resides in the biliary tree with a long-term period, residence data for childhood and adulthood are also important. Since this is a retrospective study, this information could not be obtained. Thirdly, this study was conducted at a single tertiary center retrospectively. There was the possibility of selection bias. Nevertheless, the results of this study are meaningful because this study included many patients with CACC who underwent several diagnostic tests for CS infection and had sufficient follow-up duration.
In conclusion, this study demonstrated that the clinical characteristics and prognosis of CACC compared to non-CACC. CACC was associated with early-onset, male predominance, endemic area residence, and poor survival in those under 64 years of age compared to non-CACC. The rates of CS infection have decreased in Korea; however, the incidence of CACC has not changed. Large multicenter prospective studies are needed to clarify the characteristics of CACC and the development of targeted therapies that will reverse or block CS-induced chronic inflammatory responses (32).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Samsung Medical Center (IRB No. 2015-10-129-002). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JP, JL, and SP: conceptualization (ideas, formulation or evolution of overarching research goals and aims). JP, KeL, J-IY, and SK: data curation. JP, KeL, and SP: formal analysis. JP and SP: funding acquisition. KwL, KyL, and JL: Investigation. JP, KwL, KyL, and JL: methodology. KyL and JL: project administration. KwL, KyL, and JL: resources. JP, KwL, JL, and SP: supervision. JP, KyL, JL, and SP: validation. KeL, SK, and J-IY: visualization. KeL, DK, and J-IC: writing original draft. JP, SP, KC, DK, and J-IC: writing, review, and editing. All authors contributed to the article and approved the submitted version.
This work was supported by a research program funded by the Korea Centers for Disease Control and Prevention (fund code 2015-E54004-00).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.675207/full#supplementary-material
CS, Clonorchis sinensis; CCA, cholangiocarcinoma; CACC, Clonorchis sinensis-associated CCA; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; CT, computed tomography; ELISA, enzyme-linked immunosorbent assay; AJCC, American Joint Committee on Cancer; OS, overall survival.
1. Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. (2010) 72:305–50. doi: 10.1016/S0065-308X(10)72011-X
2. Qian MB, Utzinger J, Keiser J, Zhou XN. Clonorchiasis. Lancet. (2016) 387:800–10. doi: 10.1016/S0140-6736(15)60313-0
3. Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. (2010) 25:1011–6. doi: 10.3346/jkms.2010.25.7.1011
4. Attwood HD, Chou ST. The longevity of Clonorchis sinensis. Pathology. (1978) 10:153–6. doi: 10.3109/00313027809063494
5. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol. (2009) 10:321–2. doi: 10.1016/S1470-2045(09)70096-8
6. Qian MB, Chen YD, Liang S, Yang GJ, Zhou XN. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty. (2012) 1:4. doi: 10.1186/2049-9957-1-4
7. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. (2011) 54:173–84. doi: 10.1002/hep.24351
8. Kim HG, Han J, Kim MH, Cho KH, Shin IH, Kim GH, et al. Prevalence of clonorchiasis in patients with gastrointestinal disease: a Korean nationwide multicenter survey. World J Gastroenterol. (2009) 15:86–94. doi: 10.3748/wjg.15.86
9. Lee BS, Cha BH, Park EC, Roh J. Risk factors for perihilar cholangiocarcinoma: a hospital-based case-control study. Liver Int. (2015) 35:1048–53. doi: 10.1111/liv.12618
10. Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. (2008) 103:1716–20. doi: 10.1111/j.1572-0241.2008.01796.x
11. Cho SH, Lee KY, Lee BC, Cho PY, Cheun HI, Hong ST, et al. Prevalence of clonorchiasis in southern endemic areas of Korea in 2006. Korean J Parasitol. (2008) 46:133–7. doi: 10.3347/kjp.2008.46.3.133
12. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
13. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
14. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. (2016) 49:590–7. doi: 10.5483/BMBRep.2016.49.11.109
15. Zhang GW, Lin JH, Qian JP, Zhou J. Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2014) 21:3628–37. doi: 10.1245/s10434-014-3710-x
16. Han S, Zhang X, Wen J, Li Y, Shu J, Ling H, et al. A combination of the Kato-Katz methods and ELISA to improve the diagnosis of clonorchiasis in an endemic area, China. PLoS ONE. (2012) 7:e46977. doi: 10.1371/journal.pone.0046977
17. Choi MH, Park IC, Li S, Hong ST. Excretory-secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA. Korean J Parasitol. (2003) 41:35–9. doi: 10.3347/kjp.2003.41.1.35
18. Hong ST. Changes of anti-Clonorchis sinensis IgG antibody in serum after praziquantel treatment in human clonorchiasis. Kisaengchunghak Chapchi. (1988) 26:1–8. doi: 10.3347/kjp.1988.26.1.1
19. Choi D, Hong ST. Imaging diagnosis of clonorchiasis. Korean J Parasitol. (2007) 45:77–85. doi: 10.3347/kjp.2007.45.2.77
20. Kaya M, de Groen PC, Angulo P, Nagorney DM, Gunderson LL, Gores GJ, et al. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol. (2001) 96:1164–9. doi: 10.1111/j.1572-0241.2001.03696.x
21. Sastry AV, Abbadessa B, Wayne MG, Steele JG, Cooperman AM. What is the incidence of biliary carcinoma in choledochal cysts, when do they develop, and how should it affect management? World J Surg. (2015) 39:487–92. doi: 10.1007/s00268-014-2831-5
22. Plentz RR, Malek NP. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. (2015) 29:245–52. doi: 10.1016/j.bpg.2015.02.001
23. Li M, Li J, Li P, Li H, Su T, Zhu R, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol. (2012) 27:1561–8. doi: 10.1111/j.1440-1746.2012.07207.x
24. Kim HJ, Kim JS, Suh SJ, Lee BJ, Park JJ, Lee HS, et al. Cholangiocarcinoma risk as long-term outcome after hepatic resection in the hepatolithiasis patients. World J Surg. (2015) 39:1537–42. doi: 10.1007/s00268-015-2965-0
25. Choi BI, Han JK, Hong ST, Lee KH. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev. (2004) 17:540–52. doi: 10.1128/CMR.17.3.540-552.2004
26. Huang MH, Chen CH, Yen CM, Yang JC, Yang CC, Yeh YH, et al. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol. (2005) 20:141–6. doi: 10.1111/j.1440-1746.2004.03523.x
27. Ercolani G, Dazzi A, Giovinazzo F, Ruzzenente A, Bassi C, Guglielmi A, et al. Intrahepatic, peri-hilar and distal cholangiocarcinoma: Three different locations of the same tumor or three different tumors? Eur J Surg Oncol. (2015) 41:1162–9. doi: 10.1016/j.ejso.2015.05.013
28. Sano T, Shimada K, Sakamoto Y, Ojima H, Esaki M, Kosuge T. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol. (2008) 15:590–9. doi: 10.1245/s10434-007-9687-y
29. Saxena A, Chua TC, Sarkar A, Chu F, Morris DL. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg. (2010) 14:1128–38. doi: 10.1007/s11605-010-1203-1
30. Nanashima A, Sumida Y, Abo T, Nagasaki T, Takeshita H, Fukuoka H, et al. Patient outcome and prognostic factors in intrahepatic cholangiocarcinoma after hepatectomy. Hepatogastroenterology. (2007) 54:2337–42.
31. Saito H, Noji T, Okamura K, Tsuchikawa T, Shichinohe T, Hirano S. A new prognostic scoring system using factors available preoperatively to predict survival after operative resection of perihilar cholangiocarcinoma. Surgery. (2016) 159:842–51. doi: 10.1016/j.surg.2015.10.027
Keywords: clonorchiasis, Clonorchis sinensis, cholangiocarcinoma, endemic, prognosis
Citation: Chang JI, Lee K, Kim D, Yang JI, Park JK, Choi K, Kang SH, Lee KH, Lee KT, Lee JK, Park SM and Park JK (2021) Clinical Characteristics of Clonorchis sinensis-Associated Cholangiocarcinoma: A Large-Scale, Single-Center Study. Front. Med. 8:675207. doi: 10.3389/fmed.2021.675207
Received: 02 March 2021; Accepted: 29 April 2021;
Published: 28 May 2021.
Edited by:
Fateh Bazerbachi, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Manhal Izzy, Vanderbilt University Medical Center, United StatesCopyright © 2021 Chang, Lee, Kim, Yang, Park, Choi, Kang, Lee, Lee, Lee, Park and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joo Kyung Park, bWRzb3BoaWVAZ21haWwuY29t; Seon Mee Park, c21wYXJrQGNodW5nYnVrLmFjLmty
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.