
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med. , 21 May 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.670242
 Kyuwon Kim1
Kyuwon Kim1 Kyung-Wook Jo2
Kyung-Wook Jo2 Tae Sun Shim2
Tae Sun Shim2 Jin Hwa Park1
Jin Hwa Park1 Sung Wook Hwang1,3
Sung Wook Hwang1,3 Sang Hyoung Park1,3
Sang Hyoung Park1,3 Dong-Hoon Yang1
Dong-Hoon Yang1 Jeong-Sik Byeon1
Jeong-Sik Byeon1 Seung-Jae Myung1
Seung-Jae Myung1 Suk-Kyun Yang1,3
Suk-Kyun Yang1,3 Byong Duk Ye1,3*
Byong Duk Ye1,3*Considering the risk of reactivation of latent tuberculosis infection (LTBI), not only before starting tumor necrosis factor inhibitors but also before non-TNF inhibitor therapy, LTBI screening is routinely recommended for patients with inflammatory bowel disease (IBD). However, data on the positive conversion of LTBI test results during non-TNF inhibitor therapy are scarce. Among IBD patients treated with vedolizumab and/or ustekinumab, a total of 91 patients who had negative baseline interferon-gamma release assay (IGRA) results, assessed by QuantiFERON®-TB Gold In-tube or QuantiFERON®-TB Gold Plus, were enrolled. Serial LTBI test results after starting non-TNF inhibitor therapy were collected, and patients' clinical characteristics were analyzed. Positive IGRA conversion was observed in six of 91 patients (6.6%). The cumulative IGRA conversion–free survival rates after starting therapy were 97.7% after 1 year and 86.7% after 2 years. Ulcerative colitis was more common among converters compared with non-converters (66.7 vs. 23.5%, P = 0.040). Among six converters, four had been treated with vedolizumab, one with ustekinumab, and the other with vedolizumab followed by ustekinumab. All six patients had been previously exposed to TNF inhibitors before non-TNF inhibitor therapy: five to infliximab and one to both infliximab and adalimumab. After positive IGRA conversion, none of the six converters developed active tuberculosis while maintaining non-TNF inhibitor therapy (median 6.8 months, range 0.4–32.1 months). Positive IGRA conversion among IBD patients treated with vedolizumab and/or ustekinumab appears to occur somewhat frequently, but its clinical implications remain to be elucidated.
The emergence of TNF (tumor necrosis factor) inhibitors has brought about significant improvements in clinical outcomes for patients with inflammatory bowel disease (IBD) refractory to conventional treatment. Despite the remarkable effectiveness of TNF inhibitors, however, reactivation of latent tuberculosis (TB) is a major safety concern. The risk of TB development among IBD patients treated with TNF inhibitors has been reported to increase 1.6- to 41.7-fold, depending on regional variations in TB burden and baseline risk (1, 2). Accordingly, the consensus guidelines of the European Crohn's and Colitis Organization (ECCO) (3) and the Asian Organization for Crohn's and Colitis (AOCC) and the Asia Pacific Association of Gastroenterology (APAGE) (4) recommend screening for TB and treating latent TB infection (LTBI) before the initiation of TNF inhibitor therapy.
Meanwhile, non-TNF inhibitors such as vedolizumab and ustekinumab, have shown their efficacies for inducing and maintaining clinical response and remission in patients with moderate-to-severe IBD (5–8). Owing to their mechanisms of action, vedolizumab and ustekinumab could be regarded as safer than TNF inhibitors in terms of LTBI reactivation and active TB development. In the IM-UNITI study, only one (0.25%) out of 397 patients developed active TB (7); likewise, a recent descriptive analysis of the 4 years post-marketing safety data based on the Vedolizumab Global Safety Database (VGSD) reported 9 (0.03%) events of TB in 208,050 patient-years of vedolizumab exposure (9). Hence, it is anticipated that the risk of TB is lower among IBD patients treated with vedolizumab or ustekinumab compared with those treated with TNF inhibitors. As the development of TB among patients treated with TNF inhibitors is predominantly through LTBI reactivation (10), Lee et al. (11) investigated the LTBI test conversion rate and the subsequent risk of TB during TNF inhibitor therapy. However, data on positive LTBI test result conversion among patients treated with non-TNF inhibitors are scarce. Therefore, we now report the frequency of positive conversion of interferon-gamma release assay (IGRA) results among IBD patients treated with non-TNF inhibitors, such as vedolizumab and ustekinumab, in an area with an intermediate TB burden.
We performed a retrospective observational study including patients with IBD who had been treated with vedolizumab and/or ustekinumab at the Asan Medical Center, a tertiary-care teaching hospital in South Korea, between August 2017 and December 2020. During this period, patients who had negative baseline IGRA results by QuantiFERON®-TB Gold In-tube (QFT-GIT; QIAGEN, Hilden, Germany), or QuantiFERON®-Gold Plus (QFT-Plus; QIAGEN, Hilden, Germany) were included. Vedolizumab was prescribed for patients with active Crohn's disease (CD) or ulcerative colitis (UC) and ustekinumab for patients with active CD, according to the approved indication of drugs in Korea. In total, 91 eligible patients were enrolled in our study. Among them, 11 patients had a history of LTBI and eight had a history of pulmonary TB prior to non-TNF inhibitor therapy. All patients were included in the study regardless of the history related to TB considering that (1) there is a possible risk of reinfection of TB for the residents in Korea, a country with an intermediate TB burden, and (2) the adequacy of TB treatment was not thoroughly documented in the medical records of some patients. Following the prospective registry protocol, IGRAs were performed at 30, 54, and 110 weeks after vedolizumab initiation, and at 26, 52, and 104 weeks after ustekinumab initiation. However, some IGRAs deviated from the above protocol, and those performed at the physician's discretion were also collected and analyzed. Categorical variables were expressed as numbers and percentages, and continuous variables were expressed as median and interquartile range (IQR). The crude and cumulative rates of positive IGRA conversion were calculated, and the patients' clinical characteristics were analyzed and compared according to IGRA conversion. For comparisons between IGRA positive converters and non-converters, the chi-squared test was used for categorical variables and the Mann-Whitney U-test was used for continuous variables. The ethics committee of Asan Medical Center approved the study protocol (IRB Number: 2021-0058).
Out of 91 patients, 55 were males (60.4%), and the median age at baseline was 36 years (interquartile range [IQR], 29–45.8 years) (Table 1). Sixty-seven patients (73.6%) had CD, and the median disease duration was 11.3 years (IQR, 8.1–17.0 years). A total of 89 patients (97.8%) had a previous history of exposure to TNF inhibitors. Vedolizumab, ustekinumab, and ustekinumab after vedolizumab failure were given to 47 (51.6%), 23 (25.3%), and 21 (23.1%) patients, respectively. The median duration of follow-up after non-TNF inhibitor commencement was 22.3 months (IQR, 15.5–31.1 months). During follow-up, six patients (6.6%) underwent positive IGRA conversion (Table 1). The proportion of patients with UC was significantly different between converters and non-converters (66.7 vs. 23.5%, P = 0.040), whereas other characteristics were not different between the two groups (Table 1). The cumulative IGRA conversion-free survival rate after starting non-TNF inhibitor therapy was 97.7% after 1 year and 86.7% after 2 years (Figure 1).
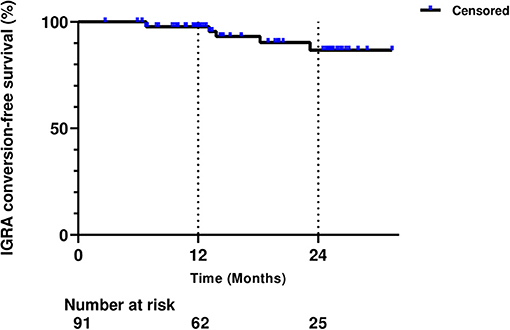
Figure 1. Cumulative interferon-gamma release assay (IGRA) conversion-free survival rate after starting non-tumor necrosis factor inhibitor therapy.
The characteristics of the six IGRA converters are summarized in Table 2. Among the six converters, four had been treated with vedolizumab, one with ustekinumab, and the other with vedolizumab followed by ustekinumab. The median interval from non-TNF inhibitor commencement to IGRA conversion was 13.5 months (range, 6.8–23.2 months). Five converters had been previously exposed to infliximab and one to both infliximab and adalimumab. Two patients (Patient 3 and Patient 6) received LTBI treatment after confirmation of IGRA conversion, but none discontinued non-TNF inhibitors. After positive IGRA conversion, none of the six converters developed active TB while maintaining non-TNF inhibitor therapy (median 6.8 months, range 0.4–32.1 months).
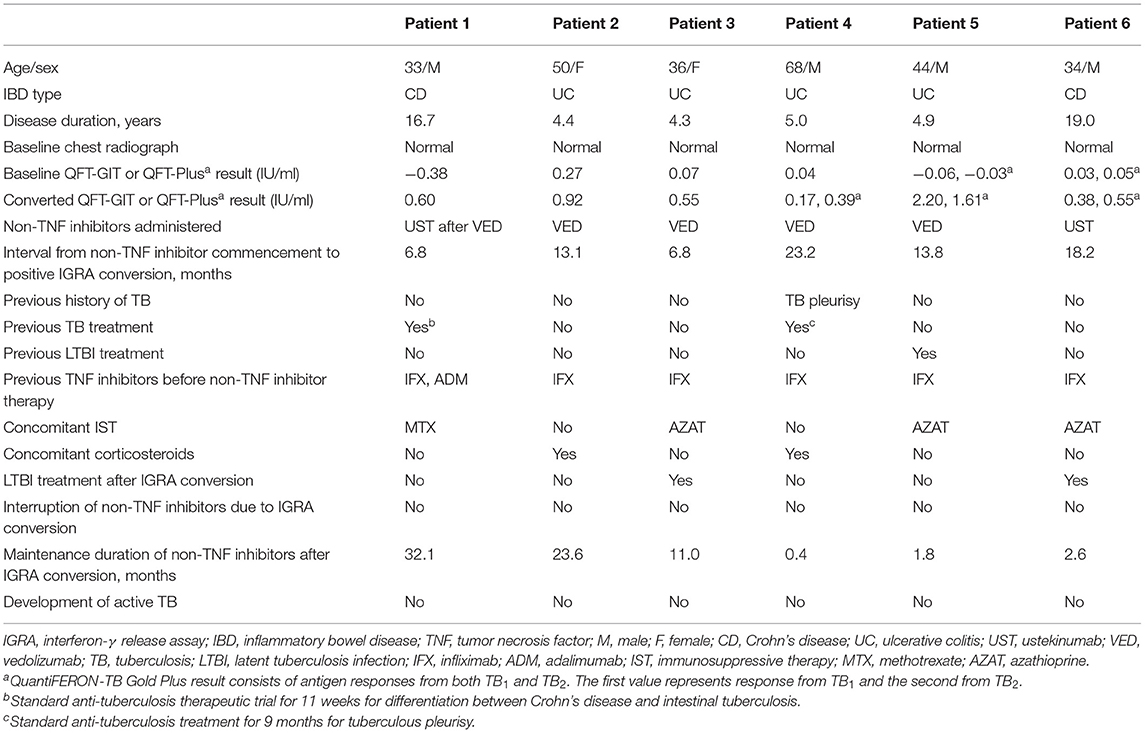
Table 2. Demographic and clinical characteristics of IGRA converters among IBD patients treated with non-TNF inhibitors.
In this study, six patients (6.6%) developed positive IGRA conversion in a median of 13.5 months after initiating non-TNF inhibitors; however, none of the converters developed active TB during a median follow-up of 6.8 months despite continued non-TNF inhibitor treatment. According to previous studies on LTBI and TB monitoring during TNF inhibitor therapy, fifteen out of 78 patients (19.2%) with IBD experienced positive conversion of any LTBI tests after a median follow-up of 16 months in Hong Kong (11), and 11.8% of patients with rheumatologic diseases underwent positive IGRA conversion after a median of 12.3 months in South Korea (12). As there was no control group treated with TNF inhibitors in our study, we could not directly compare the positive conversion rates of IGRA between patients treated with non-TNF inhibitors and those treated with TNF inhibitors. Nevertheless, by referring to the results of previous studies, we can indirectly infer that the frequency of positive IGRA conversion may be lower among patients treated with non-TNF inhibitors than those treated with TNF inhibitors. Considering that the risk of TB by TNF inhibitors is closely related to the local TB burden (13), our results cannot be readily extrapolated to the population of patients with IBD in other regions with different incidence rates of TB.
Out of six IGRA converters, two (Patient 3 and Patient 6) received LTBI treatment at the treating physician's discretion. As IGRA tests cannot distinguish recent from remote infections, the IGRA conversions in Patient 4 and Patient 5 were regarded as insignificant and probably associated with their previous history of TB (TB pleurisy in Patient 4 and LTBI in Patient 5). Although, Patient 1 and Patient 2 did not receive LTBI treatment, their clinical features, and serial follow-up chest radiographs did not reveal active TB development in both patients. This might be explained by test variability. As shown in Table 2, most IGRA converters had low-positive QFT results, which ranged from 0.35 to 0.99 IU/ml. Among the converters, Patient 1 did not receive any LTBI treatment, but a follow-up IGRA after 5.5 months showed reversion to negativity, and the negative result was replicated twice more after reversion (at 4.7 and 17.5 months). This suggests that positive IGRA conversion could have been due to test variability rather than true positive conversion. Another plausible explanation is the possibility of an insignificant impact of non-TNF inhibitors on LTBI reactivation. According to post-marketing data as well as vedolizumab clinical trial results and those of the IM-UNITI study of ustekinumab (7, 9, 14), it is anticipated that the risk of TB is low among IBD patients treated with vedolizumab or ustekinumab, similar to the incidence rates of TB in the respective areas of origin. This can also be supported by the Psoriasis Longitudinal Assessment and Registry (PSOLAR) data, which reported no cases of LTBI reactivation among ustekinumab-treated patients with psoriasis (15). Furthermore, previous national database study from Korea, which evaluated the risk of active TB disease among psoriasis patients treated with ustekinumab, revealed only three out of 2,803 patients (0.1%) developed active TB related to ustekinumab treatment (16). Overall, even if the cases of positive IGRA conversion were true conversions, the risk of LTBI reactivation appears to be low under non-TNF inhibitor therapy among IBD patients.
As mentioned above, there is uncertainty whether IGRA conversions represent de novo infections or reactivation of LTBI. In our study, five out of the six converters except Patient 2 who were lost to follow-up did not have any record of recent exposure to individuals with active TB. All patients underwent evaluations to exclude TB and no patient developed active TB although LTBI therapy was given to only two patients. Meanwhile, without an available gold standard test for detecting LTBI, there is also room for uncertainty resulting from the variability of IGRA results. According to previous studies on discriminating true Mycobacterium tuberculosis (Mtb)-specific response from test variability, 69% of borderline and 88% of low-positive IGRA results were Mtb-specific (17, 18). As shown in Table 2, except for Patient 4, all patients whose IGRA results were in the borderline zone showed a positive range of IGRA results. To overcome the inherent limitations of the test method itself, the IGRA results should be interpreted in the context of the patient's risk for TB such as epidemiologic situations. In addition, physicians should be aware of the implication of the borderline zone of IGRA value and repeat the test in cases with borderline IGRA results. The fact that we could not conduct a repeated test in Patient 4 is a limitation of our study.
In addition to the inherent limitations stemming from its retrospective design, our study was limited by its small sample size and a short follow-up duration after IGRA conversion. In particular, Patient 4 had the shortest follow-up duration because tofacitinib was initiated 2 weeks after IGRA conversion. However, even after Patient 4 switched to tofacitinib, active TB did not develop during 6.4 months of follow-up. Furthermore, we used IGRA alone to diagnose LTBI. Our domestic guidelines for LTBI diagnosis in immunocompromised patients recommend IGRA alone or IGRA combined with the tuberculin skin test (TST) (19). The TST can simply be performed via intradermal injection of 0.1 ml of tuberculin purified protein derivative. However, inconsistent skin reactions, subjective interpretation, and false-negative results from anergy or immunosuppressive drug use render the TST unreliable (20). Additionally, cross-reactions with bacillus Calmette-Guérin (BCG) can lead to TST positivity, and in Korea, BCG vaccination is mandatory for newborns (21). In light of these points, IGRA alone might be adequate for detecting LTBI.
Our study was the first real-life evaluation of IGRA conversion rate among IBD patients treated with non-TNF inhibitors. Further well-designed, prospective studies with larger sample sizes are warranted to identify the impact of non-TNF inhibitors on LTBI reactivation. Consequently, evidence-based, optimized strategies for TB prevention, including testing for and treatment of LTBI among IBD patients before non-TNF inhibitor therapy, could be established.
All data generated or analyzed during this study are included in this published article. The data underlying this article will be shared on reasonable request to the corresponding author.
The study protocol involving human participants was reviewed and approved by the ethics committee of Asan Medical Center (IRB Number: 2021-0058). The informed consent requirements were waived by the ethics committee of Asan Medical Center.
KK and BDY: study concept and design, statistical analysis, interpretation of data, and drafting of the manuscript. KK, K-WJ, TSS, JHP, SWH, SHP, D-HY, J-SB, S-JM, S-KY, and BDY: acquisition of data. K-WJ, TSS, and BDY: critical revision of the manuscript for important intellectual content. BDY: study supervision. All authors contributed to the article and approved the submitted version.
This work was supported by a Grant (No.: 2020IT0012) from the Asan Institute for Life Sciences, Seoul, Korea.
BDY has received research grants from Celltrion and Pfizer Korea; consulting fees from Abbvie Korea, Celltrion, Chong Kun Dang Pharm., Daewoong Pharma., Ferring Korea, IQVIA, Janssen Korea, Kangstem Biotech, Korea United Pharm., LG Chem., Medtronic Korea, Pfizer Korea, Shire Korea, Takeda, and Takeda Korea; speaking fees from Abbvie Korea, Celltrion, Ferring Korea, IQVIA, Janssen Korea, Pfizer Korea, Shire Korea, and Takeda Korea. S-KY received a research grant from Janssen Korea. However, all of these are not related to this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to Ji Young Park and Eun Ja Youn for data collection. We also thank Dr. Joon Seo Lim from the Scientific Publications Team at Asan Medical Center for his editorial assistance in preparing this manuscript.
IGRA, interferon-gamma release assay; IBD, inflammatory bowel disease; LTBI, latent tuberculosis infection; TNF, tumor necrosis factor; CD, Crohn's disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; BCG, bacille Calmette–Guérin; TST, tuberculin skin test; Mtb, Mycobacterium tuberculosis.
1. Byun JM, Lee CK, Rhee SY, Kim HJ, Kim JW, Shim JJ, et al. The risk of tuberculosis in Korean patients with inflammatory bowel disease receiving tumor necrosis factor-alpha blockers. J Korean Med Sci. (2015) 30:173–9. doi: 10.3346/jkms.2015.30.2.173
2. Byun JM, Lee CK, Rhee SY, Kim H-J, Im JP, Park DI, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-α inhibitor. Scand J Gastroenterol. (2015) 50:312–20. doi: 10.3109/00365521.2014.1000960
3. Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. (2021):jjab052. doi: 10.1093/ecco-jcc/jjab052. [Epub ahead of print].
4. Park DI, Hisamatsu T, Chen M, Ng SC, Ooi CJ, Wei SC, et al. Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. (2018) 16:4–16. doi: 10.5217/ir.2018.16.1.4
5. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2013) 369:699–710. doi: 10.1056/NEJMoa1215734
6. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. (2013) 369:711–21. doi: 10.1056/NEJMoa1215739
7. Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn's Disease. N Engl J Med. (2016) 375:1946–60. doi: 10.1056/NEJMoa1602773
8. Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2019) 381:1201–14. doi: 10.1056/NEJMoa1900750
9. Cohen RD, Bhayat F, Blake A, Travis S. The safety profile of vedolizumab in ulcerative colitis and Crohn's disease: 4 years of global post-marketing data. J Crohns Colitis. (2019) 14:192–204. doi: 10.1093/ecco-jcc/jjz137
10. Shim TS. Diagnosis and treatment of latent tuberculosis infection due to initiation of anti-TNF therapy. Tuberc Respir Dis. (2014) 76:261–8. doi: 10.4046/trd.2014.76.6.261
11. Lee CK, Wong SHV, Lui G, Tang W, Tam LS, Ip M, et al. A prospective study to monitor for tuberculosis during anti-tumour necrosis factor therapy in patients with inflammatory bowel disease and immune-mediated inflammatory diseases. J Crohns Colitis. (2018) 12:954–62. doi: 10.1093/ecco-jcc/jjy057
12. Kim HW, Kwon OC, Han SH, Park M-C. Positive conversion of interferon-γ release assay in patients with rheumatic diseases treated with biologics. Rheumatol Int. (2020) 40:471–9. doi: 10.1007/s00296-019-04510-6
13. Kedia S, Mouli VP, Kamat N, Sankar J, Ananthakrishnan A, Makharia G, et al. Risk of tuberculosis in patients with inflammatory bowel disease on infliximab or adalimumab is dependent on the local disease burden of tuberculosis: a systematic review and meta-analysis. Am J Gastroenterol. (2020) 115:340–9. doi: 10.14309/ajg.0000000000000527
14. Ng SC, Hilmi IN, Blake A, Bhayat F, Adsul S, Khan QR, et al. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis. (2018) 24:2431–41. doi: 10.1093/ibd/izy153
15. Kalb RE, Fiorentino DF, Lebwohl MG, Toole J, Poulin Y, Cohen AD, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatol. (2015) 151:961–9. doi: 10.1001/jamadermatol.2015.0718
16. Cho SI, Kang S, Kim YE, Lee JY, Jo SJ. Ustekinumab does not increase tuberculosis risk: results from a national database in South Korea. J Am Acad Dermatol. (2020) 82:1243–5. doi: 10.1016/j.jaad.2019.12.033
17. Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, Cattamanchi A, et al. Reproducibility of interferon gamma (IFN-γ) release assays. A systematic review. Ann Am Thorac Soc. (2014) 11:1267–76. doi: 10.1513/AnnalsATS.201405-188OC
18. Uzorka JW, Bossink AWJ, Franken WPJ, Thijsen SFT, Leyten EMS, van Haeften AC, et al. Borderline QuantiFERON results and the distinction between specific responses and test variability. Tuberculosis. (2018) 111:102–8. doi: 10.1016/j.tube.2018.06.002
19. Joint Committee for the Revision of Korean Guidelines for Tuberculosis & Korean Centers for Disease Prevention. Korean Guidelines for Tuberculosis. (2020). 4th ed. Available online at: http://tbzero.cdc.go.kr/tbzero/board/boardView.do (accessed December 10, 2020).
21. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. (2005) 293:2756–61. doi: 10.1001/jama.293.22.2756
Keywords: inflammatory bowel disease, latent tuberculosis infection, interferon-gamma release assay, vedolizumab, ustekinumab
Citation: Kim K, Jo K-W, Shim TS, Park JH, Hwang SW, Park SH, Yang D-H, Byeon J-S, Myung S-J, Yang S-K and Ye BD (2021) Frequency of Positive Conversion of Interferon-Gamma Release Assay Results Among Patients With Inflammatory Bowel Disease Treated With Non-tumor Necrosis Factor Inhibitors. Front. Med. 8:670242. doi: 10.3389/fmed.2021.670242
Received: 20 February 2021; Accepted: 27 April 2021;
Published: 21 May 2021.
Edited by:
Fernando Gomollón, University of Zaragoza, SpainReviewed by:
Sabino Riestra Menendez, Central University Hospital of Asturias, SpainCopyright © 2021 Kim, Jo, Shim, Park, Hwang, Park, Yang, Byeon, Myung, Yang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byong Duk Ye, YmR5ZUBhbWMuc2VvdWwua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.