
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 July 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.662488
This article is part of the Research Topic Game Changers in Inflammatory Bowel Diseases View all 12 articles
Background: The inflammatory bowel disease disability index (IBD-DI) was used to access body functional consequences and disease burden. However, Chinese population data are considerably limited.
Objective: We aimed to screen for disability in patients with Crohn's disease (CD) and to assess potential associations with clinical parameters as well as indices related to sarcopenia.
Methods: This cross-sectional study includes 146 CD patients from Ruijin Hospital in Shanghai, China. All patients were screened for disability and sarcopenia on the basis of the IBD-DI scale, and the criteria for Asian Working Group for Sarcopenia, respectively. Clinical and demographic variables were collected.
Results: Approximately 52.05% of the subjects suffered from moderate or severe disabilities. The prevalence of sarcopenia (48.68 vs. 31.43%, P = 0.043), Patient-Generated Subjective Global Assessment score or PG-SGA≥4 (39.47 vs. 17.14%, P = 0.003), and high-level C- reactive protein (27.63 vs. 11.43%, P = 0.021) were higher in patients with moderate to severe disability than in those without to minimal disability. By multivariate regression modeling, the following were identified as independent factors related to moderate to severe disability: disease activity (OR:10.47, 95% CI: 2.09–52.42) and body mass index (BMI) (OR:4.11, 95% CI: 1.80–9.38).
Conclusions: Disability is common in CD patients. Our study showed that moderate to severe disability is not directly associated with muscle mass or muscle quantity but is mostly correlated with disease activity as well as BMI. Thus, close monitoring and follow-up should be conducted on patients who are at high risk of disability, and effective measures should be taken, which may be the best way to prevent disability.
The prevalence of inflammatory bowel disease (IBD) has markedly increased in mainland China (1). Crohn's disease (CD) is a chronic inflammatory bowel disorder that may affect any part of the digestive tract, causing abdominal pain and diarrhea, subsequently leading to functional disability and life-threatening complications (2). CD patients are characterized by their lifelong disease, marked by episodes of remission and relapse (3). Thus, living with CD may negatively affect the physical, psychological, social, and familial quality of life (4–6). Numerous disease-related questionnaires have been developed to evaluate patient-reported outcomes in CD (7–10). Nonetheless, the majority of CD studies focus on health-related quality of life (such as the inflammatory bowel disease questionnaire) assessments, a subjective assessment about the limitations caused by the disease with high uncertainty. Disability assessment determines function loss in an individual and the social cost of CD.
The International Classification of Functioning, Disability, and Health (ICF) of the World Health Organization defines disability as an objective measure of loss of functioning and impairment in patient activity (11). The Inflammatory Bowel Disease Disability Index (IBD-DI) was developed (12) and updated in 2017 (13). The IBD-DI includes body functions, body structures, as well as activity and participation. Studies confirmed that the IBD-DI was associated with gender, clinical disease activity, and disease duration in the West (14–16). However, our literature review revealed that studies are rarely conducted on conditions and clinical results related to disability in IBD patients. This situation has been exacerbated by the allocation of greater funding for basic research instead of clinical studies. Moreover, in CD, risk factors, disease features, economic conditions, cultural background, and medical systems do not completely overlap between the East and the West. Therefore, more research on disability needs to be conducted on the Chinese population.
Inflammatory bowel diseases are often associated with malnutrition and significant alteration in body composition (17). Bryant et al. found that muscle depletion among IBD patients may affect the quality of life (18). Norman et al. suggested that malnutrition-related factors can reduce the quality of life in IBD patients (19). None of the available studies assessed the relationship between disability and body composition.
Liu et al. (20) reported that a large proportion of Chinese IBD patients experience impaired health-related quality of life. Nonetheless, considerably few studies have focused on disability and risk factors in Chinese CD patients. Thus, this study aimed to evaluate disability prevalence among CD patients, evaluated the correlation between disability and body composition, as well as the risk factors that may be associated with a CD-related disability.
In this cross-sectional study, CD outpatients (n = 197) under anti-TNF-α therapy were included at Ruijin Hospital in Shanghai from February 2019 to June 2019. All CD patients were included in the study, independent of occupational activity, gender, race, or social class. Specifically, these participants were diagnosed with CD by their treating physicians and met the criteria established by the Inflammatory Bowel Disease Group of the Chinese Society of Gastroenterology. The following exclusion criteria were applied: patients with metabolic diseases (e.g., diabetes, hyperthyroidism) whose related indices were not well-controlled after hospitalization; patients with gastrointestinal tumors or tumors at other sites; patients with impaired water and mineral metabolism caused by cortical hormone therapy; and patients with metal implants (such as cardiac pacemakers) that could influence bioelectrical impedance results. Fifty-one patients were not included for missing values in the variables of body composition, BMI or others (Figure 1). The final sample was comprised of 146 participants.
The participants were informed of the study objective before the experiments started. Informed consent was obtained from each participant, who also consented to the publication of relevant data. This study was approved by the Ethics and Research Committee of Ruijin Hospital.
Before the start of the study, a pilot study consisting of 15 participants was conducted as a preliminary investigation and to pretest the survey instrument and subsequently adjust the scale. All researchers involved in this study were trained together. The samples were described using a structured questionnaire, including sociodemographic data and lifestyle.
The following information was collected: (a) sociodemographic data, including age, gender, height, weight, and smoking and drinking habits; (b) disease characteristics, including disease duration and disease activity assessed by Crohn's disease activity index (CDAI). Patients with CDAI ≤150 were considered in clinical remission, whereas those with CDAI >150 were considered in the active phase.
We assessed body composition by multifrequency bioelectrical impedance analysis using the InBody S10 body water analyzer (InBody Korea). We also evaluated the total fat mass, body fat percentage, total muscle mass, skeletal muscle mass, and the muscle/fat ratio of the patients. This analyzer processes 30 impedance measurements by using six different frequencies (1, 5, 50, 250, 500, 1,000 kHz) in each of five segments of the body (right arm, left arm, trunk, right leg, and left leg) and 15 reactance measurements by using tetrapolar 8-point tactile electrodes at three different frequencies (5, 50, and 250 kHz) in each of the aforementioned segments.
The appendicular skeletal muscle mass (ASM) was measured as the sum of the muscle mass from the four limbs, and the ASM index (kg/m2) was calculated. Loss of skeletal muscle mass was determined based on the criteria set by the Asian Working Group for Sarcopenia (2019) (21): ASMI <7.0 kg/m2 for men and <5.7 kg/m2 for women.
Measurement was performed with the participant in the following position: seated on an armless chair, with feet supported on the floor, hips and knees flexed at 90°, arms parallel to the body, elbows flexed at 90°, and forearms and wrists in a neutral position. The dominant side was measured three times at 1-min intervals, with verbal stimulation applied. The results were expressed in kilogram-force (kg), and the mean of the three measures was used. Muscle weakness was determined based on the criteria set by the Asian Working Group for Sarcopenia (21): handgrip strength <28 kg for men, and <18 kg for women.
Patient-Generated Subjective Global Assessment (PG-SGA) was recommended as a nutritional assessment scale for IBD patients by the Inflammatory Bowel Disease Group of the Chinese Society of Gastroenterology. The PG-SGA assessment included (i) body weight, (ii) dietary intake, (iii) symptoms, (iv) movement and body function, (v) relationship between disease and nutritional requirement, (vi) metabolism requirement, and (vii) physical examination. According to the criteria, the scores indicated the following: 0–1, good nutritional status; 2–3, light malnutrition requiring nutritional education and/or nutritional intervention; 4–8, moderate malnutrition requiring nutritional intervention; and ≥9, severe malnutrition requiring symptom improvement and nutritional therapy. Patients with scores ≥4 were defined as having moderate or severe malnutrition. Those with scores <4 were enrolled in the low PG-SGA group.
CD-related disability was assessed based on the IBD-DI (13). The original IBD-DI comprises 19 items capturing five aspects of the ICF categories, including general health, body function (sleep/energy, affect, body image, pain, diarrhea, body mass index, and weight loss), body structure (presence of blood in the stool, arthralgia/arthritis), participation activity (regulating defecation, looking after own health, interpersonal activities, and work/education), and environmental factors (exacerbating effect or medication, food, family, and health care professional). A simplified version of the IBD-DI, which contains 14 items, was validated in 2017. All items were rescaled to range from 0 to 4. Binary items were coded as “0” for “no” and “4” for “yes.” BMI > 18.5 was assigned a score of “0,” whereas BMI < 18.5 was assigned a score of “4.” The total score ranged from 0 to 100 using the following formula: score × 100/(p × 4), where p represents the number of answered items. The total scores and their corresponding classifications were as follows: 0–20 (no disability), 20–35 (mild disability), 35–50 (moderate disability), and 50–100 (severe disability).
The data were analyzed using SPSS version 22.0. P < 0.05 indicated statistical significance. For normally distributed continuous data, the mean (standard deviation [SD]) was used as the measure of central location, whereas for continuous data with non-normal distribution, the median (interquartile range) was used. These characteristics were compared between the moderate to severe disability group and the no disability and minimal disability group by using Pearson's chi-square or Fisher's exact test to compare proportions. The odds ratio (OR) and its 95% confidence interval (CI) were calculated to determine the association of risk factors with moderate or severe disabilities. Statistically significant variables in the univariate analyses were then included in a multivariate regression model to identify the independent risk factors of moderate to severe disability by backward elimination analysis.
Table 1 presents the demographics and baseline characteristics of the population. All patients who completed the study allowed the use of their administrative data, which were thus included in this study. The mean age of the participants was 38.89 ± 10.68, 66.43% were male, 52.05% had moderate or severe disabilities, 28.76% had moderate or severe malnutrition (PG-SGA≥4), and 40.41% had sarcopenia. The average duration of IBD was 5.59 y. The disease was considered clinically in remission in 120 patients. The IBD disability index for all CD patients was 33.62 ± 9.63 (0–61).
Table 2 showed baseline characteristics of activity and remission phase participants of the CD patients. The active phase subjects had a higher CRP level (80.77 vs. 6.67%, P = 0.012), moderate to severe disability (92.31 vs. 43.33%, P < 0.001), PG-SGA≥4 (69.23 vs. 20.00%, P < 0.001) compared to the remission phase. The active phase subjects were also more frequently disability and sarcopenia compared with patients with the remission phase.
Table 3 lists the body composition, sociodemographic factors, age, nutritional-biochemical indexes, nutritional status of the patients by their disability status. The moderate to severe disability group had lower values for body weight, BMI, BFM, ASMI, and handgrip strength, compared with the group without to minimal disability (P < 0.05). The moderate to severe disability group had higher CDAI and PG-SGA scores, compared with the without to minimal disability group (P < 0.05). No significant differences in age, duration of disease, and hemoglobin levels were found between the moderate to severe disability group and the without to minimal disability group (P > 0.05). The prevalence of sarcopenia (48.68 vs. 31.43%, P = 0.043), PG-SGA≥4 (39.47 vs. 17.14%, P = 0.003), and high-level CRP (27.63 vs. 11.43%, P = 0.021) were higher in the moderate to severe disability group than in the without to minimal disability group.
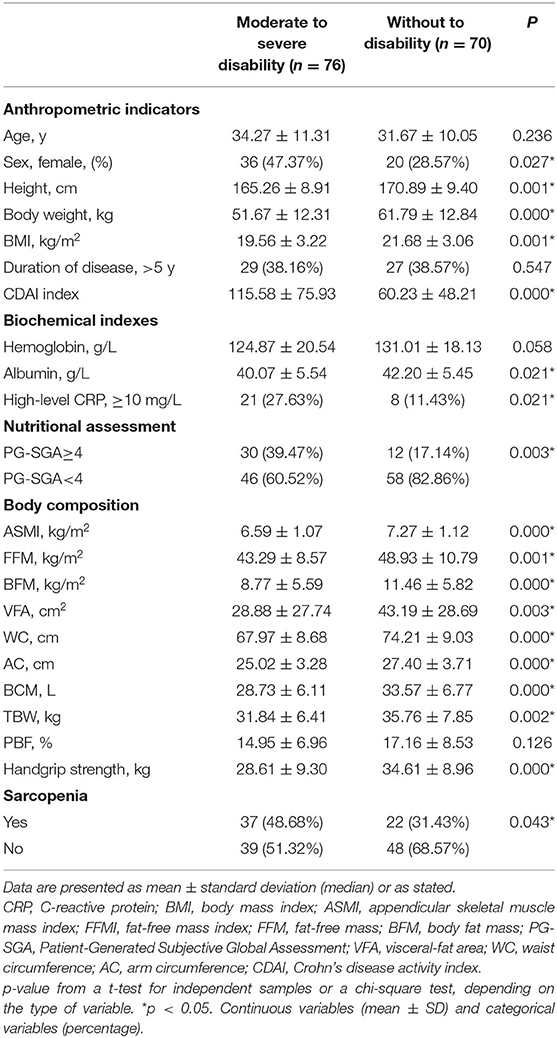
Table 3. General characteristics of CD patients with moderate to severe disability vs. Without to minimal disability.
In CD patients, the variables that were significantly correlated with the IBD-DI were BMI, CDAI, PG-SGA, ASMI, handgrip strength, and CRP (Figure 2).
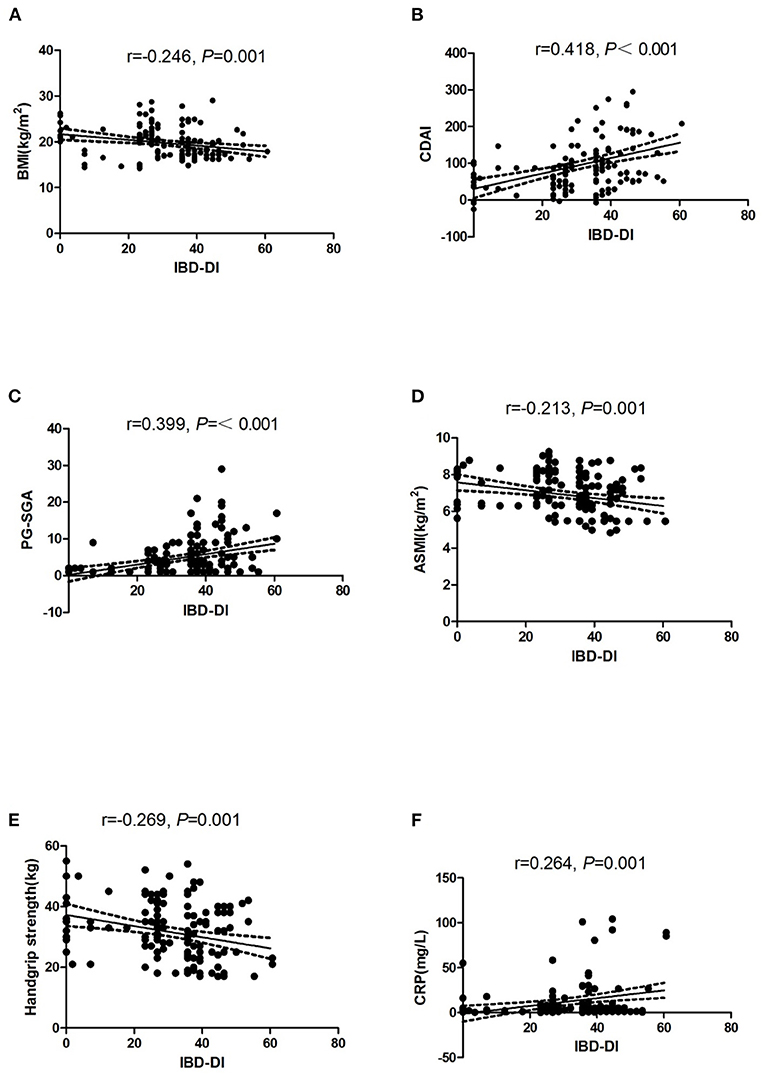
Figure 2. Dispersion graphs depicting correlations between IBD-DI and BMI (A), CDAI (B), PG-SGA (C), ASMI (D), Handgrip strength (E), and CRP (F). P-values on each graph were calculated for all CD patients. r indicates the Spearman's rank correlation coefficient. IBD-DI, inflammatory bowel disease disability index; BMI, body mass index; CDAI, Crohn's disease activity index; PG-SGA, Patient-Generated Subjective Global Assessment; ASMI, appendicular skeletal muscle mass index; CRP, C-reactive protein.
In univariate regression models, disability was significantly associated with BMI (OR: 4.60, 95% CI: 2.28–9.30), sex (OR: 2.25, 95% CI: 1.13–4.47), CDAI (OR: 15.69, 95% CI: 3.54–69.42), BMI (OR: 4.60, 95% CI: 2.28–9.30), sarcopenia (OR: 3.40, 95% CI: 1.59–7.72), and CRP (OR:1.31, 95% CI: 1.05–1.64) (Table 4).
By multivariate analysis, the BMI (OR:4.11, 95% CI: 1.80–9.38) and disease activity (OR:10.47, 95% CI: 2.09–52.42) were identified as the most significant factors predicting disability.
In the past 20 years, CD has markedly increased and become a common disease of the digestive system in China (22). About 70–80% of CD patients may suffer from intestinal obstruction, abdominal abscess, intestinal perforation, and other complications. CD patients may receive surgery, high medical costs. A complicated disease course increases disability, including serious damage to the physical and mental health of patients, a decline in the quality of life (23), and work efficiency (24–26), and even depression (27). In the current study, we confirmed CD in the active phase to be significantly associated with disability.
We used the scale developed by Gower-Rousseau et al., which consisted of 14 items. The later Korean, Portuguese, and Prevost versions containing 14 items showed good reliability and validity. We determined the average IBD-DI score, which was 33.62 ± 9.63, similar results were observed in the Gower-Rousseau study. In the current study, 52.05% of the CD patients had a moderate to severe disability, whose results were similar to those reported by Marinelli et al. (28) and Yoon et al. (23). Notably, the results of the multivariate analysis indicated that disease activity was an independent risk factor for disability in patients with CD (OR:10.47, 95%CI:2.09–52.42). In a validation study from Australia (29), disability was significantly correlated with CDAI, and lBD-Q. A study among Koreans showed that disability in patients with IBD was correlated with disease activity and poor quality of life (23, 29). A meta-analysis found a significant correlation between disease activity and disability in IBD patients (30). All of these observations confirmed that disease activity is an important factor affecting disability. Therefore, effective treatment measures should be applied to reduce the degree of disease activity in patients.
BMI was another independent predictor for CD patients with a moderate to severe disability in this study (OR:4.11, 95 CI: 1.80–9.38). The patients with moderate to severe disability had lower BMI than those without or with minimal disability (19.56 ± 3.22 vs. 21.68 ± 3.06, P = 0.001). However, this was the first study to concurrently demonstrate that BMI was significantly associated with disability. A Portugal study reported that BMI was related to the quality of life in IBD patients; moreover, the effect of BMI on the psychological and physical quality of life was mediated via the mechanisms of body image (31). An Israel study enrolled 100 IBD patients and found that a lower BMI was associated with a more severe disease course (32). A case-control study revealed that women with ulcerative colitis exhibited decreased lower limb strength and mobility limitations, which were associated with BMI (33). Besideds, a systematic review indicated that BMI was normally lower in CD patients, and regular medical therapy could not improve BMI in these patients (34). Our previous study (35) demonstrated that the dietary structure of IBD patients was unreasonable, characterized by insufficient intake of energy and protein; in addition, lack of physical activity can lead to body muscle depletion. Wardle et al. (36) found an increased prevalence of disordered eating behavior in CD and a greater prevalence of binge eating, food craving, low mood, and high anxiety. Moreover, Chan et al. (27) reported that symptoms of anxiety and depression were independently associated with IBD-related disability. Arigo et al. (37) suggested that fear and anxiety surrounding gastrointestinal symptoms can lead to disordered eating practices of a restrictive nature. In a French survey (38), nearly half of the subjects reported that the disease had changed the pleasure of eating, with only a quarter of the patients eating a normal diet during relapse. Thus, reduced dietary intake and disordered eating behavior can potentially lower BMI and aggravate disability which may associated with anxiety and depression.
Studies on the association between IBD-related disability and muscle-related sarcopenia have rarely, if ever, been reported. A previous study determined that the prevalence of sarcopenia among adult Chinese patients with CD was 60% (39). In the current study, the prevalence rates of sarcopenia were lower than the previously reported rate in Chinese patients. The discrepancies were most likely attributable to significant differences in patient selection and the methods used. We found that the muscle mass in remission was significantly higher than that in activity, which is consistent with our previous findings. Notably, patients with moderate to severe disability had lower sarcopenia-related index than patients with without to minimal disability (ASMI: 6.91 ± 1.12 vs. 7.59 ± 0.97, P = 0.002, handgrip strength: 28.61 ± 9.30 vs. 34.61 ± 8.96, p < 0.001). Research has shown that sarcopenia is a progressive and generalized syndrome characterized by the loss of skeletal muscle mass and muscle strength with adverse outcomes, such as frailty, poor quality of life, and mortality (40, 41). The pathogenesis of muscle wasting includes several elements, such as aging, systemic inflammation, mitochondrial dysfunction, increased proteolysis, decreased proteosynthesis, and insulin resistance (40). Cravo et al. reported that reduced lower muscle attenuation seemed to be associated with more severe phenotypes in patients with CD (42). These conditions may aggravate disability in CD patients. Univariate analysis showed that moderate to severe disability was significantly associated with CD-related sarcopenia; however, multivariate analysis revealed no such finding.
In the univariate analysis, moderate to severe disability was found in 2.25 times more female than male CD patients. This finding is consistent with the studies conducted among French (13) and Spanish (14) subjects, which also found a higher IBD-DI in female than male subjects. By contrast, the current study did not find the same result after multivariate analysis. Similarly, a Dutch study observed no link between disability and sex (43). In addition, the data indicated that patients in the moderate to severe disability group had higher CRP levels than those in the without to minimal disability group (27.63 vs. 11.43%, P = 0.021). However, this finding was not supported by the multivariate analysis. Nonetheless, close attention to the clinical CRP score should be given attention.
In China, CD patients have poor access to IBD treatment centers and specialist doctors. Moreover, doctors focus more on disease treatment and remission than the quality of life and disability in CD patients (44). Although an increasing number of studies have reported that disability in CD can affect their work efficiency and psychological status, reports on Chinese-related populations were limited. Thus, CD patients in China must monitor the conditions of their disability during clinical treatment. Moreover, prompt corrective measures have to be undertaken to alleviate body mass depletion and maintain remission, consequently improving the quality of life and reducing disability. A multidisciplinary assessment of patients with CD is always encouraged, and nutrition strategies should always be suitable to the needs of the patient.
We also identified several limitations. First, this study is a single-center cross-sectional study. This research sample size was small and the size of the cohort was only defined by the number of consecutive outpatients during the sample collection. Selection bias could not be excluded and not all Chinese CD patients could be represented, considering the sample; moreover, the correlation between patient disability and demographic and disease characteristics at a certain time node does not imply causality. Second, selection bias could not be avoided because not all patients who were surveyed were willing to participate. Third, a cross-sectional protocol provides limited evidence concerning changes over time, which would be better assessed by a future longitudinal study. Furthermore, multicenter, larger sample clinical observational research, longitudinal follow-up and intervention studies would be required to determine the reversal of disability for the successful management of disease activity and BMI.
We demonstrated that disability is strongly related to disease activity and body mass in CD patients. In the management of the disease, not only the regular progression of the disease but also disability and BMI should be investigated during the regular treatment of clinical outpatients. In conclusion, close monitoring and follow-up should be conducted for patients with a high risk of disability, and effective measures should be adopted, which may be the best approach to preventing disability and helping patients return to normal life. This would also allow for a novel multidisciplinary approach based on collaborative efforts between gastroenterologists, nurses, nutritionists, and psychologists to improve the quality of life in general.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics and Research Committee of Ruijin Hospital. The patients/participants provided their written informed consent to participate in this study.
DB, JZ, and YS: conceptualization. DB, YG, YJ, ZH, and YT: investigation. DB, JZ, and YS: methodology. YS: funding acquisition. DB and YJ: data curation. DB: writing—original draft preparation. YG, ZH, JZ, and YS: writing—review and editing. YT, JZ, and YS: project administration. JZ and YS: supervision. QC and ZH: validation. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all of the participants and investigators for their support and cooperation.
1. Qiu Y, Ren W, Liu Y, Chen WE, Pan XH, Ren JJ. Disease burden of inflammatory bowel disease in China from 1990 to 2017: findings from the global burden of diseases (2017). EClinicalMedicine. (2020) 27:100544. doi: 10.1016/j.eclinm.2020.100544
2. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. (2017) 389:1741–55. doi: 10.1016/S0140-6736(16)31711-1
3. Veauthier B, Hornecker JR. Crohn's disease: diagnosis and management. Am Fam Phys. (2018) 98:661–9.
4. Lo B, Julsgaard M, Vester-Andersen MK, Vind I, Burisch J. Disease activity, steroid use and extraintestinal manifestation are associated with increased disability in patients with inflammatory bowel disease using the inflammatory bowel disease disability index: a cross-sectional multicentre cohort study. Eur J Gastroenterol Hepatol. (2018) 30:1130–6. doi: 10.1097/MEG.0000000000001199
5. Vedamurthy A, Ananthakrishnan AN. Influence of environmental factors in the development and outcomes of inflammatory bowel disease. Gastroenterol Hepatol. (2019) 15:72–82.
6. Mikocka-Walus A, Pittet V, Rossel JB, von Känel R; Swiss IBD Cohort Study Group. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. (2016) 14:829–35.e1. doi: 10.1016/j.cgh.2015.12.045
7. Sands BE, Han C, Gasink C, Jacobstein D, Szapary P, Gao LL, et al. The effects of ustekinumab on health-related quality of life in patients with moderate to severe Crohn's disease. J Crohns Colitis. (2018) 12:883–95. doi: 10.1093/ecco-jcc/jjy055
8. Gater A, Kitchen H, Heron L, Pollard C, Håkan-Bloch J, Højbjerre L, et al. Development of a conceptual model evaluating the humanistic and economic burden of Crohn's disease: implications for patient-reported outcomes measurement and economic evaluation. Expert Rev Pharmacoecon Outcomes Res. (2015) 15:643–56. doi: 10.1586/14737167.2015.1045883
9. Mathias SD, Vallow S, Gipson DS, Thorneloe KS, Sprecher D. Development of focal segmental glomerulosclerosis patient-reported outcome measures: symptom diary and symptom impact questionnaire. Am J Kidney Dis. (2017) 70:532–40. doi: 10.1053/j.ajkd.2017.04.023
10. Dulai PS, Boland BS, Singh S, Chaudrey K, Koliani-Pace JL, Kochhar G, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn's disease. Gastroenterology. (2018) 155:687–95.e10. doi: 10.1053/j.gastro.2018.05.039
11. Cieza A, Stucki G. The international classification of functioning disability and health: its development process and content validity. Eur J Phys Rehabil Med. (2008) 44:303–13.
12. Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. (2012) 61:241–7. doi: 10.1136/gutjnl-2011-300049
13. Gower-Rousseau C, Sarter H, Savoye G, Tavernier N, Fumery M, Sandborn WJ, et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. (2017) 66:588–96. doi: 10.1136/gutjnl-2015-310151
14. López-Cortés R, Herrero-Hahn R, De la Rosa-Eduardo R, Montoya-Juárez R, García-Caro MP, Marín-Fernández B, et al. Cultural adaptation and validation of the inflammatory bowel disease disability index in a spanish population and its association with sociodemographic and clinical factors. Int J Environ Res Public Health. (2019) 16:635. doi: 10.3390/ijerph16040635
15. Lauriot Dit Prevost C, Azahaf M, Nachury M, Branche J, Gerard R, Wils P, et al. Bowel damage and disability in Crohn's disease: a prospective study in a tertiary referral centre of the Lémann Index and Inflammatory Bowel Disease Disability Index. Aliment Pharmacol Ther. (2020) 51:889–98. doi: 10.1111/apt.15681
16. Shafer LA, Walker JR, Chhibba T, Targownik LE, Singh H, Ivekovic M, et al. Health care indicators of moderate to severe IBD and subsequent IBD-related disability: a longitudinal study. Inflamm Bowel Dis. (2019) 25:1996–2005. doi: 10.1093/ibd/izz102
17. Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. (2008) 24:694–702. doi: 10.1016/j.nut.2008.03.018
18. Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. (2013) 38:213–5. doi: 10.1111/apt.12372
19. Norman K, Kirchner H, Lochs H, Pirlich M. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol. (2006) 12:3380–5. doi: 10.3748/wjg.12.3380
20. Liu R, Tang A, Wang X, Shen S. Assessment of quality of life in chinese patients with inflammatory bowel disease and their caregivers. Inflamm Bowel Dis. (2018) 24:2039–47. doi: 10.1093/ibd/izy099
21. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: (2019). consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
22. Cui G, Yuan A. A systematic review of epidemiology and risk factors associated with chinese inflammatory bowel disease. Front Med. (2018) 5:183. doi: 10.3389/fmed.2018.00183
23. Yoon JY, Shin JE, Park SH, Park DI, Cha JM. Disability due to inflammatory bowel disease is correlated with drug compliance, disease activity, and quality of life. Gut Liver. (2017) 11:370–6. doi: 10.5009/gnl16422
24. Høivik ML, Moum B, Solberg IC, Henriksen M, Cvancarova M, Bernklev T, et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut. (2013) 62:368–75. doi: 10.1136/gutjnl-2012-302311
25. Chao CY, Lemieux C, Restellini S, Afif W, Bitton A, Lakatos PL, et al. Maladaptive coping, low self-efficacy and disease activity are associated with poorer patient-reported outcomes in inflammatory bowel disease. Saudi J Gastroenterol. (2019) 25:159–66. doi: 10.4103/sjg.SJG_566_18
26. Shafer LA, Walker JR, Restall G, Chhibba T, Ivekovic M, Singh H, et al. Association between IBD disability and reduced work productivity (Presenteeism): a population-based study in Manitoba, Canada. Inflamm Bowel Dis. (2019) 25:352–9. doi: 10.1093/ibd/izy236
27. Chan W, Shim HH, Lim MS, Sawadjaan FLB, Isaac SP, Chuah SW, et al. Symptoms of anxiety and depression are independently associated with inflammatory bowel disease-related disability. Dig Liver Dis. (2017) 49:1314–9. doi: 10.1016/j.dld.2017.08.020
28. Marinelli C, Savarino EV, Marsilio I, Lorenzon G, Gavaruzzi T, D'Incà R, et al. Sleep disturbance in Inflammatory Bowel Disease: prevalence and risk factors - a cross-sectional study. Sci Rep. (2020) 10:507. doi: 10.1038/s41598-020-57460-6
29. Leong RW, Huang T, Ko Y, Jeon A, Chang J, Kohler F, et al. Prospective validation study of the international classification of functioning, disability and health score in Crohn's disease and ulcerative colitis. J Crohns Colitis. (2014) 8:1237–45. doi: 10.1016/j.crohns.2014.02.028
30. Lo B, Prosberg MV, Gluud LL, Chan W, Leong RW, van der List E, et al. Systematic review and meta-analysis: assessment of factors affecting disability in inflammatory bowel disease and the reliability of the inflammatory bowel disease disability index. Aliment Pharmacol Ther. (2018) 47:6–15. doi: 10.1111/apt.14373
31. Trindade IA, Ferreira C, Pinto-Gouveia J. The effects of body image impairment on the quality of life of non-operated Portuguese female IBD patients. Qual Life Res. (2017) 26:429–36. doi: 10.1007/s11136-016-1378-3
32. Yerushalmy-Feler A, Ben-Tov A, Weintraub Y, Amir A, Galai T, Moran-Lev H, et al. High and low body mass index may predict severe disease course in children with inflammatory bowel disease. Scand J Gastroenterol. (2018) 53:708–13. doi: 10.1080/00365521.2018.1464595
33. Zaltman C, Braulio VB, Outeiral R, Nunes T, de Castro CL. Lower extremity mobility limitation and impaired muscle function in women with ulcerative colitis. J Crohns Colitis. (2014) 8:529–35. doi: 10.1016/j.crohns.2013.11.006
34. Dong J, Chen Y, Tang Y, Xu F, Yu C, Li Y, et al. Body mass index is associated with inflammatory bowel disease: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0144872. doi: 10.1371/journal.pone.0144872
35. Bian D, Shi Y, Jiang Y, Zhong J, Sun J, Gu Y. Combined patient-generated subjective global assessment and body composition facilitates nutritional support in inflammatory bowel disease: an ambulatory study in Shanghai. Asia Pac J Clin Nutr. (2018) 27:1230–8. doi: 10.6133/apjcn.201811_27(6).0009
36. Wardle RA, Thapaliya G, Nowak A, Radford S, Dalton M, Finlayson G, et al. An examination of appetite and disordered eating in active Crohn's disease. J Crohns Colitis. (2018) 12:819–25. doi: 10.1093/ecco-jcc/jjy041
37. Arigo D, Anskis AM, Smyth JM. Psychiatric comorbidities in women with celiac disease. Chronic Illn. (2012) 8:45–55. doi: 10.1177/1742395311417639
38. Zallot C, Quilliot D, Chevaux JB, Peyrin-Biroulet C, Guéant-Rodriguez RM, Freling E, et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm Bowel Dis. (2013) 19:66–72. doi: 10.1002/ibd.22965
39. Zhang T, Cao L, Cao T, Yang J, Gong J, Zhu W, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn's disease undergoing bowel resection. JPEN J Parenter Enteral Nutr. (2017) 41:592–600. doi: 10.1177/0148607115612054
40. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. (2015) 22:100–6. doi: 10.1016/j.coph.2015.04.003
41. Scaldaferri F, Pizzoferrato M, Lopetuso LR, Musca T, Ingravalle F, Sicignano LL, et al. Nutrition and IBD: malnutrition and/or sarcopenia? A practical guide. Gastroenterol Res Pract. (2017) 2017:8646495. doi: 10.1155/2017/8646495
42. Cravo ML, Velho S, Torres J, Costa Santos MP, Palmela C, Cruz R, et al. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn's disease: an exploratory study. Clin Nutr ESPEN. (2017) 21:79–5. doi: 10.1016/j.clnesp.2017.04.005
43. van der Have M, Fidder HH, Leenders M, Kaptein AA, van der Valk ME, van Bodegraven AA, et al. Self-reported disability in patients with inflammatory bowel disease largely determined by disease activity and illness perceptions. Inflamm Bowel Dis. (2015) 21:369–77. doi: 10.1097/MIB.0000000000000278
Keywords: disability, Crohn's disease, risk factors, disease activity, sarcopenia, body mass index
Citation: Bian D, Jiang Y, Gu Y, He Z, Chen Q, Tang Y, Zhong J and Shi Y (2021) Body Mass Index and Disease Activity Are Associated With Moderate to Severe Disability in Crohn's Disease: A Cross-Sectional Study in Shanghai. Front. Med. 8:662488. doi: 10.3389/fmed.2021.662488
Received: 01 February 2021; Accepted: 14 June 2021;
Published: 09 July 2021.
Edited by:
Anita Bálint, University of Szeged, HungaryReviewed by:
Yuji Naito, Kyoto Prefectural University of Medicine, JapanCopyright © 2021 Bian, Jiang, Gu, He, Chen, Tang, Zhong and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Shi, c2hpLnlvbmdtZWlAMTYzLmNvbQ==; Jie Zhong, SmltbXl6ajY0QG1lZG1haWwuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.