
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med. , 15 October 2021
Sec. Ophthalmology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.657993
This article is part of the Research Topic Progressive Keratoconus: Insights into Etiopathogenesis, Diagnosis, and Treatment View all 11 articles
 Chiara Bonzano*
Chiara Bonzano* Carlo Alberto Cutolo
Carlo Alberto Cutolo Donatella Musetti
Donatella Musetti Ilaria Di Mola
Ilaria Di Mola Chiara Pizzorno
Chiara Pizzorno Riccardo Scotto
Riccardo Scotto Carlo Enrico Traverso
Carlo Enrico TraversoPurpose: To investigate the demographic and corneal factors associated with the occurrence of delayed reepithelialization (DRE) after epithelium-off crosslinking (epi-off CXL).
Design:Retrospective case series.
Methods:A chart review was performed to identify patients treated with epi-off CXL. DRE was defined as a corneal epithelial defect detected by fluorescein staining that persisted for more than 10 days. Slit-lamp examination, anterior segment optical coherence tomography, corneal topography, and corneal in vivo confocal microscopy (IVCM) were always performed preoperatively and at each follow-up visit (1, 3, 6, 12 months). A generalized estimating equation was used to assess the baseline factors associated with DRE.
Results:Data from 153 eyes were analyzed. The mean age of patients was 24.9 ± 8.5 years, and 47 (30.7%) were women. The average reepithelization time was 4.7 ± 1.8 days. Six eyes (3.9%) experienced DRE. In the multivariate model, both the age of the patient (OR = 1.30; p = 0.02) and the corneal steepest meridian (OR = 0.44, p = 0.047) were associated with DRE. Baseline nerve count was also associated with DRE (0.87, p = 0.03). Male gender was associated with a slower early nerve regrowth (1–6 months) (p = 0.048), but not with the occurrence of DRE (p = 0.27). Preoperative central corneal thickness was not related to DRE (p = 0.16). DRE was not associated with keratoconus progression after epi-off CXL (p = 0.520).
Conclusions:The association between DRE and age may reflect the age-related decrease in the corneal healing response. Also, low baseline corneal nerve count is associated with DRE. Gender seems to affect reinnervation measured by IVCM but not the reepithelization time. DRE does not seem to affect the efficacy of epi-off CXL.
Keratoconus (KC) is a non-inflammatory corneal disease characterized by progressive ectasia, in which stromal thinning and cornea weakening can lead to an increase of anterior and posterior corneal curvature (1). The resulting irregular astigmatism, myopia together with the progressive corneal scarring, is responsible for visual loss (2).
Corneal collagen crosslinking (CXL) is a parasurgical technique of corneal tissue strengthening. Riboflavin activated by irradiation with ultraviolet-A (UVA) light increases the intra and interfibrillar covalent bonds, thereby increasing the mechanical strength and slowing the progression of corneal ectasia (3).
The epithelium-off CXL (epi-off CXL) technique is deemed a safe procedure for the treatment of progressive KC (4–8).
To reduce discomfort and to promote fast and safe epithelial healing, patients are generally given daily topical antibiotics and corticosteroids for 1 to 2 weeks following the epi-off CXL with close follow-up. A bandage contact lens is usually placed following the procedure, night and day up to 3–5 consecutive days. Three days later, after lens removal, complete epithelial healing is observed at the biomicroscopic examination in most of the cases (9).
Delayed reepithelialization (DRE), defined as a corneal epithelial defect detected by fluorescein-staining that persisted for more than 10 days after treatment, is a possible complication of epi-off CXL.
This study aims to investigate the demographic and corneal factors associated with DRE.
A retrospective chart review was performed to identify patients treated with epi-off CXL at ClinicaOculistica, University of Genova, Italy. All subjects provided written informed consent.
Inclusion criteria were as follows: patients affected by grade II–III KC (Amsler-Krumeich (AK) grading); clinical and instrumental progression documented by repeated corneal topography over at least 6 months intended as an increase in the steep meridian value (Kmax) of 1.0 diopter or more; and willingness to undergo epi-off CXL.
Diagnosis of KC was established by using the AK classification, based on spectacle refraction, central keratometry, corneal transparency, and corneal thickness.
We included eyes with early to moderate progressive KC, corneal thickness >400 μm, and with minimum of 12 months follow-up after epi-off CXL.
Exclusion criteria were advanced KC with stromal scarring, corneal hydrops, herpetic keratitis, autoimmune and other systemic diseases, pregnancy, and breastfeeding.
Slit-lamp examination implemented with corneal epithelial fluorescein staining, anterior segment optical coherence tomography (AS-OCT) (RTVue, Optovue Inc., Fremont, CA), corneal topography measurement using TMS-4 topographer (Tomey Corporation, Tokyo, Japan) with surface regularity index, and corneal in vivo confocal microscopy (IVCM) (Heidelberg Retina Tomograph II, Rostock Cornea Module) were always performed preoperatively and at each follow-up visit (1, 3, 6, and 12 months).
Epi-off CXL was always performed by the same surgeon (C.B.) using the accelerated protocol that uses equivalent total irradiance [9 mW/cm for 10 min, 5.4 J/cm (A9/10-CXL)] (10).
The procedure was always performed under sterile operating conditions using topical anesthesia oxybuprocaine hydrochloride 0.4% (Alfa Intes—Ind.Ter.Splendore) anesthetic drops. Topical pilocarpine 2.0% was administered 20 min before treatment.
After the application of an eyelid speculum, epithelial removal (9-mm) was achieved using a blunt knife. Riboflavin (0.1% in 20% dextran solution; Ricrolin; Sooft, Montegiorgio, Italy) was administered topically every minute for 15 min. The administration was continued every 2 min during UVA exposure.
The UVA irradiation was performed with a CBM X-Linker Vega using a 9 mW/cm2 to obtain 10 min of UVA irradiation on balance while delivering a standard energy dose of 5.4 J/cm2.
The post-CXL medication consisted of antibiotic eye drops solution (Netilmicin 0.3%) (3mg/ml) (four times daily for 1 week) and dexamethasone sodium phosphate (0.1%) (1mg/ml) (four times daily for 1 week and tapered over the following 7 days). Preservative-free isotonic solution (hyaluronic acid (HA) 0.4% and taurine (TAU) 0.5%) and preservative-free B2 vitamin eye drops (Ribolisin free, SOOFT italia) were used for 4 weeks. Oral pain medications (Tramadol 50 mg, 1–2 per day; diclofenac 25 mg, 1–2 per a day) were prescribed on the treatment day and the day after. A specific bandage lens for injured tissues, with a regenerating, anti-inflammatory, and analgesic effect (Regenera Therapeutic Lens) (16,5 mm, hydrogel Filcon II 3 e 75% H2O, Dk = 42) was placed after the procedure to reduce the discomfort and to promote the epithelial healing. It was removed after 3 days if the epithelial healing was complete.
Delayed reepithelialization was defined as a corneal epithelial defect detected by fluorescein staining at the slit lamp examination that persisted for more than 10 days after epi-off CXL.
Delayed reepithelialization was managed conservatively with topical medication and bandage contact lens that was replaced every 3 days until complete healing. In one case, debridement was performed for redundant or loose epithelial margins. Additional surgical procedures were not needed in our cohort of patients (11, 12).
Data are reported as mean (standard deviation) for continuous parameters or as frequencies for categorical parameters.
A generalized estimating equation was used to assess the baseline factors associated with DRE and to account for the correlation between fellow eyes. DRE was considered as the dependent variable in the analysis. Then a multivariate model was built. Criteria for model selection were guided by the univariate analysis and clinical significance of the variables. Univariate linear regression was also used to assess the association between baseline characteristics and the speed of nerve regrowth between 1 and 6 months. All statistical analyses were performed with Stata version 15.1 (StataCorp LP, College Station, TX). The alpha level (type I error) was set at 0.05 for all analyses.
Based on inclusion and exclusion criteria, we analyzed data from 153 patients. The average reepithelization time was 4.7 ± 1.8 days (SD). Postoperative corneal biomicroscopic examination performed on the third day after treatment showed a clear cornea, little edema, and no opacities before and immediately after therapeutic contact lens removal. Seventy-two hours after epithelium removal, almost all the patients had complete reepithelialization as shown by the fluorescein dye test instilled in the eye, only six eyes (3.9%) experienced DRE (Figure 1). Among these no one reported either a corneal infection or KC progression after epi-off CXL. Patient demographics and baseline ocular characteristics of the two groups are summarized in Table 1. The mean age of patients was 24.5 ± 8.3 in patients who did not experience DRE, and 33.6 ± 7.5 in patients with DRE (p = 0.01). As regard ocular characteristics, nerve count was 70.4 ± 19.8 and 36.5 ± 4.9 in the patient without and with DRE (p = 0.01), respectively. No significant differences were observed between the two groups regarding other demographic or ocular characteristics. As described in the methods, a model was built to better identify ocular and demographic characteristics associated with DRE (Table 2). The age of the patient and corneal nerve count were associated with DRE in univariate analysis with OR = 1.11 (p = 0.02) and OR = 0.89 (p = 0.01), respectively. Then, we have built two different multivariate models, not including nerve count (model 1) or age (model 2), to avoid multicollinearity. In both the multivariate models, age and nerve count remained significantly associated with DRE. Nerve count and age were also found negatively correlated (r = −0.27; p = 0.028). In model 1 the steepest meridian value was associated with DRE whereas in model 2 this variable was only marginally associated with DRE. Then, the same variables were tested for association with nerve regrowth (1–6 months), and it was found that the male gender was the only variable significantly associated with a slower early nerve regrowth (p = 0.048). Of note, even if gender was included in the multivariate models, it did not associate with DRE (p = 0.27 model 1). We also tested the hypothesis that DRE could affect the efficacy of epi-off CXL, and we found that DRE was not associated with KC progression after treatment (p = 0.520).
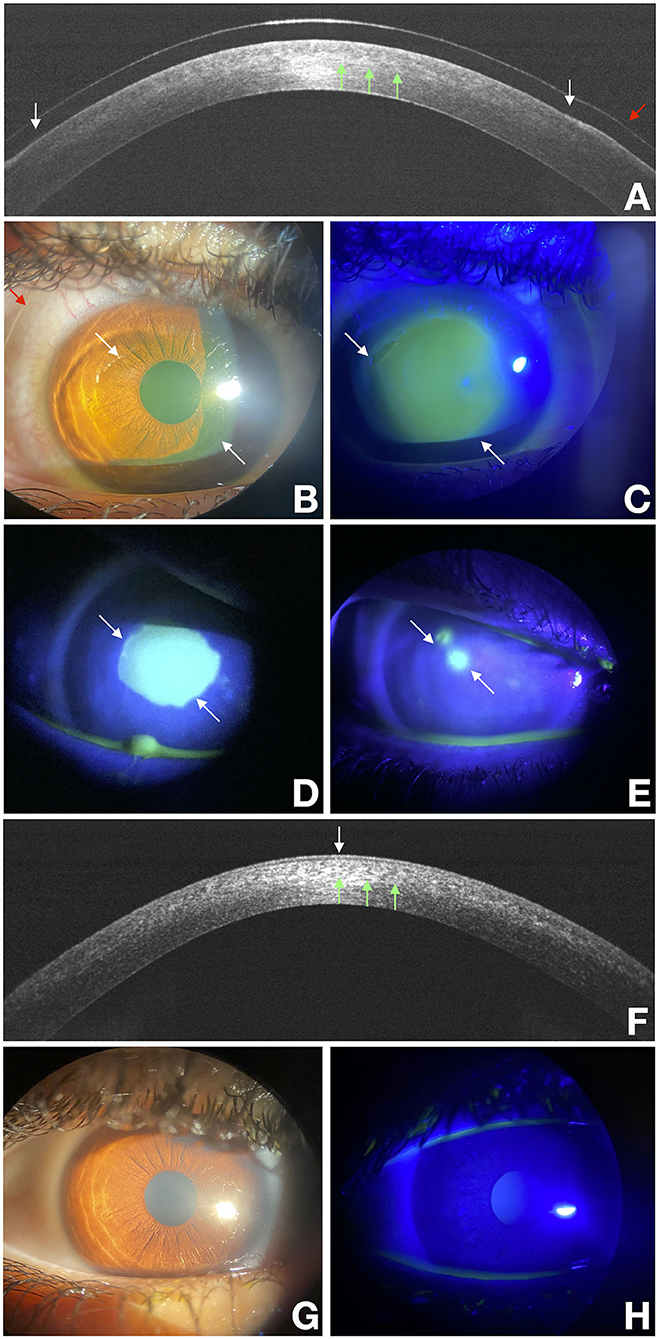
Figure 1. Delayed re-epithelialization after the epithelium-off CXL (epi-off CXL) imaged with a multimodal approach. White arrows point at the edges of the epithelial fronts and green arrows points to the demarcation line, and the red arrow points to the bandage lens. OCT scan over the disepithelized area at baseline (A). Photograph obtained at baseline with diffuse white light (B) and with fluorescein staining (green) photograph obtained under cobalt-blue light illumination (C) imaged at baseline. (D,E) were acquired at the end of treatment at 3 and 10 days respectively. They show a residual corneal epithelial disepithelialization. OCT scan over the re-epithelialization area at day 15 (F). Photograph obtained at day 15 with diffuse white light (G) and with fluorescein staining (green) photograph obtained under cobalt-blue light illumination (H).
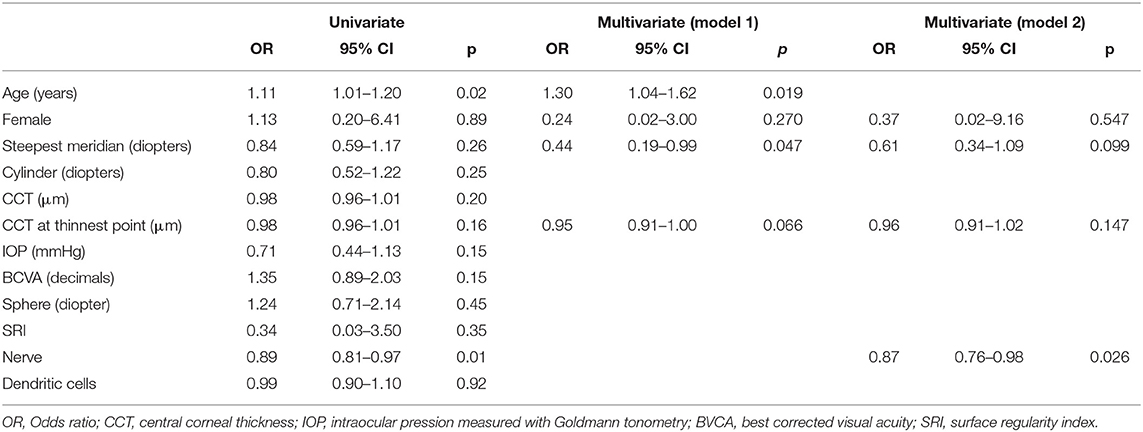
Table 2. Analysis of the association between baseline demographic and anatomical parameters with DRE.
The association between DRE and age may reflect the age-related decrease in the corneal healing response. Gipson et al. (13) and some other studies reported that corneal wound healing declines with age (13–16). Major well-known changes in the cornea with age include the thickening of both the epithelial and endothelial basement membranes.
By regulating the growth factor activity, the basement membrane plays a key role in the cellular reparative process (17). Its hemidesmosome-anchoring fibrils bind the basal cells membrane to the Bowman's layer and form anchoring complexes by binding to the stromal plaques (18). The anchoring fibrils seem to become disrupted with increasing age, and the membrane thickness exceeds fibril length, and it could effectively block linkage between the anchoring fibrils and Bowman's layer (19). Furthermore, there is a well-known diminution of sex hormones that occurs with age in both sexes that affect the glandular functions and compromise the ocular surface system, and consecutively the cascade of healing mechanisms (20). Besides, the number of nerves in the corneal epithelial subbasal plexus decreases with age, leading perhaps to the loss of sensitivity observed with age involving at first the corneal periphery and successively spreading toward the central zone (16, 21). We have to keep in mind that the corneal sensation has already nearly disappeared in the early post epi-off CXL period, it improved to its baseline levels only at sixth postoperative month according to Ozgurhan et al. (22). The lower the corneal sensitivity, the lower the trend of the corneal epithelium to heal. In our analysis, we showed that baseline nerve count seems to play a role in the corneal healing process. Last but not least, an aging-related decrease in the number of conjunctival keratocytes has been reported (23). It could mean a lower level of Muc16, conjunctival mucin, which affects the behaviors of the corneal epithelium and keratocytes (24).
Gender seems to affect reinnervation measured by IVCM but not the reepithelization time. Up to now, different studies stated that gender does not have any influence on reepithelialization as in our experience (25). Instead, no reports in literature found any impact of gender on the corneal reinnervation, unlike our observation. This study is limited by the small number of eyes who experienced complications after CXL and DRE. Even if CXL is a safe procedure, it is clinically meaningful to identify patients at risk for DRE.
The association between corneal steepest meridian readings and DRE is an interesting issue. It has been pointed out that the epithelium at the cone apex is thinner, where the stroma is steeper.
An overall thinning of the epithelium across the ectatic cornea and an apparent difference in epithelial thickness, which is lower in the central region and higher toward the inferior keratoconic cornea, is observed. Such irregularity could explain a slower reepithelialization. Vinciguerra et al. reported that the epithelium could act as a smoothing agent that reduces corneal power, astigmatism, and cornea irregularity after epi-off CXL (26). The reepithelialization and the following remodeling effect of CXL can take about 6 months to flatten and regularize the keratoconic shape of the cornea (26). This slower epithelium remodeling process when the conus is steeper could explain why topography obtained 1 month after CXL paradoxically shows an increase in the steepness of the cone.
The present study suggests that patients who experienced DRE did not derive less efficacy from epi-off CXL.
The association between DRE and age may reflect the age-related decrease in the corneal healing response. Also, low baseline corneal nerve count is associated with DRE. Gender seems to affect reinnervation measured by IVCM, but not the reepithelization time. DRE does not seem to affect the efficacy of epi-off CXL treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
CB: conceptualization, methodology, investigation, writing, and editing the final manuscript. CC: conceptualization, methodology, formal analysis, and editing the final manuscript. DM, RS, ID, and CP: resources and data curation. CT: supervision and reviewing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content of this manuscript has been presented in part at the 2019 European Society of Ophthalmology (SOE) meeting, Nice, France.
1. Sharif R, Bak-Nielsen S, Hjortdal J, Karamichos D. Pathogenesis of keratoconus: the intriguing therapeutic potential of prolactin-inducible protein. Prog Retin Eye Res. (2018) 67:150–67. doi: 10.1016/j.preteyeres.2018.05.002
2. Rabinowitz YS. Keratoconus. Surv Ophthalmol. (1998) 42:297–319. doi: 10.1016/S0039-6257(97)00119-7
3. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. (2003) 135:620–7. doi: 10.1016/S0002-9394(02)02220-1
4. Mazzotta C, Baiocchi S, Bagaglia SA, Fruschelli M, Meduri A, Rechichi M. Accelerated 15 mW pulsed-light crosslinking to treat progressive keratoconus: two-year clinical results. J Cataract Refract Surg. (2017) 43:1081–8. doi: 10.1016/j.jcrs.2017.05.030
5. Wittig-Silva C, Chan E, Islam FMA, Wu T, Whiting M, Snibson GR, et al. Randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus. Ophthalmology. (2014) 121:812–21. doi: 10.1016/j.ophtha.2013.10.028
6. Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. (2015) 41:41–6. doi: 10.1016/j.jcrs.2014.09.033
7. O'Brart DPS, Patel P, Lascaratos G, et al. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol. (2015) 160:1154–63. doi: 10.1016/j.ajo.2015.08.023
8. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. (2008) 34:796–801. doi: 10.1016/j.jcrs.2007.12.039
9. Soeters N, Wisse RPL, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. (2015) 159:821–828.e3. doi: 10.1016/j.ajo.2015.02.005
10. Lang PZ. HafeziNL, Khandelwal SS, Torres-Netto EA, Hafezi F, Randleman JB. Comparative functional outcomes after corneal crosslinking using standard, accelerated, and accelerated with higher total fluence protocols. Cornea. (2019) 38:433–41. doi: 10.1097/ICO.0000000000001878
11. Krysik K, Dobrowolski D, Wylegała EA, Lyssek-Boroń A Amniotic membrane as a main component in treatment supporting healing and patch grafts in corneal melting and perforations. J Ophthalol. (2020) 4238919:1–7. doi: 10.1155/2020/4238919
12. Leccisotti A, Mitomycin C. in photorefractive keratectomy: effect on epithelialization and predictability. Cornea. (2008) 27:288–91. doi: 10.1097/ICO.0b013e31815c5a51
13. Gipson IK. Age-related changes and diseases of the ocular surface and cornea. Investigat Opthalmol Visual Sci. (2013) 54:ORSF48. doi: 10.1167/iovs.13-12840
14. Faragher RG, Mulholland B, Tuft SJ, Sandeman S, Khaw PT. Aging and the cornea. Br J Ophthalmol. (1997) 81:814–7. doi: 10.1136/bjo.81.10.814
15. Rao SN, Chuck RS, Chang AH, LaBree L, McDonnell PJ. Effect of age on the refractive outcome of myopic photorefractive keratectomy. J Cataract Refract Surg. (2000) 26:543–6. doi: 10.1016/S0886-3350(99)00465-4
16. Niederer RL, Perumal D, Sherwin T, McGhee CNJ. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. (2007) 91:1165–9. doi: 10.1136/bjo.2006.112656
17. Torricelli AAM, Singh V, Santhiago MR, Wilson SE. The Corneal epithelial basement membrane: structure, function, and disease. Investigat Opthalmol Visual Sci. (2013) 54:6390. doi: 10.1167/iovs.13-12547
18. Mantelli F, Mauris J, Argüeso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. (2013) 13:563–8. doi: 10.1097/ACI.0b013e3283645899
19. Alvarado J, Murphy C, Juster R. Age-related changes in the basement membrane of the human corneal epithelium. Invest Ophthalmol Vis Sci. (1983) 24:1015–28.
20. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigat Opthalmol Visual Sci. (2011) 52:1938. doi: 10.1167/iovs.10-6997c
21. Roszkowska AM, Colosi P, Ferreri FMB, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. (2004) 218:350–5. doi: 10.1159/000079478
22. Ozgurhan EB, Celik U, Bozkurt E, Demirok A. Evaluation of subbasal nerve morphology and corneal sensation after accelerated corneal collagen cross-linking treatment on keratoconus. Curr Eye Res. (2015) 40:484–9. doi: 10.3109/02713683.2014.932387
23. Berlau J, Becker H-H, Stave J, Oriwol C, Guthoff RF. Depth and age-dependent distribution of keratocytes in healthy human corneas: a study using scanning-slit confocal microscopy in vivo. J Cataract Refract Surg. (2002) 28:611–6. doi: 10.1016/S0886-3350(01)01227-5
24. Shirai K, Okada Y, Cheon D-J, et al. Effects of the loss of conjunctival Muc16 on corneal epithelium and stroma in mice. Investigat Opthalmol Vis Sci. (2014) 55:3626. doi: 10.1167/iovs.13-12955
25. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. (2014) 3:445–64. doi: 10.1089/wound.2013.0473
Keywords: epithelium-off crosslinking, accelerated corneal crosslinking, keratoconus, delayed re-epithelialization, bandage contact lens
Citation: Bonzano C, Cutolo CA, Musetti D, Di Mola I, Pizzorno C, Scotto R and Traverso CE (2021) Delayed Re-epithelialization After Epithelium-Off Crosslinking: Predictors and Impact on Keratoconus Progression. Front. Med. 8:657993. doi: 10.3389/fmed.2021.657993
Received: 24 January 2021; Accepted: 16 September 2021;
Published: 15 October 2021.
Edited by:
Mee Kum Kim, Seoul National University, South KoreaReviewed by:
Betul Bayraktutar, Tufts Medical Center, United StatesCopyright © 2021 Bonzano, Cutolo, Musetti, Di Mola, Pizzorno, Scotto and Traverso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Bonzano, b2N1bGlzdGFib256YW5vQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.