
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 29 March 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.656194
This article is part of the Research Topic Application of Next Generation Sequencing (NGS) and CRISPR-Cas Systems in The Diagnosis of Infectious Diseases View all 45 articles
Background: Talaromycosis is a serious fungal infection which is rare in immunocompetent people. Since its clinical manifestations lack specificity, it is easy to escape diagnosis or be misdiagnosed leading to high mortality and poor prognosis. It is necessary to be alert to the disease when broad-spectrum antibiotics do not work well in immunocompetent patients.
Case Presentation: A 79-year-old man was admitted to our Infectious Diseases Department for recurrent fever and cough. Before admission he has been treated with piperacillin-tazobactam, moxifloxacin followed by antituberculous agents in other hospitals while his symptoms were not thoroughly eased. During the first hospitalization in another hospital, he has been ordered a series of examination including radionuclide whole body bone imaging, transbronchial needle aspiration for subcarinal nodes. However, the results were negative showing no neoplasm. After being admitted to our hospital, he underwent various routine examinations. The initial diagnosis was bacterial pneumonia, and he was given meropenem injection and tigecycline injection successively, but there were no improvement of symptoms and inflammatory indicators. In the end, the main pathogen Talaromyces marneffei was confirmed using Metagenomic Next-Generation Sequencing (mNGS), and his clinical symptoms gradually relieved after targeted antifungal treatment using voriconazole.
Conclusion: When empirical anti-infective treatment is ineffective, it is necessary to consider the possibility of opportunistic fungal infections on immunocompetent patients. mNGS, as a new generation of pathogenic testing methods, can often detect pathogenic bacteria faster than traditional methods, providing important help for clinical decision-making.
Talaromycosis is an opportunistic fungal infection caused by Talaromyces marneffei (T. marneffei), the only thermal dimorphic fungus of Penicillium, which is endemic to Southeast Asia but rare in the Ningbo city of Zhejiang province. Excrement of Rhizomys that were carrying T. marneffei may contaminates surroundings, leading to infections. Susceptible people may be infected through alimentary tract, respiratory tract and broken skin or mucosa contact (1). Since the clinical manifestations lack specificity, it is easy to escape diagnosis and be misdiagnosed especially amongst immunocompetent patients. It is one of the severe opportunistic infections in HIV patients. Recently a small but increasingly number of HIV-negative patients with abnormal immunity, such as patients of connective tissue disease, organ transplant recipients, and long-term users of immunosuppressive agents, were reported to be infected. It rarely infects immunocompetent people, though when it happens the mortality were higher, maybe due to delayed diagnosis (2, 3). Here we presented an immunocompetent Ningbo native suffered from T. marneffei which was hard to identify by conventional detection methods. Finally, the fungus was confirmed by mNGS and prolonged sputum culture.
A 79-year-old man was presented to our hospital with 1-month cough and fever. He has visited two other local hospitals for more than three times where a series of examinations were performed. His chest computerized tomography (CT) scan revealed ground-glass nodules in the upper lobe of the right lung with multiple enlarged lymph nodes in the mediastinum which were suspected of tumors (Figure 1A). In order to clarify the nature of the lesion he further took a needle biopsy of lymph nodes under bronchoscope while pathological results showed no evidence of tumors. Presumed to be pulmonary infection, he was then given piperacillin-tazobactam, moxifloxacin, and empirical anti-tuberculosis treatment successively. His body temperature gradually returned to normal, but the cough still exists. One week later, his cough worsened without fever. Outpatient blood routine examination revealed that white cells count (WBC) 13.1 x 109/L, C-reactive protein (CRP) 183.51 mg/L. Re-examination of chest CT showed infectious lesions in both lungs, left hilar shadow increased, and mediastinal lymph nodes slightly enlarged (Figure 1B). For further treatment, he was admitted to the Department of Infectious Diseases of Ningbo First hospital. His initial diagnosis was bacterial pneumonia.
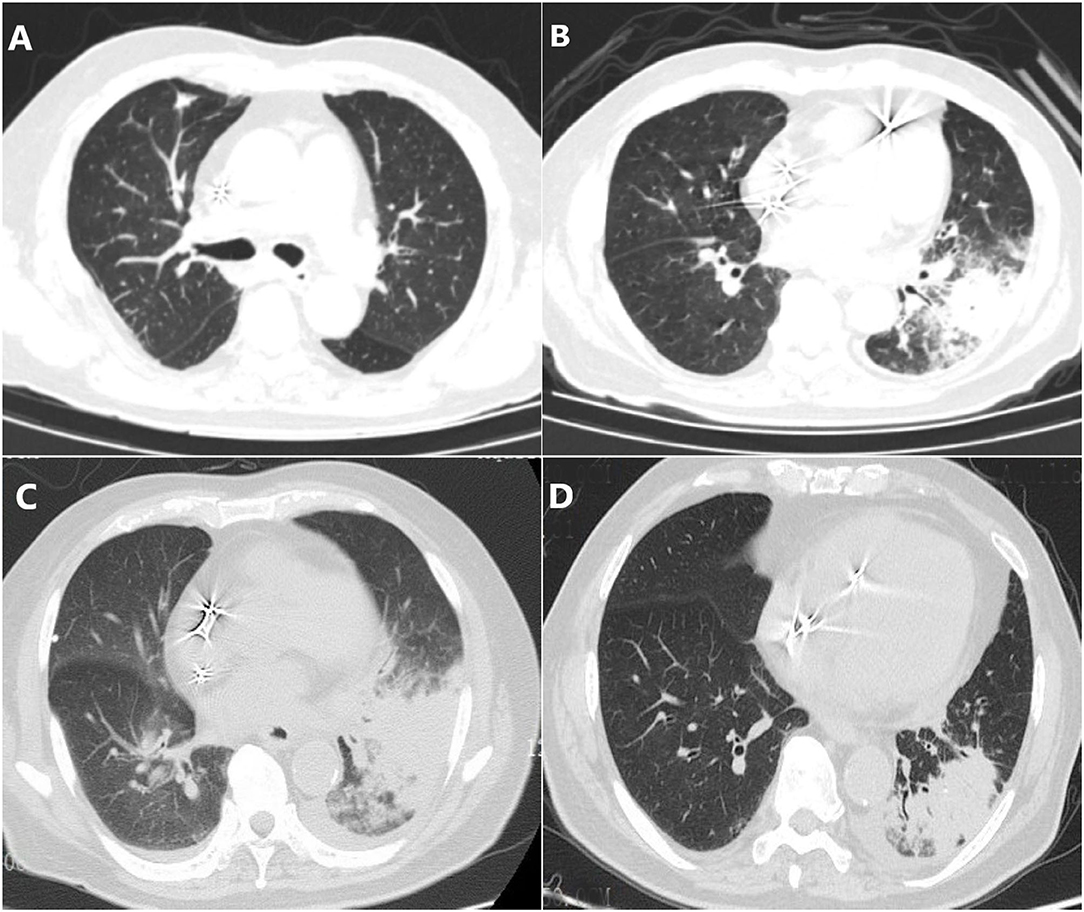
Figure 1. Chest CT scans in different phases. (A) Ground-glass nodules in the upper lobe of the right lung with multiple enlarged lymph nodes in the mediastinum which were suspected of tumors. (B) Infectious lesions in both lungs, left hilar shadow increased, and mediastinal lymph nodes slightly enlarged. (C) The area of pneumonia was larger than before and pleural fluid appeared. (D) Pneumonia and pleural effusion were both absorbed obviously.
He has not taken any immunosuppressants or glucocorticoids before. He had hypertension for more than 10 years treated by amlodipine and bisoprolol, diabetes mellitus for 3 years treated by insulin with unsatisfactory glycemic control, and eczema for 10 years without therapy. He did not have chronic airway disease although he had smoked for 20 years and quitted smoking for more than 20 years. Three years ago, he had undergone Permanent Cardiac Pacemaker implantation. As a Ningbo native, he had retired from a Metal Instrument Factory where he worked as an installer of metallic parts for more than 20 years. And he hasn't been to the wild recently. He has no prior family history of malignancy, infectious diseases or psychosis. Physical examination showed large eczema-like scars on two legs and found no other positive signs.
After admission, we examined his cellular immune function for lymphocyte subsets analysis (Table 1), immunoglobulins, cytokines, and found no obvious immune deficiency. And his tuberculosis T-SPOT. TB test, human immunodeficiency virus (HIV) test, tumor biomarkers (including carcinoembryonic antigen, carbohydrate associated antigen 199, alpha-fetoprotein, and cancer antigen 125), Antinuclear antibody (ANA) and Antineutrophil autoantibodies (ANCA), serum cryptococcal antigen colloidal gold test, and serological test for syphilis were all negative.
Since he has used broad-spectrum antibiotics with poor efficacy, we performed meropenem, tigecycline consecutively (Table 2). However, the peak temperature did not drop (Figure 2), and the inflammation indexes did not improve (Table 3). During hospitalization, his galactomannan (GM) was positive in serum. Thus, we empirically add caspofungin for antifungal treatment. Meanwhile, we replaced tigecycline with linezolid. Since then, the fever seems to have gradually eased but WBC increased slowly (Figure 3). We ordered another chest CT scan which indicated the lesions of pneumonia were larger than before and pleural fluid appeared (Figure 1C). On November 5, he underwent a fiberoptic bronchoscopic examination (Figure 4) and lavage of the basal segment of the left lower lobe. Bronchoalveolar Lavage fluid (BALF) was tested for mNGS, culture, liquid-based cytology. At the same time, blood sample was sent for mNGS examination.
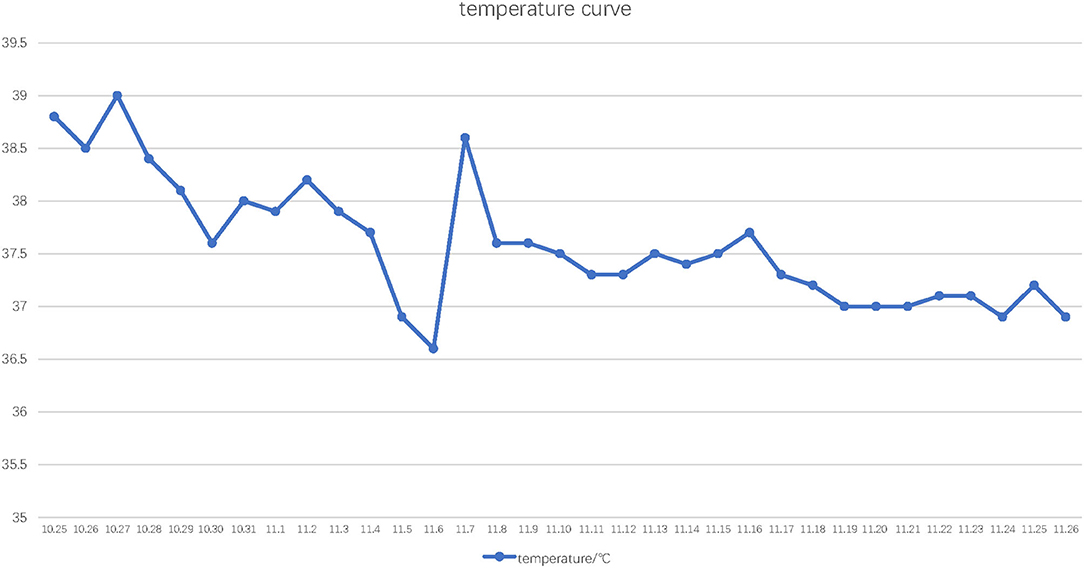
Figure 2. Temperature curve. The horizontal axis indicates the date and the vertical axis indicates highest daily ear temperature.
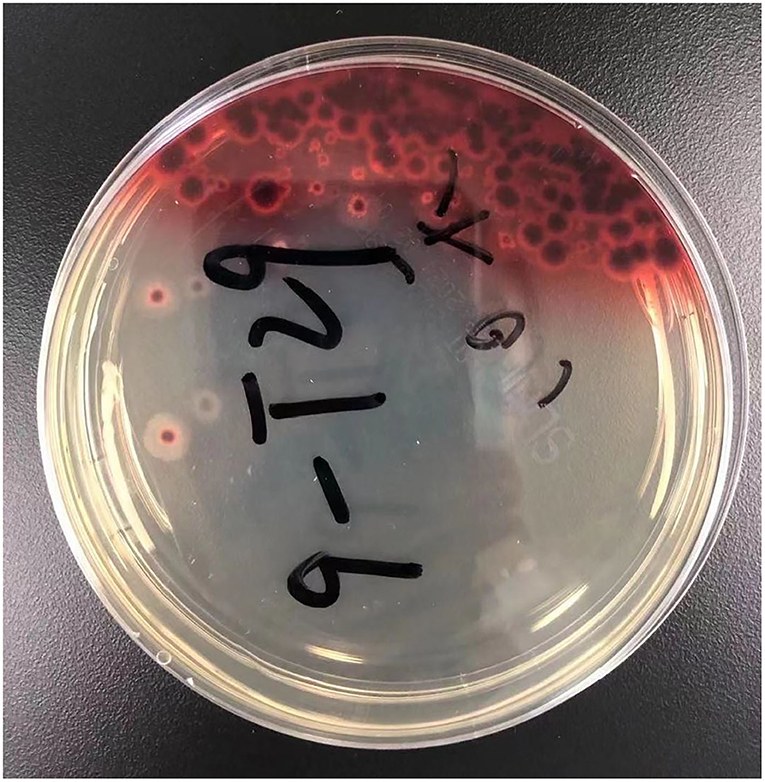
Figure 3. The result of prolonged sputum culture. A diffusible red pigment produced by Talaromyces marneffei, diffuses to the surrounding medium.
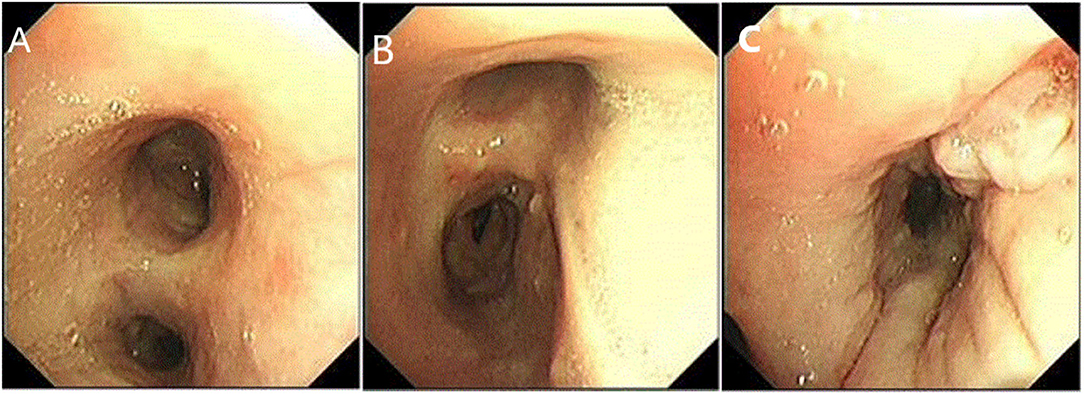
Figure 4. Fiberoptic bronchoscopy. (A,B) showed the mucosa of the left and right main bronchus is scattered with nodules. (C) Longitudinal change of mucosa in the basal segment of the left lower lobe.
Four days later, we changed antibiotics to intravenous voriconazole and piperacillin-tazobactam since mNGS suggested Penicillium marneffei infection (Table 4). Moreover, we prolonged sputum culture time to one week and the fungus was further validated by culture (Figure 3). After he was prescribed targeted anti-fungal agent of voriconazole 200 mg twice daily, the clinical presentations and laboratory indicators were gradually returning to normal (Table 3). The whole process was concluded (Figure 5).
Prior to follow-up, he carried on taking voriconazole 200 mg twice daily regularly without relapse. On December 9th, his repeated chest CT scan showed that pneumonia was cured and pleural effusion were absorbed obviously (Figure 1D).
As the only thermal dimorphic fungus of Penicillium, Talaromyces marneffei grows as yeast in vivo at 37°C as pathogenic mode and as mold in vitro at 25°C (4). It was originally taken from the hepar of Rhizomys in 1956 while three years later, the first case of human infected by Talaromyces marneffei was found derived from the laboratory (1, 2). Talaromyces marneffei proliferates in macrophages and spreads through reticuloendothelial system. T cells, especially CD4+ Cells, are vital to remove Talaromyces marneffei. In theory, Talaromyces marneffei can be cleared in 2–3 weeks in healthy hosts while it may be fatal to immunocompromised patients (1). Thus, it tends to be found on hosts with cellular immune deficiency. The most common hosts are HIV patients followed by other immunocompromised hosts, such as patients who suffer from connective tissue diseases or hematopathy, organ transplant recipients, and long-term users of immunosuppressants. It rarely persists in immunocompetent hosts.
The pathological changes caused by talaromycosis may be divided into three groups as granuloma, reactive necrosis and purulent inflammation (1). The most common clinical symptoms are fever, coughing, anemia, wasting, and characteristic umbilicated skin lesion (5, 6) which are usually nonspecific and of little help to the judgment. Chest CT scan of pulmonary talaromycosis often lacks specificity. As is reported, it can be multiple cavities with uneven thick wall and clear edge, enlarged mediastinal lymph nodes, ground glass opacity, or pleural effusion (1, 7). Thus, without enough suspicion, it is easy to be misdiagnosed as bacterial pneumonia, cancer, tuberculosis, and other fungal infection.
In recent decades, increasing incidents of talaromycosis in healthy hosts are reported (8). The fatality rate of talaromycosis in non-HIV patients is higher than HIV-infected patients which is probably due to delayed diagnoses and treatments (2, 3). Without timely diagnosis and targeted therapy, the mortality rate of talaromycosis can be above 90% (9). We herein reported a case of HIV-negative talaromycosis with pleural effusion from nonendemic region, and without history of recent contact with wildlife. The old man suffered from diabetes with unsatisfactory blood glucose control, cardiac disease and eczema which maybe risk factors of talaromycosis. However, lymphocyte subsets analysis showed his CD3, CD4/CD3, CD8, and CD4/CD8 were all normal in addition to the negative HIV test. Like many other immunocompetent talaromycosis patients, he had been misdiagnosed as bacterial pneumonia and tuberculosis until mNGS cleared away the confusion. Furthermore, prolonged sputum culture verified the pathogen showing bright red surrounding molds which is the unique feature of Talaromyces marneffei as it can product a soluble red pigment (3) whose essence maybe monascorubramine encoded by pks3 and other four genes (10). Blood dissemination can be determined from the mNGS of blood. According to literature, disseminated talaromycosis usually has worse prognosis than localized ones since it may be accompanied with multiple organ dysfunction (1). We speculated his anemia maybe related to Talaromyces marneffei (11) although it needs further investigation and verification.
Amphotericin B is the first line agent for talaromycosis with HIV suggested by guideline (12). However, severe nephrotoxicity limits the clinical use of amphotericin B. As a broad-spectrum antifungal agent, voriconazole was reported to be effective for talaromycosis (13, 14). Therefore, we chose voriconazole to treat this patient since it is safe, strong and within his economic affordability. The result was satisfactory.
There are some take-away lessons from this case. Since routine culture often cannot cover all bacteria and fungi, timely communications between clinicians and microbiologists is necessary when a particular pathogen is suspected. Microbiologists may then adjust culture methods to improve detection rate of rare pathogens. When traditional anti-infection treatments are not effective and the pathogen is difficult to be detected by routine examinations, mNGS is recommended since it may detect some rare pathogen faster than culture. Then the patient may get the appropriate treatments sooner and have a better prognosis.
Some limitations were discovered in this case. First, in the course of empirical medication, we changed agents frequently which may be influenced by patient's anxiety and high expectations. Second, we lacked suspicion to this rare fungus and initially did not have close communication with microbiology experts which we later rectified. Last, we still don't understand if his thrombocytosis and refractory pleural effusions are related to talaromycosis when his albumin concentration was above 30 g/L.
In conclusion, we should be alert of rare fungi when routine anti-infective therapies were useless even in immunocompetent hosts. As an advanced test method, mNGS may detect pathogens fast and exactly.
The datasets have been uploaded into EMBL database, accession number PRJEB43434.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
JS analyzed data and drafted the manuscript. NY collected the data. GQ revised the manuscript. All authors read and approved the final manuscript.
This research was supported by Key Program of Natural Science Foundation of Ningbo under Grant No. 202003N4019.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Ada Hoi Yan Ma for polishing the manuscript.
1. Wang YG, Cheng JM, Ding HB, Lin X, Chen GH, Zhou M, et al. Study on the clinical features and prognosis of Penicilliosis marneffei without human immunodeficiency virus infection. Mycopathologia. (2018) 183:551–8. doi: 10.1007/s11046-017-0236-3
2. Chan JF, Lau SK, Yuen KY Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. (2016) 5:e19. doi: 10.1038/emi.2016.18
3. Kawila R, Chaiwarith R Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. (2013) 13:464. doi: 10.1186/1471-2334-13-464
4. Zhou F, Bi X, Zou X, Xu Z, Zhang T. Retrospective analysis of 15 cases of Penicilliosis marneffei in a southern China hospital. Mycopathologia. (2014) 177:271–9. doi: 10.1007/s11046-014-9737-5
5. Du Q Tong CK. Talaromyces (Penicillium) marneffei infection. IDCases. (2018) 13:e00428. doi: 10.1016/j.idcr.2018.e00428
6. Furusawa H, Miyazaki Y, Sonoda S, Tsuchiya K, Yaguchi T, Kamei K, et al. Penicilliosis marneffei complicated with interstitial pneumonia. Intern Med. (2014) 53:321–3. doi: 10.2169/internalmedicine.53.1465
7. Li X, Hu W, Wan Q, Lei Q, Sun C, Hou Z, et al. Non-HIV talaromycosis: Radiological and clinical analysis. Medicine. (2020) 99:e19185. doi: 10.1097/MD.0000000000019185
8. Ye F, Luo Q, Zhou Y, Xie J, Zeng Q, Chen G, et al. Disseminated penicilliosis marneffei in immunocompetent patients: a report of two cases. Indian J Med Microbiol. (2015) 33:161–5. doi: 10.4103/0255-0857.148433
9. Liyan X, Changming L, Xianyi Z, Luxia W Suisheng X. Fifteen cases of penicilliosis in Guangdong, China. Mycopathologia. (2004) 158:151–5. doi: 10.1023/b:myco.0000041842.90633.86
10. Tam EW, Tsang CC, Lau SK Woo PC. Polyketides, toxins and pigments in Penicillium marneffei. Toxins. (2015) 7:4421–36. doi: 10.3390/toxins7114421
11. Sirisanthana V Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pediatr Infect Dis J. (1995) 14:935–40. doi: 10.1097/00006454-199511000-00003
12. Larsson M, Nguyen LH, Wertheim HF, Dao TT, Taylor W, Horby P, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther. (2012) 9:24. doi: 10.1186/1742-6405-9-24
13. Ge Y, Xu Z, Hu Y Huang M. Successful voriconazole treatment of Talaromyces marneffei infection in an HIV-negative patient with osteolytic lesions. J Infect Chemother. (2019) 25:204–7. doi: 10.1016/j.jiac.2018.08.007
Keywords: talaromyces marneffei, immunocompetent, next-generation sequencing, anti-fungal treatment, case report
Citation: Shi J, Yang N and Qian G (2021) Case Report: Metagenomic Next-Generation Sequencing in Diagnosis of Talaromycosis of an Immunocompetent Patient. Front. Med. 8:656194. doi: 10.3389/fmed.2021.656194
Received: 20 January 2021; Accepted: 05 March 2021;
Published: 29 March 2021.
Edited by:
Li Ang, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Wenjun Du, Shandong Public Health Clinical Center, ChinaCopyright © 2021 Shi, Yang and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoqing Qian, YmlsbC5xaWFuQG91dGxvb2suY29t
†ORCID: Jiejun Shiorcid orcid.org/0000-0003-0911-2773
Naibin Yang orcid.org/0000-0001-7439-723X
Guoqing Qian orcid.org/0000-0003-2427-8042
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.