
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 10 May 2021
Sec. Geriatric Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.649907
This article is part of the Research Topic Reducing the Burden of Age-related Disease in relation to Osteoporosis, Sarcopenia and Osteosarcopenia View all 13 articles
 Amir Bagheri1†
Amir Bagheri1† Rezvan Hashemi2†
Rezvan Hashemi2† Sanaz Soltani1
Sanaz Soltani1 Ramin Heshmat3
Ramin Heshmat3 Ahmadreza Dorosty Motlagh1
Ahmadreza Dorosty Motlagh1 Bagher Larijani4
Bagher Larijani4 Ahmad Esmaillzadeh1,5,6*
Ahmad Esmaillzadeh1,5,6*Background: Sarcopenia has rarely been linked to Food-based Inflammatory Potential of the Diet (FIPD) in earlier studies. This study was performed to examine the association of FIPD and sarcopenia and its components.
Method: In the cross-sectional research, dietary intakes of 300 randomly-selected elderly adults aged 55 years or older were collected through a validated food frequency questionnaire. We constructed FIPD score based on average consumptions of 28 food items. According to The European Working Group on Sarcopenia definition, sarcopenia and its components such as muscle strength, muscle mass, and gait speed were defined.
Result: No significant difference was found between the prevalence of sarcopenia (P = 0.05), low muscle mass (P = 0.27), low handgrip strength (P = 0.72), and lower gait speed (P = 0.14) across tertiles of FIPD score. Moreover, we did not find significant differences among means of handgrip strength (P = 0.65), muscle mass (P = 0.33), and walking speed (P = 0.89) across FIPD categories. However, binary logistic regression analysis indicated a significant positive relationship between FIPD score and odds of sarcopenia; such that subjects in the top vs. those in the bottom FIPD tertile had 155% greater chance of having sarcopenia (OR: 2.55; 95% CI: 1.17–5.55). After controlling for all confounding factors, this association strengthened (OR: 2.67; 95% CI: 1.18–6.01).
Conclusion: We found that greater FIPD score, which means a more pro-inflammatory diet, was positively linked with sarcopenia.
Sarcopenia is a generalized and progressive skeletal muscle disease that causes rapid loss of muscle health (1). It is related to chronic disease and known as a public health problem (1, 2). The projection for number of people with sarcopenia is its increasing rate from 50 million in 2010 to more than 200 million in 2050 worldwide (3). It is estimated that Iran's aging population will approximately reach 10% of the population in 2026 (4). Additionally, the prevalence of sarcopenia in Iran was 17 to 33% (5). Thus, diagnosis of underlying factor contributing to this condition is essential.
Aging, inactivity, poor diet, chronic disease, inflammation, and iatrogenic factors are the underlying causes of sarcopenia (1). Inflammation activates many molecular pathways involved in this disease by stimulating protein catabolism and suppressing muscle synthesis (6, 7). Therefore, high levels of inflammatory cytokines have a harmful effect on muscle strength and mass (8). Diet is a modifiable factor for both sarcopenia and inflammation (9). Although dietary inflammatory index (DII) has earlier been designed to evaluate the inflammatory properties of the diet, it mostly based on nutrients (10). There are the complex mixture of nutrients and chemicals in the diet and their effect on each other (11). This can distort the known potential of their anti- or pro-inflammatory nature. Thus, studies of single nutrients and even single foods may not fully address the complex nature of a diet (12). Therefore, the use of food-based inflammatory potential of the diet (FIPD) that considers foods together is suggested (13, 14). According to this idea, Tabung et al. constructed FIPD index based on dietary intakes of foods or food groups to survey the inflammatory capacity of the diet (14). This food-based dietary pattern could be conveniently interpreted for disease prevention and health improvement as dietary guidelines (14). Such dietary patterns have been shown to predict concentrations of plasma inflammatory factors better than DII (13).
Despite some studies on the link between food-based inflammatory potential of the diet (FIPD) and risk of some chronic disease including colorectal (15) and ovarian cancer (16), irritable bowel syndrome (17), and psychological illnesses (18), to our knowledge there is no study explored the association of this dietary index and risk of sarcopenia. Moreover, scarce data are available about the association of diet and sarcopenia in the Middle East. Therefore, this study was designed to examine the relationship between FIPD and sarcopenia and its components.
We carried out a population-based cross-sectional study from May to October 2011 in Tehran, Iran. Tehran is the largest city and capital of Iran. This metropolitan region accounts for 10.8% of Iran's total population (19). The data collection and sampling method with more details have earlier been published (20). Briefly, we recruited 300 elderly adults (150 women and 150 men) aged 55 years or older who were selected through a cluster random selection method in district 6 of Tehran. According to the postal code address, the head of each cluster was determined. It should be noted that age and gender distribution, as well as demographic status in the population of district 6 of Tehran, where the current study's population came from, are representative of Tehran's population (19). To avoid heterogeneity in the data, persons whose major cause of sarcopenia did not due to aging were not included. Subjects who had sarcopenia due to lack of moving as well as those with artificial limbs or limb prostheses were not included. In addition, subjects with debilitating diseases (for instance malignancy and organ failure) that might predispose an individual to sarcopenia were not included as well. Tehran University of Medical Sciences Ethics' Committee accepted the protocol study. First, the settings of study were elucidated to the participants and then the informed written consent were obtained.
Dietary data of study participants were gathered with a Block-format 117-item Food Frequency Questionnaire (FFQ), for which detailed information about the validity and reliability has been given elsewhere (20, 21). Trained nutritionist administered the FFQs through in-person meeting. The questionnaire contained a list of food items that their portion sizes were specified. The booklet of “household measures” was used to convert the dietary items to grams per day. We calculated daily nutrients and energy intake of each participant with Iranian modified version of Nutritionist IV software.
For construction of FIPD, we used the earlier database on 486 Tehrani female adults about the association of food and food groups contributed to systemic inflammation (22). In the mentioned study, the level of inflammatory markers was assessed with serum interleukin-6 (IL-6), Tumor Necrosis Factor alpha (TNF alpha), and high-sensitivity C-reactive protein (hs-CRP) level (22). Dietary items that loaded in the western dietary pattern were determined as pro-inflammatory foods and other food items loaded in the healthy dietary pattern were determined as anti-inflammatory items. We used this approach because that study observed the healthy dietary pattern could significantly decrease inflammatory markers as well as the western dietary pattern could significantly elevated inflammatory markers (22). First, average daily consumptions of 28 food groups including 12 anti-inflammatories (e.g., poultry, fish, tomatoes, legumes, yellow vegetables, cruciferous vegetables, other vegetables, fruits, fruit juices, green leafy vegetables, tea, and whole grains) and 16 pro-inflammatory items (eggs, dairy, potatoes, pizza, butter, red meats, coffee, French fries, sweets and desserts, refined grains, snacks, hydrogenated oils, processed meats, hydrogenated fats, soft drinks, and mayonnaise) were calculated. Then, amounts of these foods items were controlled for energy with the residual analysis (23). For each individual, we multiplied average daily consumption of each food items through the factor loadings attained in the aforementioned paper (22). Then, total FIPD score of each individual was calculated through summation of each dietary item scores. Lastly, to decrease the magnitude of the scores, the final FIPD score was divided by 100. A lower FIPD or more negative score shows a less pro-inflammatory diet, and vice versa.
Sarcopenia was determined according to the definition suggested by the European Working Group on Sarcopenia (EWGSOP) (3). We considered the combination of both low muscle mass and either weak grip strength or slow gait speed to determine sarcopenia. The ratio of whole lean mass of arms and legs of each individuals known as ASM (Appendicular Skeletal Muscle) (24) divided to their height2 (ASM/height2) was considered as the muscle mass. A dual-energy X-ray absorptiometry (DEXA) (Discovery W S/N 84430) was applied for this assessment. The muscle mass lower than 5.45 kg/m2 for women and lower than 7.26 kg/m2 for men were diagnosed as low muscle mass (3).
Muscle strength of each participant was determined by handgrip test. A pneumatic squeeze bulb dynamometer (named: c7489-02 Rolyan) adjusted in pound per inch2 (psi) was used to obtained handgrip strength. Participants had to squeeze 3 times for both hands with a 30 s rest after each squeeze. Next, the mean result of all measurement was recorded. Finally, the handgrip strength lower than 30 kg for men and lower than 20 kg for women was determined as low muscle strength (25). We applied a 4-m walk gait speed exam to evaluate physical performance of participants (3). Participants with gait speeds < 0.8 m/s was diagnosed as slow gait speed (3).
Required information about non-dietary data including age, sex, socio-economic status, alcohol consumption, and smoking habits were obtained with a general questionnaire. The former medical history including history of arthritis, stroke, asthma, myocardial infarction, and diabetes, and also a history of medicine use including statins, insulin angiotensin-converting enzyme inhibitors, sexual hormones, and corticosteroid were collected as well. The physical activity performance was evaluated by the short form of the International Physical Activity Questionnaire (IPAQ) (26). Next, in accordance with recommendation of IPAQ, we calculated a metabolic equivalent-hour per week (MET-h/week) for all contributors (27). We used a digital scale to measure weight while participants were minimally clothed. We measured height in a standing position without shoes by a wall tape meter. While contributors were standing and usually breathe, their waist circumference was measured in the middle of the iliac crest and lower rib margin. Weight (kg) divided by height squared (m2) was applied to compute body mass index (BMI).
Participants were categorized based on tertile cut-off points of FIPD score. We used tertiles instead of quartiles or quintiles due to not having a large sample size. In addition, we reached the best associations when we considered participants across tertiles of FIPD score. To examine the differences in distribution of the participant's characteristics across tertiles of FIPD, we used Chi-square and ANOVA analyses for categorical and continuous variables, respectively. Dietary intakes were assessed through ANCOVA across FIPD score categories, in which we adjusted for sex, age, and energy. Binary logistic regression was applied to find the link of FIPD score with sarcopenia in crude and multivariable-adjusted models. In the first model, energy intake (kcal/d), sex (male/female), and age (continuous) were controlled for. In the second model, further adjustments were performed for alcohol consumption (yes/no), smoking (yes/no), history of chronic illness (cerebrovascular accident, arthritis, asthma, diabetes, and myocardial infarction), physical activity (MET-h/wk), and medication use (corticosteroid, statin, testosterone, and estrogen). Moreover, the odds ratio for sarcopenia in the second and third tertiles were compared with the lowest tertile (reference category). We included the tertile categories of the FIPD score as an ordinal variable in the logistic regression analysis to attain P for trends. All aforementioned analyses were done with SPSS (version 26). P-values lower than 0.05 were considered significant.
In the present investigation, total FIPD score was between −48.4 and −0.45. The cut-off points of FIPD score across increasing tertiles was <-11.25, −11.25 to −7.37, and >-7.37, respectively. Distribution of participants as well as mean values of continuous variables across tertiles of FIPD is shown in Table 1. Subjects in the third tertile compared to subjects in the first tertile of FIPD had less physical activity (P = 0.005). No significant difference was detected in mean age and BMI across FIPD categories. The distribution of subjects in alcohol use, smoking, medical or medication history between the highest vs. lowest categories of FIPD was not significantly different.
Age-, gender- and energy-adjusted means of selected food items throughout FIPD categories are displayed in Table 2. We observed a higher score of FIPD significantly related with a lower intake of cruciferous vegetables, fruits, yellow vegetables, green leafy vegetables, tomatoes, other vegetables, fish, tea, and low-fat dairy. Moreover, subjects in the highest rank of FIPD score had significantly greater consumptions of refined grains, high-fat dairy, soft drinks, red meats, potatoes, and French fries than subjects in the lowest rank.
Table 3 indicates the distribution of sarcopenia, low muscle mass, low muscle strength, and low gait speed across FIPD categories. We did not find a significant difference in the distribution of sarcopenia (P = 0.05), low muscle mass (P = 0.27), low handgrip strength (P = 0.72), and lower gait speed (P = 0.14) throughout categories of FIPD score. In addition, no significant differences were found among means of handgrip strength, muscle mass, and walking speed across FIPD categories even after adjusting for all covariates.
Odds ratios (ORs) and 95% CIs from crude and multivariable-adjusted model for sarcopenia across FIPD tertiles are depicted in Table 4. A significant relationship between a higher FIPD score and increasing odds of sarcopenia (OR: 2.55; 95% CI: 1.17–5.55) was identified in the unadjusted model. After controlling for energy intake, sex, and age (model 1), the relationship remained significant (OR: 2.67; 95% CI: 1.18–6.01). Even after additional controlling for all covariates, we found that subjects in the top vs. the bottom FIPD tertile had higher odds of sarcopenia (OR: 2.57; 95% CI: 1.11–5.89).
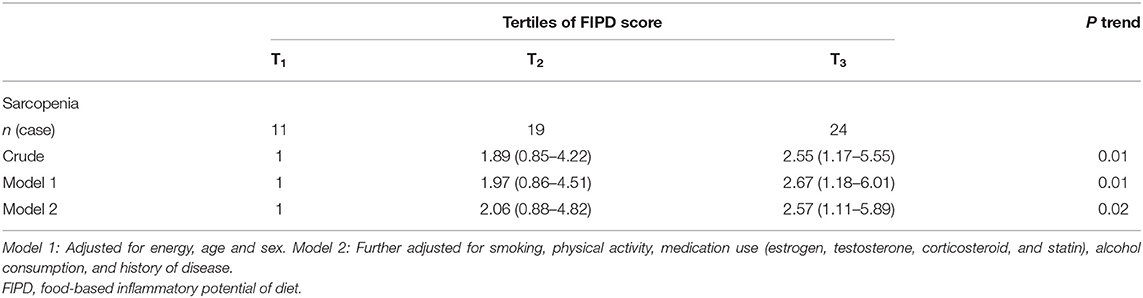
Table 4. Multivariable-adjusted odds ratios (95% CIs) for sarcopenia across tertile FIPD categories.
We evaluated the relationship between the food-based inflammatory potential of diet and sarcopenia and its components. A higher FIPD score or a more pro-inflammatory diet was linked with elevated likelihood of sarcopenia. Nevertheless, we did not find any relationship between FIPD and low gait speed, low muscle strength, and low muscle mass. As far as we know, this is the first study elucidate the role of the food-based inflammatory score in sarcopenia.
Sarcopenia is known as a disease, associated with increment hazard of disability, mortality, and fall (28, 29). It appears that level of inflammatory marker has been increased during the aging period that might cause muscle weakness or sarcopenia (30); however, dietary intakes can also contribute to elevated inflammation and muscle deterioration (9). FIPD was validated and expanded to clarify the whole dietary inflammatory capacity (14). We found that subjects with high FIPD score had a greater chance of having sarcopenia. It must be mentioned that data are very limited on the association between diet and sarcopenia especially about the inflammatory capacity of the diet. A large cross-sectional study from the United State demonstrated that higher nutrient-based DII score was related to increased risk of sarcopenia (31). Our recent publication in the current population revealed that nutrient-based DII was positively associated with sarcopenia (32). However, the current analysis is different from previous ones because unlike previous one (32), here we considered foods to compute dietary inflammatory capacity. Although both DII based on nutrient and food-based FIPD indexes evaluate the inflammatory properties of diet, nutrient-based index might have some limitations to assess this potential due to the complex synergistic and interactions effect of nutrients and chemicals in the diet (11, 14). The FIPD is a novel index constructed based on foods and food groups and is more appropriate for clinical practice and public recommendations (14). Earlier studies have shown that the food-based assessment of dietary inflammatory potential predicts systemic inflammation better than nutrient-based DII (13). However, additional investigation is needed to elucidate the association between dietary inflammatory capacity and components of sarcopenia.
As expected, we found individuals with high adherence to pro-inflammatory diet (the top FIPD tertile) had significantly lower consumptions of food groups with anti-inflammatory properties including fish, tomatoes, cruciferous vegetables, yellow vegetables, green leafy vegetables, other vegetables, fruits, tea, and low-fat dairy. Additionally, subjects in the third vs. the first FIPD tertile had significantly greater consumptions of potatoes, high-fat dairy, refined grains, red meats, soft drinks, and French fries. Based on these findings, a dietary pattern with a high intake of fish, vegetables, fruit, and low-fat dairy along with a fewer intake of red meats, refined grains, potatoes, high-fat dairy, and soft drinks might be beneficial for maintaining muscles. In line with this results, a cohort study conducted in England, Wales, and Scotland reported that a healthy dietary pattern (means greater consumption of leafy vegetables, fresh fruit, and whole-grain bread, but lesser consumption of processed meat, white bread, and added sugar) in adulthood (ages 36, 43, 53) was linked with improved muscle performance in older age (60–64) (33). A Swedish cohort study (34), that investigated the relationship of dietary patterns at study baseline with average age 71 years, suggested that eating a healthy dietary pattern especially “the Mediterranean diet” tended to reduce the progress of sarcopenia 16 years later.
There is growing evidence that increasing pro-inflammatory markers such as TNF-α, IL-1β, IL-6 are the main cause of skeletal muscle wasting and sarcopenia (35). High serum levels of these inflammatory markers can negatively influence skeletal muscle by inhibiting the expression and activity of GH and IGF-I (35, 36). Of note, studies conducted to determine the effect of anti-inflammatory medications on skeletal muscle and inflammation, showed that these anti-inflammatory drugs can significantly reduce loss of muscle mass and keep muscle strength (37). It appears that our findings provide further evidence on the contribution of dietary inflammatory potential to sarcopenia.
This study had some strengths and limitations. For the first time, we used a novel food-based IPD to predict sarcopenia and its components. Evidence found that FIPD better predicts the body's inflammation and its related co-morbidities than nutrient-based dietary inflammatory index (13). Several potential confounders were considered in the analyses. Moreover, we used a validated FFQ for the evaluation of participants dietary intakes. Along with strengths, our study had some limitations as well. First, the cross-sectional design of the study prohibits us to reach a causal relationship, because longitudinal data is not available as well as exposure and outcome are identified in each individual simultaneously. Second, the possibility of misclassification of study subjects, due to measurement errors, cannot be avoided. Third, despite considering numerous confounding factors, residual confounders cannot be ignored. Fourth, we did not measure inflammatory cytokines in the current study. The FIPD was constructed based on factor loadings of food items in healthy or Western dietary patterns among Iranian female teachers, not the current study population. It is better to construct FIPD based on coefficients between food groups and inflammatory biomarkers obtained in the same study population. Finally, the study was performed on a small sample (maximum 300 cases), due to inaccessibility to more DEXA machine in Tehran and budget limitations. Thus, caution is required to the extrapolation of our findings to the total Iranian population.
Findings from this population-based cross-sectional investigation support the role of food-based inflammatory potential of the diet in sarcopenia. Strategies to reduce consumption of foods with a greater pro-inflammatory capacity along with increasing consumption of foods with anti-inflammatory features may have benefits for older adults to prevent muscle loss. Prospective studies however are needed to verify these findings.
The raw data supporting the conclusions of this article will be made available by the authors, on reasonable request to the corresponding author.
The studies involving human participants were reviewed and approved by Tehran University of Medical Sciences Ethics' Committee. The patients/participants provided their written informed consent to participate in this study.
AB, RHa, AD, BL, RHe, SS, and AE participated to the data collection, statistical analyses, design, conception, data explanation, manuscript writing, and approval of the final version of the manuscript. All authors contributed to the article and approved the submitted version.
The financial support for this study comes from the Tehran Endocrine and Metabolism Research Center and the Tehran University of Medical Science.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all subjects participated in the study.
1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
2. Ethgen O, Beaudart C, Buckinx F, Bruyère O, Reginster J-Y. The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int. (2017) 100:229–34. doi: 10.1007/s00223-016-0220-9
3. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis report of the european working group on sarcopenia in older people. Age Ageing. (2010) 39:412–3. doi: 10.1093/ageing/afq034
4. Tanjani PT, Motlagh ME, Nazar MM, Najafi F. The health status of the elderly population of Iran in 2012. Arch Gerontol Geriatr. (2015) 60:281–7. doi: 10.1016/j.archger.2015.01.004
5. Shafiee G, Heshmat R, Ostovar A, Nabipour I, Larijani B. Sarcopenia disease in Iran: an overview. J Diabetes Metab Disord. (2019) 18:665–74. doi: 10.1007/s40200-019-00452-9
6. Jo E, Lee S-R, Park B-S, Kim J-S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin Exp Res. (2012) 24:412–22. doi: 10.3275/8464
7. Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. (2015) 12:22. doi: 10.11138/ccmbm/2015.12.1.022
8. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. (2006) 119:526. e529–6. e517. doi: 10.1016/j.amjmed.2005.10.049
9. Dodds RM, Roberts HC, Cooper C, Sayer AA. The epidemiology of sarcopenia. J Clin Densitom. (2015) 18:461–6. doi: 10.1016/j.jocd.2015.04.012
10. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
12. Cespedes EM, Hu FB. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am J Clin Nutr. (2015) 101:899–900. doi: 10.3945/ajcn.115.110213
13. Tabung FK, Smith-Warner SA, Chavarro JE, Fung TT, Hu FB, Willett WC, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. (2017) 147:1567–77. doi: 10.3945/jn.117.248377
14. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. (2016) 146:1560–70. doi: 10.3945/jn.115.228718
15. Tabung FK, Liu L, Wang W, Fung TT, Wu K, Smith-Warner SA, et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. (2018) 4:366–73. doi: 10.1001/jamaoncol.2017.4844
16. Tabung FK, Huang T, Giovannucci EL, Smith-Warner SA, Tworoger SS, Poole EM. The inflammatory potential of diet and ovarian cancer risk: results from two prospective cohort studies. Br J Cancer. (2017) 117:907–11. doi: 10.1038/bjc.2017.246
17. Salari-Moghaddam A, Keshteli AH, Esmaillzadeh A, Adibi P. Empirically derived food-based inflammatory potential of the diet, irritable bowel syndrome, and its severity. Nutrition. (2019) 63:141–7. doi: 10.1016/j.nut.2019.02.004
18. Salari-Moghaddam A, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Empirically derived food-based dietary inflammatory index is associated with increased risk of psychological disorders in women. Nutr Neurosci. (2019) 24:260–8. doi: 10.1080/1028415X.2019.1621044
19. Nourollahi T. National population and housing census in IR of Iran. In: Paper Presented at the 24th Population Census Conference. Tehran (2009).
20. Hashemi R, Heshmat R, Motlagh AD, Payab M, Esmaillzadeh A, Baigy F, et al. Sarcopenia and its determinants among Iranian elderly (SARIR): study protocol. J Diabetes Metab Disord. (2012) 11:23. doi: 10.1186/2251-6581-11-23
21. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
22. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett W. C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. (2007) 85:910–8. doi: 10.1093/ajcn/85.3.910
23. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65:1220S−8S. doi: 10.1093/ajcn/65.4.1220S
24. Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. (1990) 52:214–8. doi: 10.1093/ajcn/52.2.214
25. individuals and patients with immune-mediated polyneuropathies. Muscle Nerve (2000) 23:1393–401. doi: 10.1002/1097-4598(200009)23:9<1393::aid-mus10>3.0.co;2-o
26. Vasheghani-Farahani A, Tahmasbi M, Asheri H, Ashraf H, Nedjat S, Kordi R. The Persian, last 7-day, long form of the International Physical Activity Questionnaire: translation and validation study. Asian J Sports Med. (2011) 2:106. doi: 10.5812/asjsm.34781
27. Committee IR. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. (2005). Available online at: http://www.ipaq.ki.se/.
28. Chen L-K, Lee W-J, Peng L-N, Liu L-K, Arai H, Akishita M, et al. Recent advances in Sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2016) 17:767. e761–7. e767. doi: 10.1016/j.jamda.2016.05.016
29. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. (2016) 7:512–4. doi: 10.1002/jcsm.12147
30. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. (2017) 96:10–5. doi: 10.1016/j.maturitas.2016.11.006
31. Geng J, Deng L, Qiu S, Bian H, Cai B, Jin K, et al. Dietary inflammatory potential and risk of sarcopenia: data from national health and nutrition examination surveys. Aging. (2020) 13:1913–28. doi: 10.18632/aging.202141
32. Bagheri A, Soltani S, Hashemi R, Heshmat R, Motlagh AD, Esmaillzadeh A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr J. (2020) 19:1–8. doi: 10.1186/s12937-020-00649-2
33. Robinson SM, Westbury LD, Cooper R, Kuh D, Ward K, Syddall HE, et al. Adult lifetime diet quality and physical performance in older age: findings from a British birth cohort. J Gerontol A Biol Sci Med Sci. (2018) 73:1532–7. doi: 10.1093/gerona/glx179
34. Karlsson M, Becker W, Michaëlsson K, Cederholm T, Sjögren P. Associations between dietary patterns at age 71 and the prevalence of sarcopenia 16 years later. Clin Nutr. (2020) 39:1077–84. doi: 10.1016/j.clnu.2019.04.009
35. Sakuma K, Aoi W, Yamaguchi A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch. (2017) 469:573–91. doi: 10.1007/s00424-016-1933-3
36. Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. (2009) 64:1183–9. doi: 10.1093/gerona/glp097
Keywords: food-based inflammatory potential of the diet, sarcopenia, muscle mass, muscle strength, gait speed
Citation: Bagheri A, Hashemi R, Soltani S, Heshmat R, Dorosty Motlagh A, Larijani B and Esmaillzadeh A (2021) The Relationship Between Food-Based Pro-inflammatory Diet and Sarcopenia: Findings From a Cross-Sectional Study in Iranian Elderly People. Front. Med. 8:649907. doi: 10.3389/fmed.2021.649907
Received: 05 January 2021; Accepted: 12 April 2021;
Published: 10 May 2021.
Edited by:
Mario Ulises Pérez-Zepeda, Dalhousie University, CanadaReviewed by:
Carlos Montes Paixao Junior, Rio de Janeiro State University, BrazilCopyright © 2021 Bagheri, Hashemi, Soltani, Heshmat, Dorosty Motlagh, Larijani and Esmaillzadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Esmaillzadeh, YS1lc21haWxsemFkZWhAdHVtcy5hYy5pcg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.