
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 13 May 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.642749
This article is part of the Research TopicSpotlight on the Relationship between Sepsis and Infection: from Mechanisms to TherapyView all 26 articles
Introduction: Fission1 (Fis1) and parkin are key proteins related to mitochondrial fission and mitophagy, respectively. This study aimed to assess the prognostic value of the Fis1/parkin ratio as a biomarker in patients with sepsis.
Methods: Consecutive patients with sepsis (n = 133) or simple infection (n = 24) were enrolled within 24 h of arrival at the intensive care unit (ICU). Serum levels of Fis1, parkin, mitofusin2 (Mfn2), and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) were measured by enzyme-linked immunosorbent assay (ELISA) upon ICU admission. Clinical parameters and standard laboratory test data were also collected. All patients received follow-up for at least 28 days.
Results: Patients with sepsis presented with significantly decreased serum levels of parkin, Mfn2, and PGC-1α, but an increased serum Fis1 level and Fis1/parkin, Fis1/Mfn2, and Fis1/PGC-1α ratios at ICU admission. Relative to patients with simple infections, the ratios were remarkably elevated in septic patients—particularly septic shock patients. The area under the receiver operating characteristic (ROC) curve of the Fis1/parkin ratio was greater than that of Fis1, parkin, Mfn2, and PGC-1α levels as well as that of the Fis1/Mfn2 and Fis1/PGC-1α ratios for prediction of 28-day mortality due to sepsis. All of the ratios were significantly higher in non-survivors than survivors at the 28-day follow-up examination. Fis1/parkin ratio was found to be an independent predictor of 28-day mortality in patients with sepsis.
Conclusions: The Fis1/parkin ratio is valuable for risk stratification in patients with sepsis and is associated with poor clinical outcomes for sepsis in the ICU.
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection (1). Each year there are an estimated 48.9 million incident cases of sepsis worldwide and 11.0 million sepsis-related deaths, representing 19.7% of all global deaths (2). However, effective management of sepsis and resource allocation remain a challenge due to the inability to accurately diagnose the severity and risk of sepsis. Therefore, prognostic biomarkers are needed for early identification of patients at high risk of sepsis. Such patients could be transferred to the ICU and receive optimized hospital resources and therapies.
Among the complex mechanisms of sepsis and its heterogeneous nature, defective mitochondrial quality control (MQC) plays an important role in the severity of sepsis and sepsis-induced multiple organ dysfunction syndrome (MODS) (3–6). The MQC system aims to maintain mitochondrial homeostasis, allowing the mitochondrial network to segregate, recognize, and eliminate damaged mitochondria and to generate new mitochondria. MQC processes include mitochondrial biogenesis, mitochondrial dynamics (mitochondrial fission and fusion), and mitophagy (7, 8). Previous studies have demonstrated that sepsis is ameliorated by the recovery of mitochondria homeostasis (4, 9, 10). Therefore, indicators related to mitochondrial homeostasis may be useful for risk stratification and prognostic evaluation of patients with sepsis.
Fission 1 (Fis1), parkin, mitofusin2 (Mfn2), and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) are four key proteins involved in mitochondrial fission, mitophagy, mitochondrial fusion, and mitochondrial biogenesis, respectively (10). It has been shown that recovery of mitophagy, mitochondrial fusion, and biogenesis can partly reverse organ failure under septic conditions, whereas worsening of sepsis is accompanied by activation of mitochondrial fission (3, 11–14). In other words, elevations of parkin, Mfn2, and PGC-1α appear to protect against organ dysfunction in animal models of sepsis, whereas Fis1 is associated with sepsis severity and multiple organ dysfunction.
The peripheral blood samples are an attractive tissue for biomarker discovery as they are easily obtained and analyzed. It has been proved that the expression of Fis1, Mfn2, parkin and PGC-1α in peripheral blood mononuclear cells (PBMCs) could give us some information about the mitochondrial quality control status (15–19), and considering the immune cell death during sepsis (20, 21), MQC-related proteins in the immune cells could continuously release into circulation, making the detection of these proteins in serum possible.
Simultaneous measurement of multiple biomarkers may be useful for overcoming the limitations of using a single biomarker. Assessment of multiple biomarkers associated with different sepsis-related pathways may be particularly useful. Thus, in the present study, we use the Fis1/parkin, Fis1/Mfn2, and Fis1/ PGC-1α ratios to reflect the severity of mitochondrial homeostasis disbalance. This study aimed to investigate the feasibility of using the Fis1/parkin, Fis1/Mfn2, and Fis1/ PGC-1α ratios to predict the prognosis of septic patients and to identify what ratio provided the best performance.
This prospective study was carried out at Peking Union Medical College Hospital (PUMCH) between June 2019 and August 2020. The study was approved by the PUMCH institutional review board (approval number JS-2421) and informed consent was obtained from all enrolled patients or their relatives. Patient records were anonymized and deidentified before analysis.
The inclusion criteria were: (1) age ≥18 years and (2) diagnosis of sepsis [according to The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (1)]. The exclusion criteria were: (1) age <18 years, (2) massive bleeding or pulmonary embolism, (3) heart attack or acute exacerbation of previous heart disease in the previous week, (4) heart surgery in the previous week, and (5) lack of informed consent by the patient or their relatives.
A total of 133 septic patients, who were followed for 28 days or until death, were enrolled in this study. Septic patients were divided into non-shock and shock subgroups. The criteria for inclusion in the shock subgroup were: a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP ≥ 65 mmHg and a serum lactate level >2 mmol/L (18 mg/dL) despite adequate volume resuscitation on the day of ICU admission (1). Septic patients who did not meet these criteria were assigned to the septic non-shock group. Additionally, 24 patients with simple infection, but who did not meet the criteria for sepsis, admitted to the intensive care unit (ICU) served as a control group.
Baseline clinical and laboratory characteristics were obtained from medical records and routine ICU tests, including patient age, sex, hemodynamic parameters, blood chemistry, arterial blood gas analysis, Acute Physiology and Chronic Health Evaluation (APACHE) II score, and SOFA score.
Peripheral blood samples were collected within 24 h of ICU admission and centrifuged immediately. Serum was withdrawn and stored at −80°C until assessment by enzyme-linked immunosorbent assay (ELISA). The serum levels of Fis1, parkin, Mfn2, and PGC-1α were determined using commercially available ELISA kits following the instructions of the manufacturer (Fis1: Abbexa abx151559 Cambridge, UK; parkin: Abcam ab212159 Shanghai, China; Mfn2: Abebio AE33636HU Wuhan, China; PGC-1α: Cusabio CSB-E11761h Wuhan, China). According to the manufacturers' specifications, the ELISA assays were specific for native proteins, with no significant cross-reactivity with known analogs. Each well of the ELISA plate was loaded with 100 μL undiluted blood sample, and we did not measure the protein concentration before loading the plate.
Results are presented as the mean and standard deviation or median and range (interquartile range). Group differences for continuous variables with a non-normal distribution were tested using the Kolmogorov-Smirnov test and Mann–Whitney U test. Categorical variables are reported as proportions and compared using the χ2 or Fisher's exact tests. Logistic regression analysis was used to determine independent predictors of 28-day mortality. The receiver operating characteristic (ROC) curve was used to assess the accuracy of the variables for prediction of 28-day mortality. The Kaplan–Meier method was used to analyze the survival data. Comparisons between groups were performed using the log-rank test. P < 0.05 was considered to be statistically significant. All statistical analyses were performed with SPSS 22.0 software (IBM Inc.).
Patient recruitment for this study is shown in Figure 1. A total of 133 septic patients and 24 infected patients (controls) were enrolled in the study.
There were no significant differences in age, central venous blood oxygen saturation (ScvO2), or arteriovenous carbon dioxide partial pressure difference (Pv-aCO2) between the two groups (infection and sepsis). APACHE II and SOFA scores differed between groups, with the sepsis group scoring significantly higher (p < 0.001). The basic characteristics all enrolled patients are listed in Table 1.
Table 2 shows, for each study group, the serum Fis1, parkin, Mfn2 and PGC-1α levels; Fis1/parkin, Fis1/Mfn2, and Fis1/ PGC-1α ratios; and the PCT level. Figure 2 presents the Fis1/parkin, Fis1/Mfn2, Fis1/ PGC-1α ratios and serum PGC-1α, Fis1, Mfn2, parkin levels for each subgroup (infection, sepsis nonshock and septic shock groups). Serum PCT, Fis1, parkin, Mfn2, and PGC-1α levels, as well as the Fis1/parkin, Fis1/Mfn2, and Fis1/ PGC-1α ratios, at ICU admission differed significantly among the groups. Relative to the control group, the sepsis group had significantly higher serum mediator levels and ratios (p < 0.001). Additionally, the Fis1/parkin, Fis1/Mfn2, Fis1/ PGC-1α ratios and serum PGC-1α, Fis1, Mfn2, parkin levels were markedly different among the sepsis non-shock, septic shock, and control groups (p < 0.001). Moreover, for patients with sepsis, we compared the ratios and serum levels between survivors and non-survivors. We found that all three ratios were significantly lower in survivors compared to non-survivors (Figure 3). Survivors had significantly higher serum parkin, PGC-1α levels and lower Fis1 level.
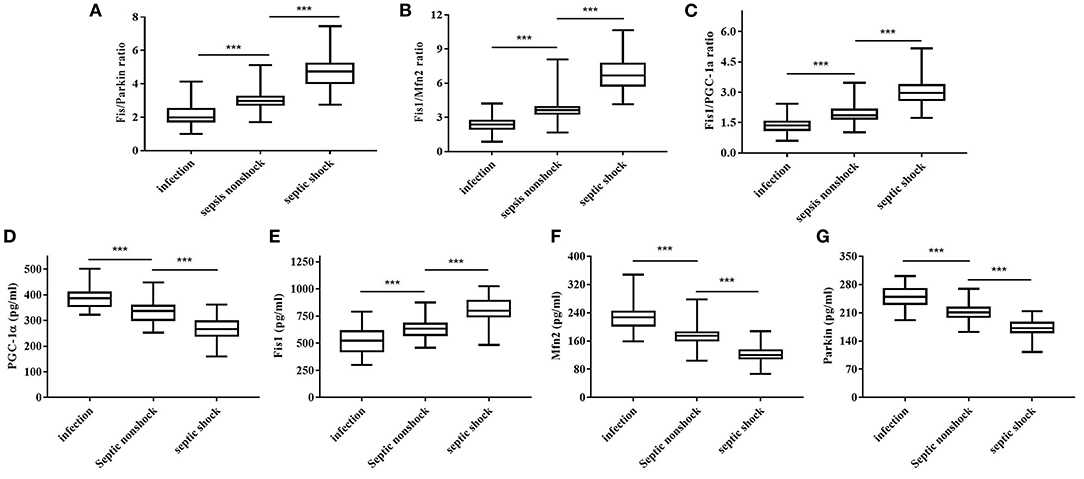
Figure 2. Fis1/parkin ratio (A), Fis1/Mfn2 ratio (B), Fis1/PGC-1α ratio (C) and serum PGC-1α (D), Fis1 (E), Mfn2 (F), parkin (G) levels at ICU admission in the patient subgroups.
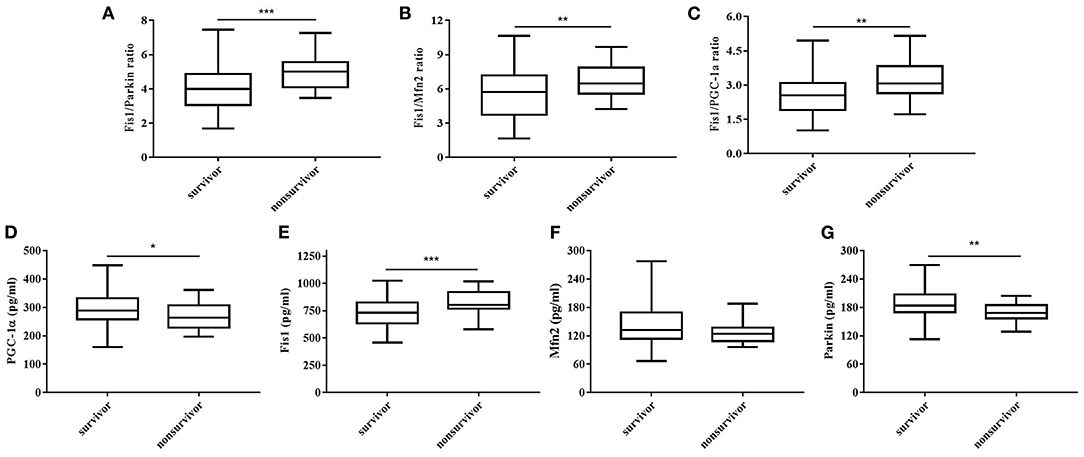
Figure 3. Fis1/parkin ratio (A), Fis1/Mfn2 ratio (B), Fis1/PGC-1α ratio (C) and serum PGC-1α (D), Fis1 (E), Mfn2 (F), parkin (G) levels at ICU admission in survivor and non-survivor groups of patients with sepsis at 28-day follow-up.
Considering that the ratios differed significantly between survivors and non-survivors, we calculated the AUCs of the mediator levels and ratios as predictors of 28-day mortality using the ROC curve. The results are presented in Table 3. As shown, the Fis1/parkin ratio was a better predictor than serum PCT, Fis1, parkin, Mfn2, and PGC-1α levels or Fis1/Mfn2 and Fis1/ PGC-1α ratios (Figure 4). For serum Fis1/parkin ratio at ICU admission, the AUC to predict 28-day mortality was 0.792 (95% CI, 0.695–0.890, P < 0.001), and the optimal cut-off value was 4.0 (sensitivity 94.4%, specificity 49.6%).
Next, the SOFA score, APACHE II score, and Fis1/parkin, Fis1/Mfn2, and Fis1/PGC-1α ratios were included in a multivariate logistic regression model to determine the independent predictors of 28-day mortality (Table 4). A high Fis1/parkin ratio (β = 1.347, odds ratio [OR] = 3.845, p = 0.021) was a significant independent risk factor for 28-day mortality in septic patients.
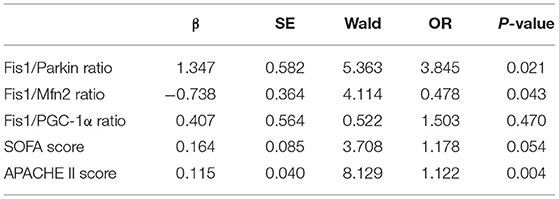
Table 4. Multivariate logistic regression analysis of factors predicting 28-day mortality in sepsis patients.
We also performed a Kaplan–Meier survival analysis using the Fis1/parkin ratio cut-off (4.00) in septic patients (Figure 5). The results indicated that septic patients with a Fis1/parkin ratio above the cut-off value had a significantly lower survival rate (p = 0.001).
There is an urgent need for biomarkers to improve early diagnosis and risk stratification for patients with sepsis. In our study, patients with sepsis presented with significantly decreased serum levels of parkin, Mfn2, and PGC-1α and significantly increased serum Fis1 level and Fis1/parkin, Fis1/Mfn2, Fis1/PGC-1α ratios. Notably, higher ratios were associated with increased severity of disease. Relative to ICU patients with other infections, septic patients showed remarkably elevated ratios, with septic shock patients having the highest ratios. It has been proved in the clinical and experimental studies, that the persistence of low mitochondrial mitophagy, biogenesis, fusion levels and a high level of fission during sepsis suggested a poor outcome (3, 6, 14, 22), which agrees with the changes observed in our study.
Our findings support that high ratios at ICU admission can identify the severity of sepsis. In addition, septic patients with poor outcomes (non-survivors) had higher Fis1/parkin, Fis1/Mfn2, and Fis1/PGC-1α ratios. The ratios were greater than the absolute Fis1, parkin, Mfn2, and PGC-1α levels for prognostic purposes and had significant prognostic value for 28-day mortality. The Fis1/parkin ratio performed best among all of the ratios, as reflected by the AUC. Additionally, Fis1/parkin ratio was proved to be an independent risk predictor for patients with sepsis. Septic patients with a higher Fis1/parkin ratio (>4.0) had a significantly lower survival rate. Therefore, we chosen Fis1/parkin ratio to represent the mitochondrial homeostasis, indicating the balance between risk factor and protective factor.
Mitochondria are highly dynamic organelles and frequently undergo fission and fusion to modulate mitochondrial morphology, number, and size. Fission (in which mitochondria divide) is essential for cell growth and division, as it promotes adequate numbers of mitochondria. Fission may also occur when there is significant mitochondrial damage, as fission will allow the cell to segregate the damaged portion (23). During sepsis, mitochondrial dysfunction results in activation of mitochondrial fission and inactivation of mitochondrial fusion, which each or both promote dysfunctional mitochondrial fragmentation (13). It remains uncertain whether the elevation of fission is a physiological adaptation or a manifestation of decompensation under septic conditions (4), although persistent elevation of fission during sepsis is associated with the deterioration of sepsis both in vivo and in vitro (13). The pretreatment with fission inhibitor (mdivi-1) significantly attenuated mitochondrial dysfunction and apoptosis in septic animal model (3). In this profile, the overactivation of fission during sepsis may be a risk factor. Mitophagy refers to selective autophagy of mitochondria, and its main function is to recognize damaged mitochondria for degradation. In sepsis, mitochondrial dysfunction induces a loss of mitochondrial membrane potential that triggers mitophagy. Upregulation of mitophagy may improve organ function and reduce organ inflammation in response to lipopolysaccharide (LPS), whereas inhibition of mitophagy has been shown to increase mortality in septic mice (9, 22, 24). This phenomenon suggests a protective role of mitophagy during sepsis. Therefore, a higher Fis1/parkin ratio is expected to reflect a more severe imbalance of mitochondrial homeostasis. This would result in poorer outcomes for patients, which is consistent with our results. An elevation of Fis1/parkin ratio could be used to alert clinicians that mitochondrial dysfunction is existed, and clinical adjustments are needed to prevent the progression of sepsis. The reasons for the elevation of MQC-related biomarkers may be considered as: the persistent mitochondrial damage and the cell death (apoptosis, pyroptosis, or necroptosis) under septic exposure, which lead to continuous release of MQC-related proteins into circulation. In a clinical study in multiple sclerosis (MS) patients, the serum levels of parkin are remarkably augmented and associated with disease activity, indicating the use of parkin as biomarker of mitophagy (15). The researchers had previously proved that parkin levels were increased within the CNS and at the systemic level in patients with MS compared to other neurological disorders and healthy individuals (16). Though these studies were not in septic setting, but suggesting the possibility of use serum parkin level as mitophagy related biomarker in septic patients. There are also some studies investigated the fission protein (Fis1, Drp1) in peripheral blood lymphocytes (PBL) and also the gene and protein expression of mitochondrial fusion (Mfn2)/fission (Fis1)/biogenesis (PGC-1α) in peripheral blood mononuclear cells (PBMCs) as biomarker to reflect the MQC status (17–19). Immune cells may be the main source of MQC-related proteins release. Of course, further reevaluation of the patient and repeated exploration for a possible source are still needed, and our research is ongoing.
Given the crucial role of mitochondria in sepsis (25, 26), numerous studies have evaluated other mitochondrial function biomarkers, such as electron transport chain (ETC) enzyme activity in platelets or circulating mitochondrial DNA (mtDNA) in plasma or serum, to monitor mitochondrial function. However, ETC has not been found to be useful for evaluating the prognosis of sepsis (27, 28). Quite a few studies have investigated the prognostic value of mtDNA in septic patients (29–33). Most studies that performed AUC analysis found a statistically significant association between mtDNA levels and mortality. However, steps need to be taken to standardize how mtDNA is measured to facilitate large, prospective, multicenter trials to better assess the ability of mtDNA to predict outcomes. In a previous study, we evaluated the potential utility of serum uncoupling protein-2 (UCP2) level as a mitochondrial function biomarker in septic patients (34). However, it did not outperform the Fis1/parkin ratio (the AUC of serum UCP2 level for predicting 28-day mortality was 0.704, compared with 0.792 for the Fis1/parkin ratio). There are also studies investigated the mitochondria in circulating cells, such as respiratory chain biochemistry in platelets (35) or the complex activity in platelets (28) or mitochondrial bioenergetic reserve in PBMCs (36, 37), aiming to provide insight into sepsis-associated temporal changes and how these changes relate to recovery. However, they were all required further validation. At present, monitoring of mitochondrial function is still limited to experimental work.
In a word, use of the serum Fis1/parkin ratio has several advantages: (1) Serum Fis1 and parkin levels can be accurately determined with high reproducibility using the ELISA technique, relative to biomarkers measured by western blot (WB) or polymerase chain reaction (PCR). (2) Serum samples are easier to obtain than tissue/organ samples or blood mitochondria containing cells (i.e., immune cells). (3) The Fis1/parkin ratio is specifically related to sepsis pathophysiology rather than more general inflammatory reactions. The use of this biomarker may help transform our understanding of sepsis from a “physiological syndrome” to a “group of distinct biochemical disorders” and lead to advances in the search for adjunctive sepsis therapies (38).
Some limitations of this study merit consideration. First, an important limitation of the study is that the source of the proteins identified in the serum samples was not validated. Second, we did not compare the Fis1/parkin ratio with other classic biomarkers of mitochondrial function (i.e., mtDNA, complex of respiratory chain). Third, comparisons of Fis1 and parkin levels before and after treatment were not performed during patients' stays in the ICU. Fourth, complications of the patients' underlying diseases may have had an effect on the results. Lastly, this study was conducted at a single center with a relatively small sample size. Thus, larger studies at multiple centers are warranted to confirm our findings.
This study aimed to assess the use of the serum Fis1/parkin ratio as a biomarker of prognosis in septic patients. We found that the serum Fis1/parkin ratio is an independent risk factor and a predictor of 28-day mortality in patients with sepsis. The higher the Fis1/parkin ratio is, the severer the disease is. These results suggest that this ratio is valuable for rapid risk stratification when patients are admitted to the ICU. Further study is needed to verify the potential utility and beneficial effects of this biomarker to answer specific clinical questions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by This study was approved by the Ethical Committee of PUMCH (approval number: JS-2421). The patients/participants provided their written informed consent to participate in this study.
WH conceived and designed the study, enrolled the patients, collected the blood samples, analyzed and interpreted data, performed the statistical analysis, and drafted the manuscript. XW conceived and designed the study, enrolled the patients, interpreted data and revised the manuscript. HZ enrolled the patients, analyzed data and revised the manuscript. GW enrolled the patients, collected blood samples, and analyzed data. DL conceived and designed the study, interpreted data and revised the manuscript. All authors read and approved the final manuscript.
This work was supported by the Chinese National Natural Science Foundation (No. 81671878) and Key Project of Central Health Care Scientific Research (2020ZD08).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MQC, mitochondrial quality control; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; Fis1, fission protein 1; Mfn2, mitofusin2; ICU, intensive care unit (ICU); APACHE II score, Acute Physiology and Chronic Health Evaluation II score; CRRT, continuous renal replacement therapy; HR, heart rate; MAP, mean arterial blood pressure; MV, mechanical ventilation; NE, norepinephrine; SOFA, sequential organ failure assessment score; CVP, central venous pressure; Lac, lactic acid; PaO2, arterial partial pressure of oxygen; PI, perfusion index; Pv-aCO2, arteriovenous carbon dioxide partial pressure difference; ScvO2, central venous blood oxygen saturation; PCT, procalcitonin; cTnI, cardiac troponin I; NT-ProBNP, N-terminal pro-brain natriuretic peptide.
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, Miriuka SG, et al. Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radic Res. (2014) 48:769–83. doi: 10.3109/10715762.2014.906592
4. Wu Y, Yao YM, Lu ZQ. Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J Mol Med. (2019) 97:451–62. doi: 10.1007/s00109-019-01756-2
5. Oami T, Watanabe E, Hatano M, Teratake Y, Fujimura L, Sakamoto A, et al. Blocking liver autophagy accelerates apoptosis and mitochondrial injury in hepatocytes and reduces time to mortality in a murine sepsis model. Shock. (2018) 50:427–34. doi: 10.1097/SHK.0000000000001040
6. Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. (2010) 182:745–51. doi: 10.1164/rccm.201003-0326OC
7. Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. (2018) 15:543–54. doi: 10.1038/s41569-018-0059-z
8. Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. (2018) 17:865–86. doi: 10.1038/nrd.2018.174
9. Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. (2018) 138:2247–62. doi: 10.1161/CIRCULATIONAHA.117.032821
10. Stallons LJ, Funk JA, Schnellmann RG. Mitochondrial homeostasis in acute organ failure. Curr Pathobiol Rep. (2013) 1:10.1007/s40139-013-0023-x. doi: 10.1007/s40139-013-0023-x
11. MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. (2012) 185:851–61. doi: 10.1164/rccm.201106-1152OC
12. Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. (2011) 121:4003–14. doi: 10.1172/JCI58662
13. Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. (2013) 83:568–81. doi: 10.1038/ki.2012.441
14. Piquereau J, Godin R, Deschenes S, Bessi VL, Mofarrahi M, Hussain SN, et al. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. (2013) 9:1837–51. doi: 10.4161/auto.26502
15. Castellazzi M, Patergnani S, Donadio M, Giorgi C, Bonora M, Fainardi E, et al. Correlation between auto/mitophagic processes and magnetic resonance imaging activity in multiple sclerosis patients. J Neuroinflammation. (2019) 16:131. doi: 10.1186/s12974-019-1526-0
16. Patergnani S, Castellazzi M, Bonora M, Marchi S, Casetta I, Pugliatti M, et al. Autophagy and mitophagy elements are increased in body fluids of multiple sclerosis-affected individuals. J Neurol Neurosurg Psychiatry. (2018) 89:439–41. doi: 10.1136/jnnp-2017-316234
17. Scaini G, Fries GR, Valvassori SS, Zeni CP, Zunta-Soares G, Berk M, et al. Perturbations in the apoptotic pathway and mitochondrial network dynamics in peripheral blood mononuclear cells from bipolar disorder patients. Transl Psychiatry. (2017) 7:e1111. doi: 10.1038/tp.2017.83
18. Wang S, Song J, Tan M, Albers KM, Jia J. Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer's disease. Eur J Neurol. (2012) 19:1015–22. doi: 10.1111/j.1468-1331.2012.03670.x
19. Busquets-Cortes C, Capo X, Martorell M, Tur JA, Sureda A, Pons A. Training and acute exercise modulates mitochondrial dynamics in football players' blood mononuclear cells. Eur J Appl Physiol. (2017) 117:1977–87. doi: 10.1007/s00421-017-3684-z
20. Park DW, Zmijewski JW. Mitochondrial dysfunction and immune cell metabolism in sepsis. Infect Chemother. (2017) 49:10–21. doi: 10.3947/ic.2017.49.1.10
21. Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. (2005) 174:5110–8. doi: 10.4049/jimmunol.174.8.5110
22. Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, Bohanon M, et al. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol. (2014) 306: L604–19. doi: 10.1152/ajplung.00272.2013
23. Jang DH, Greenwood JC, Owiredu S, Ranganathan A, Eckmann DM. Mitochondrial networking in human blood cells with application in acute care illnesses. Mitochondrion. (2019) 44:27–34. doi: 10.1016/j.mito.2017.12.009
24. Chang AL, Ulrich A, Suliman HB, Piantadosi CA. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic Biol Med. (2015) 78:179–89. doi: 10.1016/j.freeradbiomed.2014.10.582
25. Duran-Bedolla J, Montes DOM, Saldana-Navor V, Villalobos-Silva JA, Rodriguez MC, Rivas-Arancibia S. Sepsis, mitochondrial failure and multiple organ dysfunction. Clin Invest Med. (2014) 37:E58–69. doi: 10.25011/cim.v37i2.21087
26. Zhang H, Feng YW, Yao YM. Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res. (2018) 5:41. doi: 10.1186/s40779-018-0187-0
27. Protti A, Fortunato F, Artoni A, Lecchi A, Motta G, Mistraletti G, et al. Platelet mitochondrial dysfunction in critically ill patients: comparison between sepsis and cardiogenic shock. Crit Care. (2015) 19:39. doi: 10.1186/s13054-015-0762-7
28. Sjovall F, Morota S, Hansson MJ, Friberg H, Gnaiger E, Elmer E. Temporal increase of platelet mitochondrial respiration is negatively associated with clinical outcome in patients with sepsis. Crit Care. (2010) 14:R214. doi: 10.1186/cc9337
29. Timmermans K, Kox M, Scheffer GJ, Pickkers P. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock. (2016) 45:607–12. doi: 10.1097/SHK.0000000000000549
30. Di Caro V, Walko TR, Bola RA, Hong JD, Pang D, Hsue V, et al. Plasma mitochondrial DNA–a novel DAMP in pediatric sepsis. Shock. (2016) 45:506–11. doi: 10.1097/SHK.0000000000000539
31. Schafer ST, Franken L, Adamzik M, Schumak B, Scherag A, Engler A, et al. Mitochondrial DNA: an endogenous trigger for immune paralysis. Anesthesiology. (2016) 124:923–33. doi: 10.1097/ALN.0000000000001008
32. Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Jones AE. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock. (2012) 38:337–40. doi: 10.1097/SHK.0b013e318266a169
33. Harrington JS, Huh JW, Schenck EJ, Nakahira K, Siempos II, Choi A. Circulating mitochondrial DNA as predictor of mortality in critically Ill patients: a systematic review of clinical studies. Chest. (2019) 156:1120–36. doi: 10.1016/j.chest.2019.07.014
34. Huang W, Wang X, Zhang H, Wang C, Liu D. The value of serum uncoupling protein-2 level for the patients with sepsis. Shock. (2020) 54:301–7. doi: 10.1097/SHK.0000000000001523
35. Protti A, Fortunato F, Caspani ML, Pluderi M, Lucchini V, Grimoldi N, et al. Mitochondrial changes in platelets are not related to those in skeletal muscle during human septic shock. PLoS ONE. (2014) 9:e96205. doi: 10.1371/journal.pone.0096205
36. Weiss SL, Zhang D, Bush J, Graham K, Starr J, Tuluc F, et al. Persistent mitochondrial dysfunction linked to prolonged organ dysfunction in pediatric sepsis. Crit Care Med. (2019) 47:1433–41. doi: 10.1097/CCM.0000000000003931
37. Weiss SL, Selak MA, Tuluc F, Perales VJ, Nadkarni VM, Deutschman CS, et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med. (2015) 16:e4–12. doi: 10.1097/PCC.0000000000000277
Keywords: mitochondrial homeostasis, fission1/parkin ratio, sepsis, biomarker, prognosis
Citation: Huang W, Wang X, Zhang H, Wang G and Liu D (2021) Prognostic Significance of the Fission1/Parkin Ratio for Sepsis: A Prospective Cohort Study. Front. Med. 8:642749. doi: 10.3389/fmed.2021.642749
Received: 07 January 2021; Accepted: 08 April 2021;
Published: 13 May 2021.
Edited by:
Alessandro Russo, University of Pisa, ItalyReviewed by:
Rita M. Cowell, Southern Research Institute, United StatesCopyright © 2021 Huang, Wang, Zhang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoting Wang, aWN1dGluZ0AxNjMuY29t; Dawei Liu, ZHdsaXU5OEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.