- 1Department of Intensive Care Unit, Central South University, Xiangya Hospital, Changsha, China
- 2Department of Intensive Care Unit, Peking University, Shenzhen Hospital, Shenzhen, China
Background: Septic shock patients have tendencies toward impairment in cerebral autoregulation and imbalanced cerebral oxygen metabolism. Tissue Oxygen Saturation (StO2) and Transcranial Doppler (TCD) monitoring were undertaken to observe the variations of cerebral hemodynamic indices and cerebral/peripheral StO2 to find risk factors that increase the sepsis-associated delirium (SAD).
Materials and Methods: The research cohort was chosen from septic shock patients received in the Department of Critical Care Medicine, Xiangya Hospital, Central South University between May 2018 and March 2019. These patients were separated into two groups, SAD and non-SAD as assessed by using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Comparisons were made between the two groups in terms of peripheral StO2, fluctuations in regional cerebral oxygen saturation (rSO2), cerebral vascular automatic regulation function [Transient Hyperemic Response Ratio (THRR) index], cerebral hemodynamic index, organ function indicators, blood gas analysis indices, and patient characteristics.
Results: About 39% of the patients (20/51) suffered from SAD. Nearly 43% of the patients died within 28 days of admission (22/51). Individuals in the SAD cohort needed a longer period of mechanical ventilation [5 (95% CI 2, 6) vs. 1 days (95% CI 1, 4), p = 0.015] and more time in ICU [9 (95% CI 5, 20) vs. 5 days (95% CI 3, 9), p = 0.042]; they also experienced more deaths over the 28-day period (65 vs. 29%, p = 0.011). The multivariate regression analysis indicated that independent variables associated with SAD were THRR index [odds ratio (OR) = 5.770, 95% CI: 1.222–27.255; p = 0.027] and the mean value for rSO2 was < 55% (OR = 3.864, 95% CI: 1.026–14.550; p = 0.046).
Conclusion: Independent risk factors for SAD were mean cerebral oxygen saturation below 55% and cerebrovascular dysregulation (THRR < 1.09).
Introduction
The sepsis-associated delirium (SAD) is regarded as a diffuse cerebral dysfunction, caused by inflammatory responses of the body to infections taking place with no discernible cause of central nervous system, i.e., infection. SAD may have a rapid onset and levels may fluctuate over time (1, 2). Research has demonstrated that pathogenesis of SAD can involve neuronal degeneration, neurotransmitter imbalance, abnormal cerebral perfusion, and neuroinflammation (3–6). Some researchers have proposed that there is a higher likelihood of SAD in patients experiencing cerebral edema, blood-brain barrier disruption, lower brain oxygen uptake, and variations in cerebral blood flow as a result of hemodynamic instabilities (7, 8). For sepsis patients, variations in cerebral autoregulation and perfusion can be visually detected by using transcranial Doppler (TCD) ultrasound. Significant links between SAD and cerebral vascular autoregulation disorders were revealed by Pfister et al. (9). Some study has found a close correlation between clinical manifestations of SAD and variations in cerebral hemodynamics found by using TCD ultrasound. Pierrakos et al. (10) found that pulsatility index (PI) on the first day could predict the positive Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) test in sepsis patients (p < 0.01) and only on the first day, the mean blood velocity in the middle cerebral artery (MCA) and cerebral blood flow index (CBFi) was found to be lower in those patients with a high initial PI.
Hypoxemia and hypotension lead to an increase in neuronal apoptosis and, further, have direct associations with poor outcomes (11). Current methods for monitoring mixed venous oxygen saturation (SvO2) and central venous oxygen saturation (ScvO2) are non-continuous, demand invasive procedures, and do not permit pinpointed local brain tissue monitoring, meaning that rapid and effective identification of sepsis patients with higher risks of suffering brain/brain-tissue hypoxemia is not possible (12). Monitoring tissue oxygen saturation (StO2) and regional cerebral oxygen saturation (rSO2) by using near-infrared spectroscopy (NIRS) offers a noninvasive means of assessing cerebral and local tissue oxygen metabolism, offering continuous real-time data regarding the balance between demand and supply for oxygen. Additionally, rSO2 is a sensitive marker for global cerebral hypoperfusion (13). This study has demonstrated that monitoring of StO2 is a useful means of clinically evaluating sepsis/septic shock (14, 15).
In light of the above, this research employs TCD and StO2 monitoring for observation of the variations in the cerebral hemodynamic indices of the MCA and also variations in cerebral/peripheral StO2 for identification of elements that increase the risk of patients developing SAD.
Materials and Methods
Patients
This study selected patients suffering from septic shock accepted in the Department of Intensive Care Unit, Xiangya Hospital, Central South University between May 2018 and March 2019. This study represents a pilot observational cohort study; criteria for inclusion were set in alignment with the standard definition for sepsis 3.0. Every patient received treatment in accordance with the 2016 International Guidelines for the Treatment of Sepsis and Septic Shock (16), these being a mean arterial pressure (MAP) that reaches 65 mm Hg, with lactic acid normalization being the aim of the initial resuscitation procedures. All the patients in this study met the septic shock diagnostic criteria (17). Patients were excluded for any of the following criteria: aged below 18 years; past history of craniotomy or mental disorder; the presence of neurological disease (obvious intracranial lesions, e.g., intracranial infection, stroke, craniocerebral trauma, subarachnoid hemorrhage, cerebral hemorrhage); liver failure with suspicion of hepatic encephalopathy; pregnancy; abnormal outcomes of cervical vascular assessment with TCD or MRI/CT (e.g., carotid plaques or thrombosis with hemodynamically significant stenosis); and blood flow signals that could not be detected by using TCD within the time frame. Primary outcomes were either being discharged from ICU or developing delirium within 7 days of ICU admission; the secondary outcome was the mortality rate after 28 days.
Ethical Approval/Informed Written Consent
This study was undertaken in accordance with accepted medical ethical standards. Approval was granted by the Xiangya Hospital, Central South University Ethics Committee (ethics no.: 2018101082); immediate relatives of patients provided informed written consent.
Method for Evaluating Delirium
Delirium was diagnosed based on the CAM-ICU undertaken two times daily (10–11 a.m. and 4–5 p.m.) by qualified researchers in the ICU from the initial day of admission up to 7 days stay. Patients were allocated to the SAD cohort if they had two positive CAM-ICU screens carried out by the two investigators. Every septic shock patient was allocated the SAD or non-SAD group based on their suffering or not suffering delirium.
Data Collection
General data were harvested for all the selected septic shock patients and also specific data regarding 28-day mortality rate (%), time spent in ICU (days), time spent on mechanical ventilation (days), continuous renal replacement therapy (CRRT), sedation/analgesia medication administered, the sequential (sepsis-related) organ failure assessment (SOFA) score, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score.
Indicators of Circulating Hemodynamic Management
Indicators included urine output, total resuscitation fluid, norepinephrine dose level, central venous pressure (CVP), lactate clearance, and arterial/central venous blood gas indicators. The septic shock patient cohort for this research was given critical echocardiography within 60 min of being admitted measuring cardiac output (CO), left ventricular ejection fraction (LVEF), inferior vena cava diameter (IVCD), and inferior vena cava-collapse index (IVC-CI).
Organ Function/Biochemical Markers
Monitoring of blood samples began as soon as the patient was admitted to ICU, which included organ function assessment with routine blood indicators, regulation indicators, and kidney and liver function indicators. The biomarker indicators associated with sepsis measured were central nervous system-specific protein (S100β), neuron-specific enolase (NSE), and procalcitonin (PCT).
Transcranial Doppler Monitoring Index
Bilateral MCAs flow signals were obtained by using TCD ultrasound [Shenzhen Delikai (Nanshan District, Shenzhen, Guangdong Province) EMS-9A dual channel, 1.6 MHz TCD probe] once 6 h of initial resuscitation had been undertaken with the septic shock patient. The dynamic assessment for cerebral vascular autoregulation was completed, CBFi was calculated (CBFi = 10 × MAP/1.47 PI), and a record was made of PI (PI = VsMCA−VdMCA/VmMCA), systolic velocity of MCAs (VsMCA), mean blood flow velocity [mean velocity of MCAs (VmMCA)], and the diastolic velocity of MCAs (VdMCA). The THRR method was employed for assessing dynamic cerebral vascular autoregulation, i.e., MCA blood flow underwent stable lowering between 30 and 50% of baseline values employing confirmed carotid artery compression between 3 and 9 s; the blood flow velocity: baseline blood flow velocity ratio was also then measured. The THRR index above 1.09 is regarded as indicating dynamic cerebral vascular autoregulation function; if the levels fall below 1.09, this is regarded as indicating impairment to cerebral vascular autoregulation (18).
Tissue oxygen saturation levels were assessed by employing a noninvasive StO2 monitor (CAS Medical Systems, Inc.) for continuous monitoring of forehead rSO2 (large probe, 2.5 cm below skull detection depth) and thenar eminence StO2 (small probe, detection depth between 0.5 and 2 cm). The aforementioned values underwent continuous recording from several 60 mins once the septic shock patients had 6 h of initial resuscitation; uniform processing of the data to place in the later period. To find a level of rSO2 and StO2 data suited to comparison purposes, they were subjected to multiple threshold analysis (<50, <55, <60, and <65%) (19).
Data Analysis
The data were analyzed by using the Statistical Package for the Social Sciences (SPSS) version 24.0 (SPSS Incorporation, Armonk, New York, USA). Normality testing of the data was undertaken. Continuous variable data that matched or were close to normal distribution were noted as mean ± SD (χ ± s). An independent Student's t-test was employed for comparing the two samples. Nonconforming data in terms of the normal distribution were noted as median [interquartile range (IQR)] and the two samples were compared employing the Wilcoxon rank-sum test. The chi-squared (X2) test was employed for comparisons of categorical data. A continuous correction methodology was used when the theoretical frequency was below five; an exact probability methodology was used when the theoretical frequency was below one. Independent predictors for delirium were detected by using the multivariate logistic regression analysis with predictor variables being chosen from the risk elements appearing in tables as p < 0.05. The correlation between the normal distribution variables mentioned above was analyzed by using the Pearson analysis method. p < 0.05 was regarded as having statistical significance.
Results
A total of 121 septic shock patients were evaluated for this study; a total of 66 of them fulfilled one or more of the exclusion criteria and four patients did not undergo a full delirium evaluation. Finally having successfully completed the CAM-ICU evaluation, a cohort of 51 patients was selected for the research. About 39% experienced SAD (Figure 1).
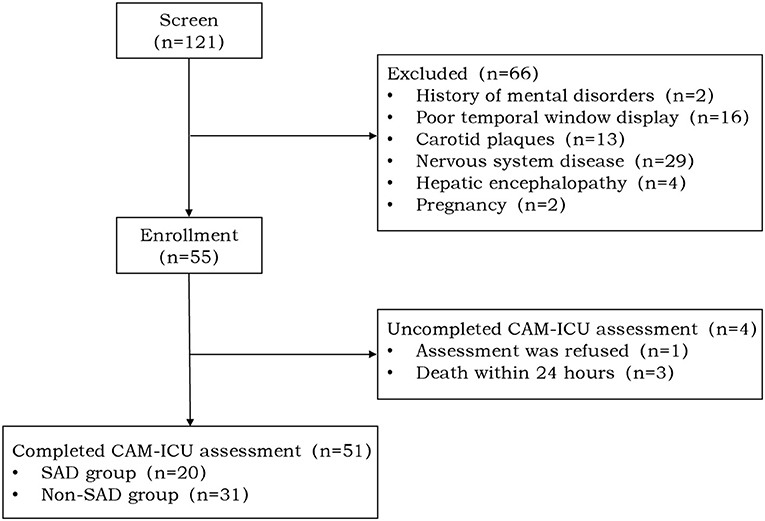
Figure 1. Study flow diagram. A total of 121 patients with septic shock were screened of which 66 patients met various exclusion criteria and four patients did not complete the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) assessment. A total of 51 patients were enrolled in our study of which 20 (39%) patients were positive for the CAM-ICU [sepsis-associated delirium (SAD) group] and 31 (61%) patients were negative for the CAM-ICU (non-SAD group).
General Clinical Data/Diagnosis
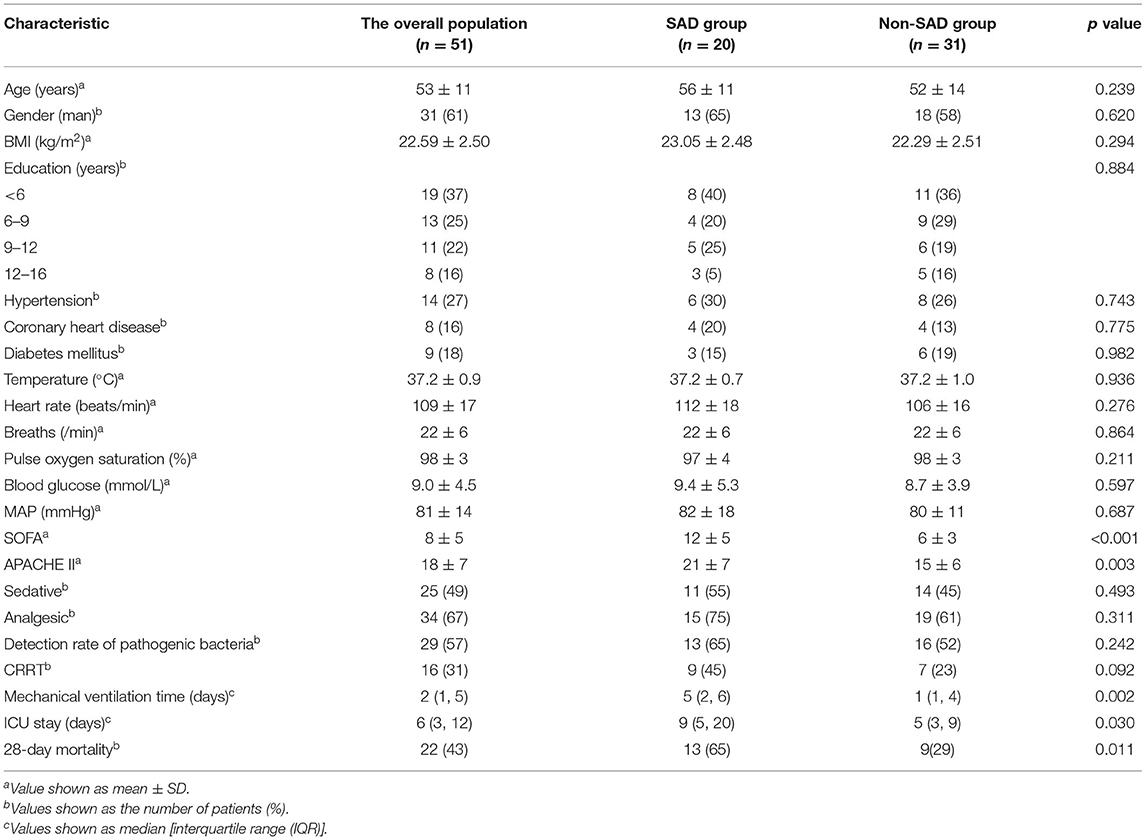
Of the 51 patients suffering septic shock, 31 (61%) patients were men; the average age for the whole group was 53 ± 11 years. The APACHE II scores (21 ± 7 vs. 15 ± 6, p = 0.003) and the SOFA scores (12 ± 5 vs. 6 ± 3, p = 0.001) were higher for the individuals in the SAD group (Table 1). The median time for the overall length of mechanical ventilation was 5 (1, 5) days; the median time for stay in ICU was 6 (3, 12) days. The SAD group required more time on mechanical ventilation compared to the non-SAD group [5 (2, 6) vs. 1 (1, 4) days, p = 0.015] and a longer stay in ICU [9 (5, 20) vs. 5 (3, 9) days, p = 0.042]. For the group as a whole, 28-day mortality was 43%; mortality was significantly greater among those in the SAD group (65 vs. 29%, p = 0.011) (Table 1).
Circulating Hemodynamics Indicators
The SAD group had a significantly lower oxygenation index (t6h) (230 ± 116 vs. 322 ± 121, p = 0.01), lower lactate clearance rate (t6h) (−0.36 ± 0.73 vs. −0.002 ± 0.35 mmol/L, p = 0.049), and higher blood lactate (Lac) (t6h) (5.2 ± 4.3 vs. 2.1 ± 1.3 mmol/L, p = 0.005). The critical echocardiography indicators showed no significant variations (p > 0.05). Additional File 1 (found in Supplementary Materials) gives greater detail.
Organ Function/Biochemical Markers
The SAD group exhibited a significantly raised white blood cell count compared to the non-SAD group (21.38 ± 14.16 vs. 12.55 ± 11.61, p = 0.019); they also had significantly higher levels of NSE [18.24 (13.29, 27.08) vs. 9.55 (6.13, 18.11), p = 0.031] and blood urea nitrogen (BUN) [9.6 (5.7, 16.9) vs. 5.8 (2.8, 11.2) mmol/L, p = 0.046]. Additional File 2 (found in Supplementary Materials) gives greater detail.
Transcranial Doppler-Detected Cerebral Hemodynamics in the MCA
The SAD group exhibited lower levels of VdMCA compared to the non-SAD group (49.7 ± 20.3 vs. 61.9 ± 17.3 cm/s, p = 0.026) with a higher PI (0.98 ± 0.19 vs. 0.84 ± 0.20, p = 0.019). Across both the groups, the dynamic cerebral vascular dysfunction (THRR index < 1.09) was 22%; the SAD group was at a significantly higher level (40 vs. 10%, p = 0.01) (Table 2).
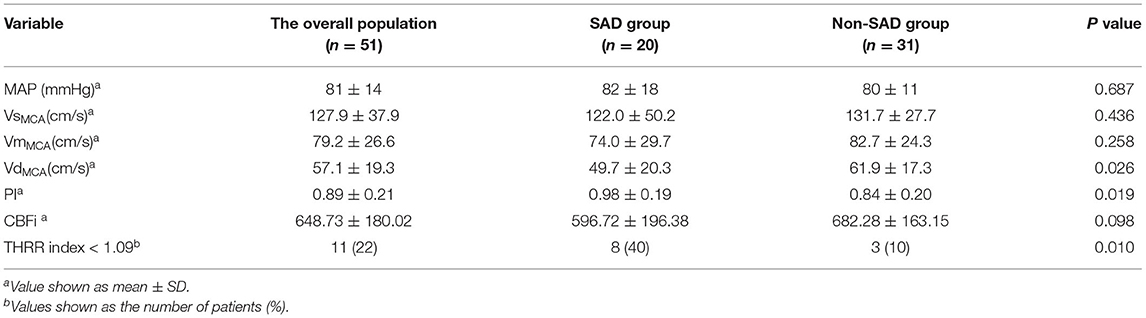
Table 2. Comparison of cerebral blood flow parameters of transcranial Doppler (TCD) between the sepsis-associated delirium (SAD) group and the non-SAD group.
StO2 Monitoring
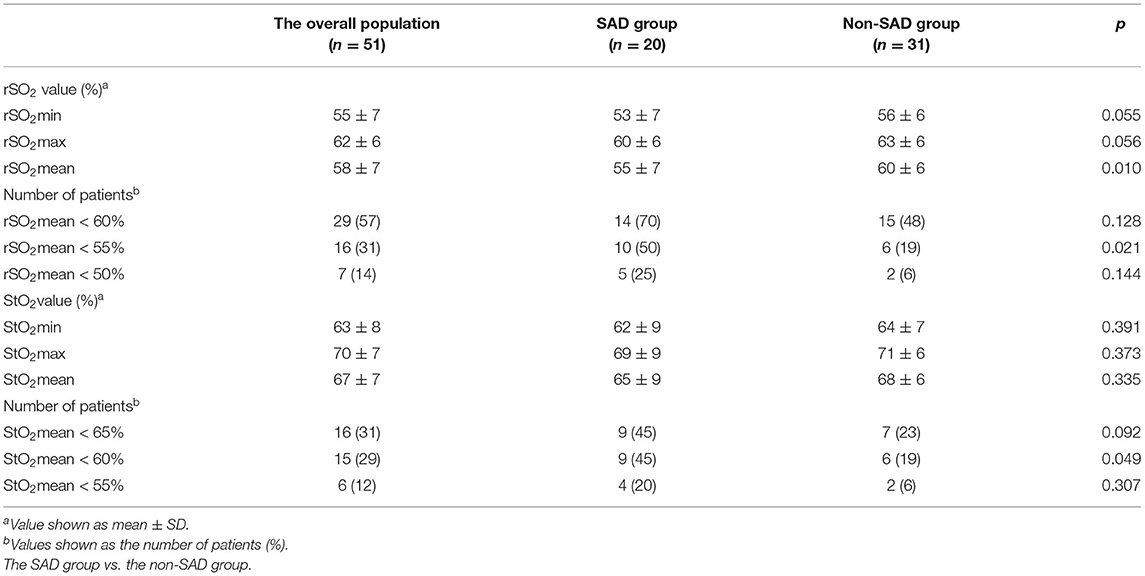
The SAD group had a lower mean rSO2 value compared to the non-SAD group (55 ± 7 vs. 60 ± 6, p = 0.01). Of the 51 patients, 16 patients had average rSO2 < 55% with significantly higher levels in the SAD group (50 vs. 19%, p = 0.021). Additionally, 15 patients had mean StO2 < 60%; again, a significantly greater number of the SAD group fall into this range (45 vs. 19%, p = 0.049) (Table 3).
Multivariate Analysis of SAD Risk Factors
In the multivariate regression analysis for risk factors focused on SAD brain parameters, the logistic regression analysis demonstrated that several independent risks were SAD predictors: these included mean rSO2 < 55% [odds ratio (OR) = 3.864, 95% CI: 1.026 to 14.550, p = 0.046] and the THRR index < 1.09 (OR = 5.77, 95% CI: 1.222–27.255, p = 0.027) (Table 4).

Table 4. The multivariate regression analysis of risk factors focuses on the brain parameters for SAD.
Discussion
Imbalances in cerebral oxygen metabolism and impairment of cerebral hemodynamics are key factors in whether or not patients develop SAD. This study confirms that there are high rates of SAD in septic shock patients and it has associations with poor outcomes. It has been shown that independent risk factors for the condition are mean cerebral oxygen saturation below 55% and cerebral vascular autoregulation dysfunction (THRR index < 1.09).
The first point to note in this study is that 39% of septic shock patients were suffering from SAD (20/51); this group had the higher APACHE II and the SOFA scores, needed more time on mechanical ventilators and longer stays in ICU, and their 28-day survival rate was significantly lower. This demonstrated that SAD has a close correlation with severe illness and poor outcomes. So, it is important to identify patients who were at risk for developing delirium. Mori et al. (20) showed a high incidence of ICU delirium associated with older age, use of sedatives and analgesics, emphasizing the need for relevant nursing care to prevent and identify early patients presenting these characteristics. Tse et al. (21) have suggested that delirium following cardiac surgery is influenced by age and several underlying diseases/conditions. Drugs and drug regimens for the prevention of delirium have not currently existed, so clinicians should concentrate on the non-medication interventions; these include keeping lines of communication open, identifying any psychological difficulties patients have early, keeping patients in touch with their family promoting healthy sleep, keeping noise to a minimum, and attempting to get patients active early (22).
Second, it was demonstrated that with septic shock patients, cerebral vascular autoregulation dysfunction is an independent risk factor. This would suggest that 6 h following initial resuscitation, patients experiencing SAD have a fall in cerebral vascular compliance and cerebral perfusion. These fluctuations could have an additional impact on cerebral vascular autoregulation. Cerebral oxygen metabolism and cerebral hemodynamic disorders have a key part in developing SAD, which should inform clinical practice in managing hemodynamics with a focus on cerebral perfusion. There is a close correlation between the central nervous system dysfunction seen in sepsis and cerebral hypoperfusion as a result of a fall in cerebral blood flow. Cerebral perfusion can be evaluated through monitoring of systolic, diastolic, and mean blood flow velocity in the anterior, middle, and posterior cerebral arteries by using TCD ultrasound (23). TCD also offers an indirect means of assessing cerebral circulation, which includes cerebral vascular autoregulation. With impairment in cerebral vascular autoregulation, fluctuations in perfusion pressure can cause cerebral congestion or ischemia resulting in nerve damage, which can have a negative impact on outcomes. In 1997, Smielewski et al. (24) discovered a correlation between a vanished transient cerebral congestion response rate (THRR) and poor outcomes for patients who had suffered serious craniocerebral injuries. While the value of brain imaging cannot be completely substituted for TCD and THRR index evaluation, they provide a means of continually monitoring cerebrovascular autoregulation in the early stages of treatment for sepsis/septic shock patients (25). Early identification of cerebrovascular autoregulation dysfunction will allow clinical interventions to take place earlier.
Third, the oxygen saturation of the brain has to be dynamically monitored in real time if cerebral perfusion is to be optimized by determining any oxygen supply/demand incompatibilities. This study demonstrated that the group with SAD had lower mean rSO2 values than those who did not; the multivariate regression illustrated that for septic shock patients, a mean rSO2 < 55% increases the likelihood of delirium. It appears that generally, patients with SAD have low levels of cerebral oxygen saturation. Discrepancies between oxygen consumption and cerebral oxygen supply are key to patients developing SAD and so septic shock patients must be continually monitored for cerebral oxygen saturation levels. As with heart rate, respiration, blood pressure, and pulse oxygen saturation, cerebral oxygen saturation level should become a standard monitoring indicator. The study has shown that intraoperative regional rSO2 levels below 40% represent a significant independent risk factor for cognitive impairment following cardiac surgery (26). According to De Tournay-Jette et al. (27), it has been reported that subjects with a rSO2 below 50% in the course of surgery had greater levels of cognitive dysfunction postoperatively 4 to 7 days after surgery (p = 0.04) and that patients who have experienced a reduction in relation to their baseline rSO2 of more than 30% were more likely to display cognitive impairment 1 month after their surgery (p = 0.03). When oxygen saturation is monitored, it offers essential data regarding brain tissue levels of oxygen that can be impacted by several diseases (28).
Finally, mean StO2 values varied from 60 to 74% following the initial resuscitation of septic shock patients. While there are normalized global parameters for hemodynamics, optimization of them may result in changes in regional perfusion and microcirculation. If these changes persist, a poor outcome is more likely. StO2 may function as an early alert to the fact that tissue hypoxia/low perfusion is beginning (29).
This study has some limitations. First, the ability of the technician can influence how effective TCD ultrasound can be. Second, evaluation was only undertaken of variations in cerebral hemodynamics and oxygen saturation in cerebral and peripheral tissue 6 h after initial resuscitation; we did not combine this with neurophysiological findings or neuroimaging. Third, patients with unattainable TCD flow spectrum or with a carotid plaque were excluded, which will affect its application to the entire septic shock patient population. As this study was observational in design and only involved a small patient cohort, randomized controlled trials (RCTs) on a larger scale may be necessary to verify our findings.
Conclusion
Patients with SAD have a close correlation with poor outcomes. Independent risk factors for SAD were mean cerebral oxygen saturation below 55% and cerebrovascular dysregulation (THRR < 1.09). This would justify the provision of RCT with adequate power for patient treatment in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This prospective observation study was undertaken at an academic hospital in an urban area of China. All patients involved gave written informed consent for their participation in this study. The Ethics Committee of Xiangya Hospital of Central South University approved all elements of this research (Ethics no: 2018101082). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QF undertook the statistical analysis, researched the scientific literature, and drafted the manuscript. MA, LH, and QP assisted in data collection. YA made contributions to the initial concept, design, and data interpretation. LZ also made contributions to the initial concept, design, data interpretation, editing, revision of the manuscript, and supervised this study. The manuscript has been reviewed and approved by all the authors.
Funding
This study benefitted from support in the form of grants from the Chinese National Natural Science Foundation, China (81873956).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.641104/full#supplementary-material
References
1. Tsuruta R, Oda Y. A clinical perspective of sepsis-associated delirium. J Intensive Care. (2016) 4:18. doi: 10.1186/s40560-016-0145-4
2. Souza-Dantas VC, Póvoa P, Bozza F, Soares M, Salluh J. Preventive strategies and potential therapeutic interventions for delirium in sepsis. Hosp Pract (Minneap). (2016) 44:190–202. doi: 10.1080/21548331.2016.1192453
3. Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what's the cause of all this confusion? Curr Opin Crit Care. (2012) 18:518–26. doi: 10.1097/MCC.0b013e328357effa
4. Burkhart CS, Siegemund M, Steiner LA. Cerebral perfusion in sepsis. Crit Care. (2010) 14:215. doi: 10.1186/cc8856
5. Gool W, Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. (2010) 375:773–5. doi: 10.1016/S0140-6736(09)61158-2
6. Ritter C, Tomasi CD, Dal-Pizzol F. Inflammation biomarkers and delirium in critically ill patients. Crit Care. (2014)18:R106. doi: 10.1186/cc13887
7. Papadopoulos MC, Davies DC, Moss RF., Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med. (2000) 28:3019–24. doi: 10.1097/00003246-200008000-00057
8. Schramm P, Klein KU, Falkenberg L., Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. (2012) 16:R181. doi: 10.1186/cc11665
9. Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Rüegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. (2008) 12:R63. doi: 10.1186/cc6891
10. Pierrakos C, Attou R, Decorte L, Kolyviras A, Malinverni S, Gottignies P, et al. Transcranial doppler to assess sepsis-associated encephalopathy in critically ill patients. BMC Anesthesiol. (2014) 14:45. doi: 10.1186/1471-2253-14-45
11. Stocchetti N, Taccone FS, Citerio G, Pepe PE, Le-Roux PD, Oddo M, et al. Neuroprotection in acute brain injury: an up-to-date review. Crit Care. (2015) 19:186. doi: 10.1186/s13054-015-0887-8
12. Pölönen P, Ruokonen E, Hippeläinen M, Pöyhönen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. (2000) 90:1052–9. doi: 10.1097/00000539-200005000-00010
13. Fischer WG. Recent advances in application of cerebral oximetry in adult cardiovascular surgery. Semin Cardiothorac Vasc Anesth. (2008) 12:60–9. doi: 10.1177/1089253208316443
14. Al-Tayar A, Abouelela A, Mohiuddeen K. Can the cerebral regional oxygen saturation be a perfusion parameter in shock? J Crit Care. (2017) 38:164–7. doi: 10.1016/j.jcrc.2016.11.006
15. Schenkman KA, Carlbom DJ, Bulger EM, Ciesielski WA, Fisk DM, Sheehan KL, et al. Muscle oxygenation as an indicator of shock severity in patients with suspected severe sepsis or septic shock. PLoS ONE. (2017) 12:e0182351. doi: 10.1371/journal.pone.0182351
16. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. (2017) 45:486–552. doi: 10.1097/CCM.0000000000002255
17. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
18. Cavill G, Simpson EJ, Mahajan RP. Factors affecting assessment of cerebral autoregulation using the transient hyperaemic response test. Br J Anaesth. (1998) 81:317–21. doi: 10.1093/bja/81.3.317
19. Kim J, Shim JK, Song JW, Kim EK. Postoperative cognitive dysfunction and the change of regional cerebral oxygen saturation in elderly patients undergoing spinal surgery. Anesth Analg. (2016) 123:436–44. doi: 10.1213/ANE.0000000000001352
20. Mori S, Takeda JR, Carrara FS, Cohrs CR, Zanei SS, Whitaker IY. Incidence and factors related to delirium in an intensive care unit. Rev Esc Enferm USP. (2016) 50:587–93. doi: 10.1590/S0080-623420160000500007
21. Tse L, Schwarz SK, Bowering JB, Moore RL, Barr AM. Incidence of and risk factors for delirium after cardiac surgery at a quaternary care center: a retrospective cohort study. J Cardiothorac Vasc Anesth. (2015) 29:1472–9. doi: 10.1053/j.jvca.2015.06.018
22. Whitehorne K, Gaudine A, Meadus R, Solberg S. Lived experience of the intensive care unit for patients who experienced delirium. Am J Crit Care. (2015) 24:474–9. doi: 10.4037/ajcc2015435
23. Pierrakos C, Antoine A, Velissaris D, Michaux I, Bulpa P, Evrard P, et al. Transcranial doppler assessment of cerebral perfusion in critically ill septic patients: a pilot study. Ann Intensive Care. (2013) 3:28. doi: 10.1186/2110-5820-3-28
24. Smielewski P, Czosnyka M, Kirkpatrick P, Pickard JD. Evaluation of the transient hyperemic response test in head-injured patients. J Neurosurg. (1997) 86:773–8. doi: 10.3171/jns.1997.86.5.0773
25. Sharma A, Aryal D. Problems with interpretation of transient hyperemic response ratio (THRR). Crit Ultrasound J. (2018) 10:11. doi: 10.1186/s13089-018-0095-2
26. Yao FS, Tseng CC, Ho CY, Levin SK, IIIner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. (2004) 18:552–8. doi: 10.1053/j.jvca.2004.07.007
27. Tournay-Jette ED, Dupuis G, Bherer L, Deschamps A, Cartier R, Denault A. The relationship between cerebral oxygen saturation changes and postoperative cognitive dysfunction in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. (2011) 25:95–104. doi: 10.1053/j.jvca.2010.03.019
28. Chen H, Wang XT. Noninvasive cerebral oxygen monitoring: clinical relevance and challenges. Chinese J Crit Care Intensive Care Med. (2018) 4:231–7. doi: 10.3877/j.issn.2096-1537.2018.03.005
Keywords: sepsis, septic shock, transcranial Doppler ultrasound, cerebral oxygen saturation, tissue oxygen saturation, delirium, sepsis-associated delirium
Citation: Feng Q, Ai M, Huang L, Peng Q, Ai Y and Zhang L (2021) Relationship Between Cerebral Hemodynamics, Tissue Oxygen Saturation, and Delirium in Patients With Septic Shock: A Pilot Observational Cohort Study. Front. Med. 8:641104. doi: 10.3389/fmed.2021.641104
Received: 14 December 2020; Accepted: 11 October 2021;
Published: 26 November 2021.
Edited by:
Bin Du, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Hamza Rayes, University of Cincinnati, United StatesSelma Tromp, St. Antonius Hospital, Netherlands
Copyright © 2021 Feng, Ai, Huang, Peng, Ai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Zhang, emxuNzA5NSYjeDAwMDQwO2NzdS5lZHUuY24=
 Qing Feng1,2
Qing Feng1,2 Lina Zhang
Lina Zhang