As a result of the continuous demographic changes, internal medicine is increasingly dealing with elderly patients suffering from chronic—and often multiple—diseases, and it is therefore the medical specialty in the closest relationship with clinical complexity (1).
Although in medicine a univocal definition of complexity has not been agreed so far (2), a complex system can be provisionally described as a network of individual factors from whose dynamic interaction new properties of the system itself emerge (3). Its most qualifying feature is represented by interconnectedness, and other essential features are non-linearity, unpredictability, adaptability, coevolution, and context sensitivity (4). It is now accepted that clinical complexity is something more and different from multimorbidity (5), and a recent consensus document (6) has identified a series of relevant determinants of complexity, either inherent (i.e., biological, such as aging, multimorbidity, frailty, disease severity, resilience) or contextual (i.e., non-biological, such as socioeconomic, cultural, environmental, behavioral factors) to the patient.
For many years, the best-known graphic conceptualization of complexity has been the Stacey diagram (7). It was conceived in the financial field, and it distinguishes an area of rationality, ruled by the usual mechanistic relationships of cause/effect, an area of chaos characterized by afinalistic turbulence and the interruption of normal connections, and a large intermediate area, that of complexity. It is within this latter, characterized by low levels of certainty and agreement, hence not evidence-based, that a large part of our complex patients fit in. This is the case, for example, of older patients with geriatric syndromes, who often suffer from frailty, cognitive impairment, higher fall risk and fall-related injuries, motor impairment, and poor health outcomes (e.g., hospital admission, mortality) (8, 9).
An additional evolution in the field of “knowledge management” is the so-called Cynefin framework (CF), which emerged from research conducted in the field of complex adaptive system theory developed by Snowden for decision-making in economics (10). As already happened for the Stacey diagram, it soon became clear that CF could prove useful for making sense of complexity even in the biomedical field (11–13). In fact, the CF provides a reference language to dynamically contextualize situations located in different domains (contexts) that require distinguished responses and characterized by different patterns of cause–effect relationship. More in depth, the CF was described as a “sense-making” tool, able to provide a reasoning pathway rather than a solution to a specific problem (10). Figure 1 shows how these four domains, which only partially overlap with the areas of the Stacey diagram, are embedded to each other. The simple domain is the only legitimate space of the best practice in which cause and effect are in a linear relation and easy to understand. Inside this area, obvious troubles and straightforward clinical problems can be solved through validated protocols, without the need for specialized medical knowledge and for further analysis or experimenting. In the complicated domain, only a good practice is possible by experts or focused physicians capable of grasping, by analytical tools and specialized knowledge, apparently hidden causalities and relationships which can, however, still be recognized. The complex domain is a fluid space of varying stabilities over time and space, and this is the case, for example, of patients with multiple chronic conditions, presenting with multifaceted clinical complaints, with complex social circumstances. The most correct attitude toward biological and non-biological factors is to abandon a reductionist mindset, and to reason in terms of systems and elements in continuous interaction (14), in order to probe the emergent causality and to get insight for a proper diagnosis. A cause–effect relationship can only be unveiled in retrospect, as no physician would immediately know a priori which solution is best, and this represents an opportunity for emergent practice. The chaotic domain is the space of medical urgencies or late-stage or novel diseases against which an expeditious and often innovative action is required to steer the system and stabilize the situation without targeting insight and decision making. An emblematic example is that of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, which has put a lot of pressure at multiple levels, including the healthcare and the economic and political systems, also representing an unprecedented challenge for the whole scientific community (15). In this case, the fact that very little knowledge was available at first strongly favored innovative action that was needed to face the initial phases of the pandemic, especially for understanding SARS-CoV-2 infection clinical characteristics and outcomes. Table 1 reports four different clinical scenarios according to the four Cynefin domains. Indeed, these four domains should not be considered as “waterproof” compartments. If it is true that different domains make use of different thinking strategies, in reality they communicate through the circular flow of knowledge that is gradually generated, and CF actually provides a context-driven and flexible approach to medical care (16).
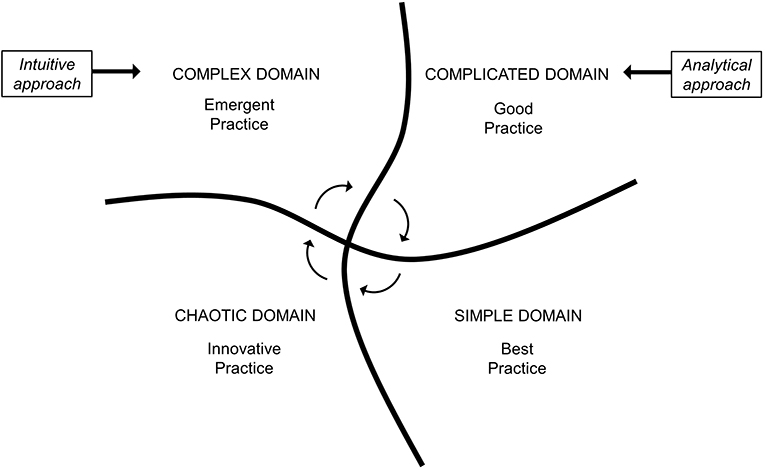
Figure 1. Cynefin identifies four different domains of medical knowledge. Each of them consents different standards of clinical practice and generates circular fluxes of knowledge. Different domains require different diagnostic approaches. Adapted and modified from Snowden & Boone (10).
Having said that, the most important novelty of CF consists in having kept the boundaries between “complex” and “complicated.” Even in the scientific literature, the colloquial use of the term “complex” instead of “complicated” is not uncommon, but while the latter points to the general difficulty of understanding, the former implies the presence of interconnections that constitute the functional characteristic of the systems (17). In clinical practice, not realizing of being in the wrong context can have dire consequences. Due to the tendency to get an immediate control of a certain disease or condition, medicine usually treats many problems as complicated, thus requiring many tests and analyses (13) and ultimately getting overdiagnosis and resource waste (18). However, this tendency would not allow patterns to emerge, causing a final detrimental effect. This is truly a critical issue: complex problems cannot be addressed as the complicated sum of solvable subproblems, and, for facing them, an analytical and reductionist approach would dissolute those interactions that constitute their essence.
CF, representing a suitable conceptual platform to talk about clinical complexity, can contribute to better-oriented diagnostic processes. Internal medicine has a lot to do with the diagnostic processes (19) which are particularly susceptible to errors (20) due to the great dissimilarity among patients (21), as well as due to their individual complexity (4). Clinical diagnosis is a multistage process that takes advantage of a series of strategies (21, 22). Although maximum flexibility of thought has long been recommended, these strategies can essentially be traced back to an intuitive (23) and analytical (24) approach. The intuitive approach is based on the so-called tacit knowledge arising from a wealth of experience enriched by personal perceptions and beliefs which are hard to transfer. The analytic approach is based on an explicit knowledge based on facts, rules, and procedures codified in paper or electronic form and already widely accepted (25). This analytical approach consciously uses a hypothetical–deductive logic and is time- and resource-consuming. The intuitive approach, on the contrary, unconsciously uses short mental circuits through which the patient's clinical picture as a whole is automatically compared, according to a pattern recognition process to a prototype of disease which is already present in the physician's experiential memory.
Patients with rare diseases, with atypical presentation, with an unusual course or who require instrumental tests with advanced technology for being diagnosed, place themselves in the CF in a complicated context and require an analytical approach based on evidence-based algorithms managed by doctors with specialized training (Figure 1). The same reductionist approach is not as effective, and above all efficient, for the diagnosis of those complex patients who currently make up the vast majority in outpatient clinics and internal medicine wards. Older, fragile patients with multimorbidity and polytherapy, perhaps accompanied by abnormal behaviors and social distress, even if characterized by unusual disease course or atypical presentation (26), must be studied through an intuitive, multidimensional and holistic approach, which constitutes an essential qualifier of internal medicine and is crucial for the embracement of clinical complexity (27). This approach is faster and tends to consider contextual aspects and to avoid, as far as possible, expensive and invasive tests. In other terms, this represents an emergent practice, which is an action-oriented approach (28) that generates a new, patient-centered, opportunity. Indeed, the recognition of complex patients remains the most difficult challenge in clinical practice. Therefore, rather than with the use of a clinical guideline, patients can be categorized within the Cynefin framework by applying a score which quantifies clinical complexity. However, a universally accepted tool for assessing clinical complexity is yet to be developed (4).
Concerning the potential role of machine learning in aiding clinical decisions, this has been widely discussed (29, 30). In brief, machine learning is an algorithm that is able to learn by using a huge amount of data which certainly exceed the capacity of a human mind (29). However, if from a theoretical point of view this could potentially allow a more precise diagnosis, convincing evidence about its feasibility and usefulness is still lacking.
If CF does not offer concrete scientific or clinical solutions, it certainly represents a useful conceptual framework to capture the critical consequences of clinical complexity (12). In the day-by-day medical activity, it must be kept in mind that complex and complicated domains are different contexts, characterized by different clinical practice standards and that require different diagnostic reasoning approaches.
Author Contributions
All authors participated in the drafting of the manuscript or critical revision of the manuscript for important intellectual content and provided approval of the final submitted version.
Funding
This research was part of a project for the study of clinical complexity (SMAC study) funded by San Matteo Hospital Foundation - Italian Ministry of Health (Progetto di Ricerca Corrente 2017 - GC). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CF, cynefin framework.
References
1. Weinberger SE. Challenges for internal medicine as the American College of Physicians celebrates its 100th anniversary. Ann Intern Med. (2015) 162:585–6. doi: 10.7326/M14-2905
2. Turner BJ, Cuttler L. The complexity of measuring clinical complexity. Ann Intern Med. (2011) 155:851–2. doi: 10.7326/0003-4819-155-12-201112200-00009
3. Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ. (2001) 323:625–8. doi: 10.1136/bmj.323.7313.625
4. Corazza GR, Formagnana P, Lenti MV. Bringing complexity into clinical practice: an internistic approach. Eur J Intern Med. (2019) 61:9–14. doi: 10.1016/j.ejim.2018.11.009
5. Hong CS, Atlas SJ, Ashburner JM, Chang Y, He W, Ferris TG, et al. Evaluating a model to predict primary care physician-defined complexity in a large academic primary care practice-based research network. J Gen Intern Med. (2015) 30:1741–7. doi: 10.1007/s11606-015-3357-8
6. Corazza GR, Klersy C, Formagnana P, Lenti MV, Padula D, Consensus Panel. A consensus for the development of a vector model to assess clinical complexity. Intern Emerg Med. (2017) 12:1313–8. doi: 10.1007/s11739-017-1709-6
7. Stacey RD. Strategic Management and Organizational Dynamics: The Challenge of Complexity. London: Financial Times (1999).
8. Vetrano DL, Calderón-Larrañaga A, Marengoni A, Onder G, Bauer JM, Cesari M, et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci. (2018) 73:1350–6. doi: 10.1093/gerona/glx178
9. Xu X, Mishra GD, Jones M. Evidence on multimorbidity from definition to intervention: an overview of systematic reviews. Ageing Res Rev. (2017) 37:53–68. doi: 10.1016/j.arr.2017.05.003
11. Sturmberg JP, Martin CM. Knowing–in medicine. J Eval Clin Pract. (2008) 14:767–70. doi: 10.1111/j.1365-2753.2008.01011.x
12. Kempermann G. Cynefin as reference framework to facilitate insight and decision-making in complex contexts of biomedical research. Front Neurosci. (2017) 11:634. doi: 10.3389/fnins.2017.00634
13. Gray B. The Cynefin framework: applying an understanding of complexity to medicine. J Prim Health Care. (2017) 9:258–61. doi: 10.1071/HC17002
14. Sturmberg JP. Complexity mindsets at work. J Eval Clin Pract. (2016) 22:101–2. doi: 10.1111/jep.12488
15. Lambert H, Gupte J, Fletcher H, Hammond L, Lowe N, Pelling M, et al. COVID-19 as a global challenge: towards an inclusive and sustainable future. Lancet Planet Health. (2020) 4:e312–4. doi: 10.1016/S2542-5196(20)30168-6
16. Snowden D. Complex act of knowing: paradox and descriptive self-awareness. J Knowledge Manage. (2002) 6:1–14. doi: 10.1108/13673270210424639
17. Sturmberg JP, Martin CM, Katerndahl DA. It is complicated! - misunderstanding the complexities of ‘complex'. J Eval Clin Pract. (2017) 23:426–9. doi: 10.1111/jep.12579
20. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. (2013) 24:525–9. doi: 10.1016/j.ejim.2013.03.006
21. Croskerry P. From mindless to mindful practice–cognitive bias and clinical decision making. N Engl J Med. (2013) 368:2445–8. doi: 10.1056/NEJMp1303712
22. Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. (2006) 355:2217–25. doi: 10.1056/NEJMra054782
23. Brush JE Jr, Sherbino J, Norman GR. How expert clinicians intuitively recognize a medical diagnosis. Am J Med. (2017) 130:629–34. doi: 10.1016/j.amjmed.2017.01.045
24. Round A. Introduction to clinical reasoning. J Eval Clin Pract. (2001) 7:109–17. doi: 10.1046/j.1365-2753.2001.00252.x
25. Wyatt JC. Management of explicit and tacit knowledge. J R Soc Med. (2001) 94:6–9. doi: 10.1177/014107680109400102
26. Jarrett PG, Rockwood K, Carver D, Stolee P, Cosway S. Illness presentation in elderly patients. Arch Intern Med. (1995) 155:1060–4. doi: 10.1001/archinte.1995.00430100086010
27. Wilson T, Holt T, Greenhalgh T. Complexity science: complexity and clinical care. BMJ. (2001) 323:685–8. doi: 10.1136/bmj.323.7314.685
28. Van Beurden EK, Kia AM, Zask A, Dietrich U, Rose L. Making sense in a complex landscape: how the cynefin framework from complex adaptive systems theory can inform health promotion practice. Health Promot Int. (2013) 28:73–83. doi: 10.1093/heapro/dar089
29. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. (2019) 380:1347–58. doi: 10.1056/NEJMra1814259
Keywords: aging, clinical complexity, conceptual framework, decision-making, multimorbidity
Citation: Corazza GR and Lenti MV (2021) Diagnostic Reasoning in Internal Medicine. Cynefin Framework Makes Sense of Clinical Complexity. Front. Med. 8:641093. doi: 10.3389/fmed.2021.641093
Received: 14 December 2020; Accepted: 01 March 2021;
Published: 22 April 2021.
Edited by:
Tzvi Dwolatzky, Technion Israel Institute of Technology, IsraelReviewed by:
Karolina Maria Piotrowicz, Jagiellonian University Medical College, PolandShusaku Tsumoto, Shimane University, Japan
Copyright © 2021 Corazza and Lenti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gino Roberto Corazza, Z3IuY29yYXp6YUBzbWF0dGVvLnB2Lml0
 Gino Roberto Corazza
Gino Roberto Corazza Marco Vincenzo Lenti
Marco Vincenzo Lenti