- 1Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Department of Respiratory Medicine, Ruijin North Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Pulmonary and Critical Care Medicine, Changhai Hospital of Shanghai, Second Military Medical University, Shanghai, China
- 4Department of Respiratory Medicine, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 5Department of Respiratory Medicine, Tongren Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 6Department of Respiratory Medicine, Central Hospital of Minhang District, Shanghai, Fudan University, Shanghai, China
- 7Department of Respiratory Medicine, Central Hospital of Jiading District, Shanghai, Fudan University, Shanghai, China
- 8Department of Respiratory Medicine, Shanghai Putuo District People's Hospital, Shanghai, China
Background: Patients with chronic obstructive pulmonary disease (COPD) are more susceptible to Aspergillus colonization or infection. Several studies have demonstrated that invasive pulmonary Aspergillosis (IPA) and Aspergillus hypersensitivity (AH) have a detrimental effect on COPD. However, it remains to be clarified whether Aspergillus colonization is associated with acute exacerbation of COPD (AECOPD). This study aimed to explore the impact of Aspergillus colonization in the lower respiratory tract on AECOPD.
Method: Patients with Aspergillus colonization were identified from a retrospective cohort of hospitalized AECOPD from 2011 to 2016 in eight centers in Shanghai, China. The demographic information, conditions of the stable stage, clinical characteristics during hospitalization, and 1-year follow-up information after discharge were collected and compared to participants without fungi colonization.
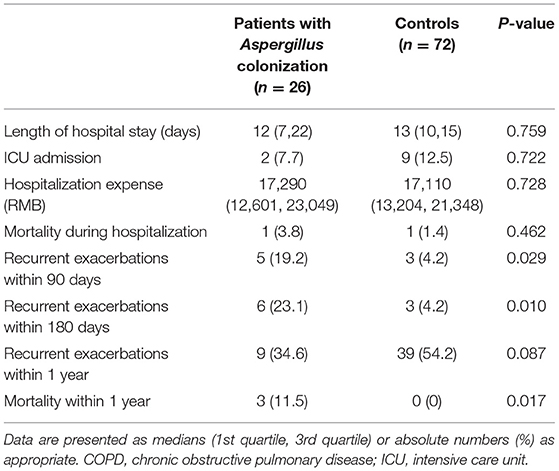
Result: Twenty-six hospitalized AECOPD patients with Aspergillus colonization and 72 controls were included in the final analysis after excluding patients with other fungi isolation and matching. The rates of recurrence of acute exacerbation within 90 days and 180 days after discharge in the patients with Aspergillus colonization were both significantly higher than that in the fungi negative patients (90 days: 19.2 vs. 4.2%, p = 0.029; 180 days: 23.1 vs. 4.2%, p = 0.010), and the all-cause mortality within 1 year was also higher (11.5 vs. 0.0%, p = 0.017). Multivariate logistic regression analysis showed that Aspergillus colonization was an independent risk factor for the recurrence of acute exacerbation within 90 days and 180 days (90 days: OR = 8.661, 95% CI: 1.496-50.159, p = 0.016; 180 days: OR =10.723, 95% CI: 1.936-59.394, p = 0.007).
Conclusion: Aspergillus colonization may predict poor prognosis of AECOPD while leading to an increased risk of recurrent AECOPD in a short period.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory disease characterized by progressive and irreversible airflow limitation. Due to the aging population, the expanding epidemic of cigarette smoking, and other reasons, COPD's prevalence and mortality are among the highest worldwide while causing significant economic and social burden (1). Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) refers to the deterioration of respiratory symptoms in a short period and requiring additional treatment. Exacerbation is the primary factor of poor outcomes of this disease, including loss of working capacity, a decline in lung function, hospitalization, and sometimes even death (2–4). AECOPD is mainly induced by pathogenic microorganisms and environmental factors. Bacteria and viruses are considered as the usual triggers for acute exacerbations rather than fungi.
Aspergillus is an airborne fungal pathogen that spreads all over the world. The conidia can reach the pulmonary parenchyma through the airway as its diameter is only 2-3 μm. Meanwhile, chronic airway inflammation in COPD results in damage to the epithelium of the lower respiratory tract (LRT) and further weakens the defense of the airways. As a result, COPD patients are susceptible to Aspergillus colonization or infection (5). Aspergillus usually colonize the airways without generating infection. It is reported that the prevalence of Aspergillus colonization in COPD patients is from 8.33 (6) to 16.6% (7). According to the host immune response, Aspergillus can cause invasive pulmonary aspergillosis (IPA), allergic bronchopulmonary aspergillosis (ABPA), or other clinical syndromes (8). Growing evidence support that COPD is the risk factor of IPA, which carries an abysmal prognosis (5, 9). A study between COPD patients and healthy volunteers demonstrated that COPD might predispose Aspergillus hypersensitivity (AH) and ABPA (10). It has been reported that allergy and AH are associated with severe symptoms and reduction of lung function in COPD patients (11, 12).
It is necessary to find out the significance of Aspergillus colonization in COPD patients for clinical decision making. But the effect of Aspergillus colonization in COPD remains to be studied and clarified, especially on the prognosis. Therefore, we carried out this study to investigate the impact of LRT Aspergillus colonization on the prognosis of patients hospitalized for AECOPD, which might help with the management strategies.
Patients and Methods
Study Design
This multicenter retrospective cohort study was initiated by the Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Fudan University in Shanghai, China. We collected patients hospitalized with AECOPD from January 2011 to December 2016 in eight hospitals in Shanghai. From the cohort, those with LRT Aspergillus colonization were identified (Aspergillus positive group) and those without evidence of Aspergillus colonization were classified as controls (Aspergillus negative group). The LRT Aspergillus colonization was defined as culture positive for Aspergillus from samples including sputum, endotracheal suction, bronchoscopic flushing, and/or lavage fluid (BALF), and had no clinical presentation of fungal infection during hospitalization. Propensity score matching was applied if there was a significant difference in baseline characteristics between the two groups. The ethics committee of Zhongshan Hospital of Fudan University approved this study (Approval notice number: B2015-119R).
Culture of Aspergillus
The microbiologic findings of LRT samples were carried out in the following way. After obtaining the sample from the patient, it was quickly sent to the microbiology laboratory at room temperature, and processed on the same day. About 10 μl samples were pipetted directly from the submitted specimen and inoculated onto sabouraud dextrose agar (SDA) containing chloramphenicol and incubated at 28°C~35°C for 7 days. The eight units that performed the Aspergillus culture were all under the quality control of Shanghai Center For Clinical Laboratory, and the examiners had all received professional training.
Inclusion Criteria
Those with all the following criteria were enrolled: (1) Age over 18 years old. (2) The hospitalized patient met the diagnostic criteria for COPD and acute exacerbations in the 2017 GOLD report. (3) The patients' LRT samples had been detected for Aspergillus.
Exclusion Criteria
Those who met any of the following items were excluded: (1) Chest CT findings suggested chronic pulmonary aspergillosis (CPA), IPA or ABPA. (2) A positive result of serum or BALF galactomannan determination. (3) Treatment of anti-fungal therapy. (4) A positive culture of other fungi except for Aspergillus from LRT specimens. (5) Sputum smears showed spores indicating Candida. (6) Patients with mental diseases that made themselves unable to cooperate in data collection. (7) Combined with other underlying lung diseases, such as lung cancer, bronchiectasis, interstitial pulmonary diseases, lung abscess, and tuberculosis. (8) Patients with immunocompromised diseases.
Data Collection
The patients' clinical data who met the inclusion criteria through their hospitals' electronic and paper medical records were collected and put into the Epidata system (The EpiData Association, Odense, Denmark). An independent investigator would check the collected data. If inconsistent data were found, the examiner would correct it after checking the original case history.
The information to be collected was as follows: basic patient characteristics (sex, age, smoking history, etc.), CAT and mMRC scores, treatment during the stable stage, complications, lung function, microbiologic findings of LRT samples, chest CT findings, laboratory test results, length of hospitalization or ICU stay, hospitalization expense, mortality during hospitalization, recurrent exacerbations within 90 days, 180 days, 1 year, and the mortality within 1 year. As for smoking history, we collected the information on whether the patient had smoked, how much he/she smoked, and how long he/she had smoked. Smoking history was defined as over 10 pack-years, regardless of current smoking status.
Statistical Analysis
Statistical analysis was performed on Stata 14 (StataCorp., College Station, TX).
Categorical variables were presented as absolute numbers and rates or percentages. Chi-square test or Fisher's exact test was used for comparison. The Shapiro-Wilk test would evaluate the normality of continuous variables. The continuous variables with normal distribution were presented as means and standard deviations, and the Student t-test was performed in comparison. The non-normally distributed continuous variables were presented as medians and quartiles, and the difference between the two groups was compared using Mann-Whitney U-test. Logistic regression was applied to explore possible risk factors for Aspergillus colonization and the recurrence of exacerbations. Variables with p < 0.10 in the univariate analysis would enter the multivariate analysis. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
A total of 1,116 patients hospitalized for AECOPD in the retrospective cohort were screened, and 626 patients met the inclusion and exclusion criteria. Thirty-one patients with Aspergillus and no other fungi identified in their LRT samples and 459 with no LRT fungi were kept in the remaining cases. As the baseline characteristics were significantly different in these two groups (shown in Table 1), we adopted propensity score matching at a ratio of 1:3 (caliper = 0.05). Finally, 26 cases were included in the positive group and 72 cases in the negative group for the final analysis.
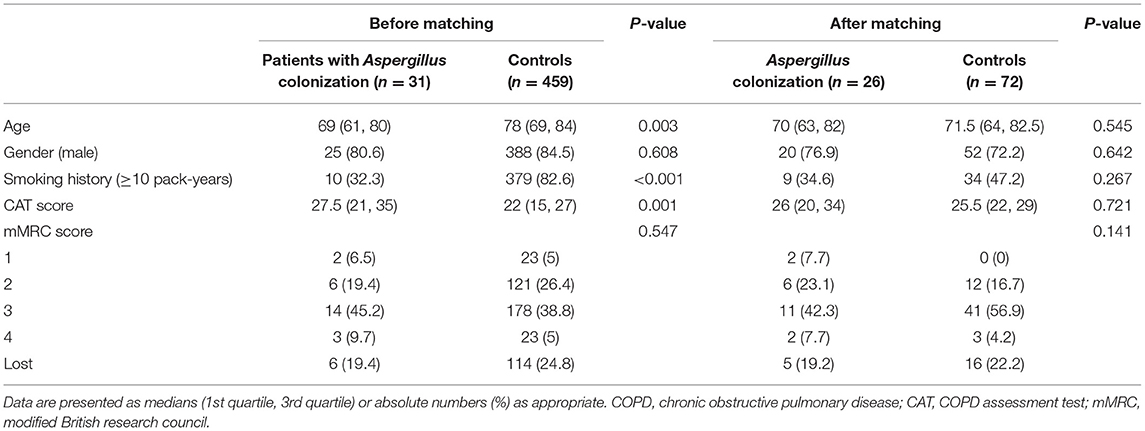
Table 1. Baseline characteristics of hospitalized COPD patients with and without lower respiratory Aspergillus colonization before and after propensity score matching.
Baseline Characteristics Before and After Matching
Table 1 showed the baseline data of the Aspergillus positive group and Aspergillus negative group, including the age, gender, smoking history, and the CAT scores and mMRC scores before exacerbation, which were used to assess the patients' symptom burden during the stable stage.
Before applying propensity score matching, the age in the positive group was considerably lower than that of the negative group (69 vs. 78, p = 0.003), and the percentage of patients with smoking history was also dramatically lower (32 vs. 83%, p < 0.001). The CAT scores of the positive group were notably higher compared with the negative group (27.5 vs. 22, p = 0.001), which presented that COPD patients with Aspergillus colonization had a higher symptom burden.
The baseline characteristics of the two groups were controlled at a similar level after matching of age, gender, smoking history, and CAT score and mMRC score of stable stage, which was more conducive for us to assess the clinical characteristics and prognosis of AECOPD patients based on Aspergillus colonization.
Corticosteroids Use Was Related to Aspergillus Colonization
Inhaled corticosteroids use in patients with positive Aspergillus culture during the stable phase was distinctly higher than that of negative patients (65.4 vs. 27.8%, p = 0.001, Table 2). Univariate logistic regression analysis indicated that inhaled corticosteroids use was a risk factor for Aspergillus colonization (OR = 4.911, 95% CI: 1.883-12.807, p = 0.001). In terms of other drugs and treatment methods, we found no statistical differences. There was no remarkable difference in the prevalence of comorbidities, either.
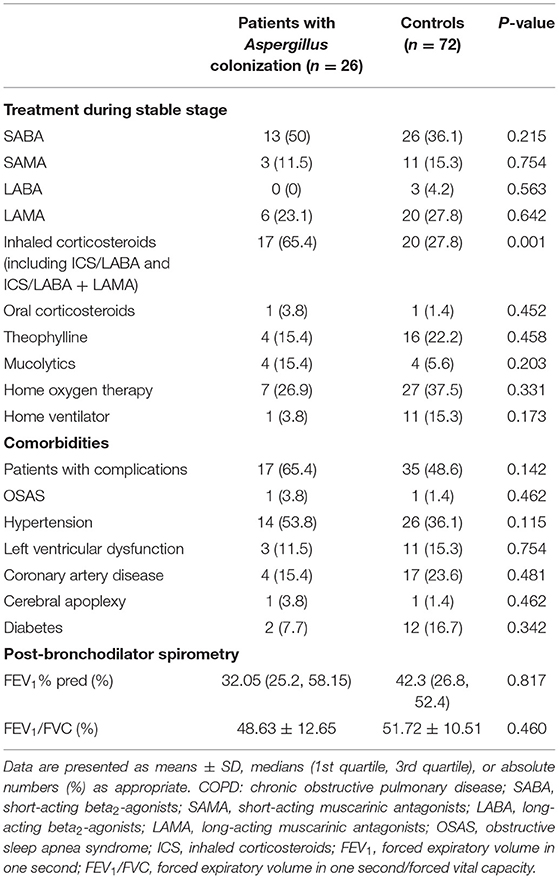
Table 2. Treatment during the stable stage, comorbidities, and airflow limitation of hospitalized COPD patients with or without Aspergillus colonization.
The airflow limitation was assessed by forced expiratory volume in one second (FEV1)% predicted and forced expiratory volume in one second/forced vital capacity (FEV1/FVC) post-bronchodilators recorded in stable stage. FEV1% predicted and FEV1/FVC values of the two groups after matching showed no difference. The FEV1/FVC values were <70%, which met the diagnostic criteria of COPD and both groups had severe airflow obstruction.
Table 3 summarized the examination results of AECOPD patients on admission. Laboratory test results showed a difference between patients with LRT Aspergillus colonization and controls in the number and percentage of eosinophils (Eosinophil count: 33.21 vs. 70.4, p = 0.030; Eosinophils%: 0.3 vs. 0.7, p = 0.019). Accordingly, there was a trend of an increase in neutrophil counts and percentage in the Aspergillus colonization group on admission, which could be explained by the bacterial infection which served as the trigger of exacerbations. No significant difference was identified in other clinical characteristics. No laboratory results on admission were found to be a risk factor or protective factor of LRT Aspergillus colonization.
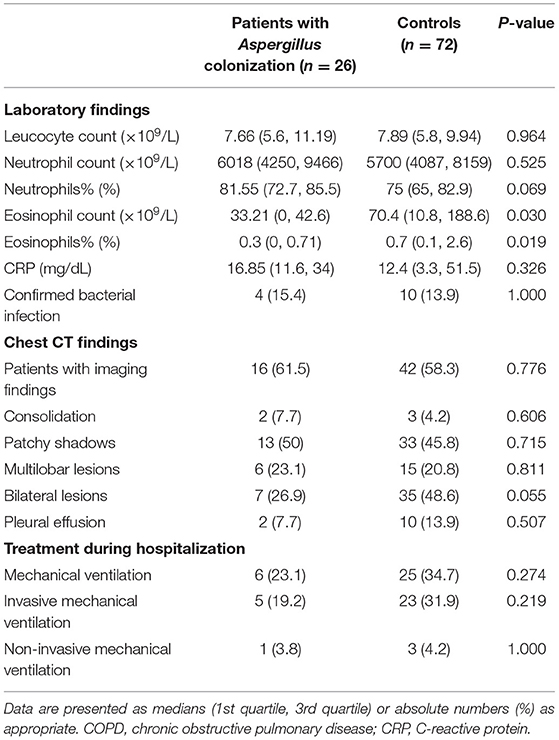
Table 3. Clinical characteristics and treatments of COPD patients on admission with or without Aspergillus colonization.
Patients With Aspergillus Colonization Were at a High Risk of Relapse of AECOPD
Table 4 presented the clinical outcomes of the patients during hospitalization and within 1 year after discharge. We did not find notable differences in length of hospitalization, length of ICU stays, hospitalization costs, and in-hospital mortality. Compared with those Aspergillus cultured-negative, the rate of recurrent exacerbation within 90 days of the Aspergillus positive group was significantly higher (19.2 vs. 4.2%, p = 0.029). The recurrence rate within 180 days was obviously higher, too (23.1 vs. 4.2%, p = 0.010). There was no considerable difference in the rate of recurrence of AECOPD within 1 year. The all-cause 1-year mortality was higher in Aspergillus positive group than the negative group (11.5 vs. 0.0%, p = 0.017).
Tables 5, 6 manifested the results of logistic regression analysis. Univariate analysis proved that Aspergillus colonization was a risk factor for relapse of acute exacerbation within 90 days and 180 days (90 days: OR = 5.476, 95% CI: 1.207-24.849, p = 0.028; 180 days: OR = 6.9, 95% CI: 1.582-30.087, p = 0.010). Besides, pleural effusion in chest CT findings was also a risk factor of recurrent exacerbations in COPD patients (90 days: OR = 5.4, 95% CI: 1.103-26.438, p = 0.037; 180 days: OR = 4.444, 95% CI: 0.945-20.893, p = 0.059). Multivariate analysis agreed with the results of univariate analysis. Variables associated with the relapse of acute exacerbation were Aspergillus colonization (90 days: OR = 8.661, 95% CI: 1.496-50.159, p = 0.016; 180 days: OR =10.723, 95% CI: 1.936-59.394, p = 0.007) and pleural effusion (90 days: OR = 9.793, 95% CI: 1.470-65.237, p = 0.018; 180 days: OR = 8.791, 95% CI: 1.317-58.689, p = 0.025).
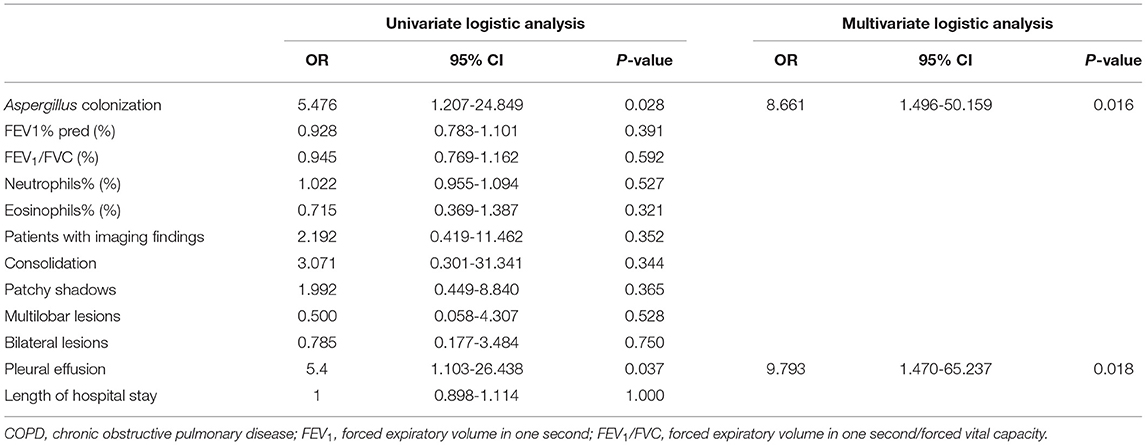
Table 5. Univariate and multivariate logistic analysis for relapse of acute exacerbation in COPD patients within 90 days after discharge.
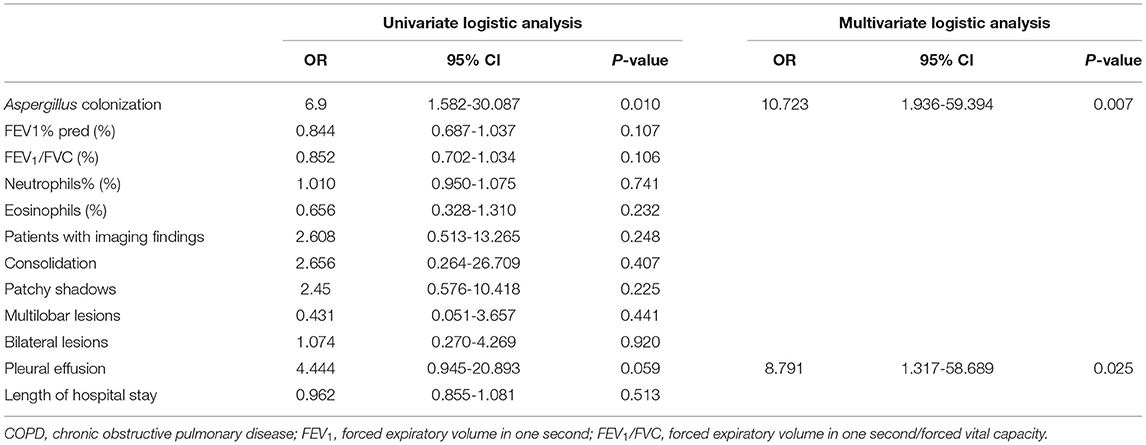
Table 6. Univariate and multivariate logistic analysis for relapse of acute exacerbation in COPD patients within 180 days after discharge.
Discussion
Aspergillus Colonization Predict Poor Outcomes of AECOPD
We investigated a large sample of more than 1,000 patients with severe exacerbation of COPD from eight centers and collected abundant information on the stable stage, acute exacerbation stage, clinical outcomes, and prognosis. While other researchers focused on the risk factors and clinical manifestations of COPD patients colonized or infected by Aspergillus, we were the first to concentrate on the prognosis of COPD patients after hospitalization discharge. We established three follow-up periods of 90 days, 180 days, and 1 year to observe the short-term and long-term prognosis, which was a new attempt in similar researches. The results of this study suggested that Aspergillus colonization is associated with poor clinical outcomes of hospitalized AECOPD, including higher acute exacerbation rates within 90 days, 180 days, and higher mortality within 1 year after discharge.
We speculated that the worse prognosis is attributed to AH. Firstly, COPD patients are more likely to have an allergic reaction to Aspergillus than those without the disease. A prospective multicenter study demonstrated that COPD had a high frequency of sensitization in various allergens, including Aspergillus (13). Agarwal et al. found AH in 8.5% (17 in 200) COPD patients, while none in healthy volunteers (10). Secondly, COPD patients with Aspergillus were at higher levels of specific-IgE (14), related to AH. A study of Aspergillus sensitization and asthma's relationships indicated that Aspergillus's long-term colonization is related to sensitization (15). For COPD patients, it is also possible that persistent Aspergillus colonization has a stimulating effect on AH. Thirdly, AH can lead to decreased lung function and severe respiratory symptoms (11, 12). Tiew et al. found that fungal sensitization was associated with the frequency of exacerbations (13). Moreover, another study suggested that the cluster characterized by Aspergillus, Curvularia, and Penicillium had increased exacerbations and higher mortality than another cluster with Saccharomyces (14). These findings are consistent with ours, indicating that it is long-term colonization of Aspergillus in the airways of COPD patients that cause sensitization and make patients more susceptible to acute exacerbations under specific stimulation.
Furthermore, researchers found that sensitization to recombinant antigens of Aspergillus increases the risk for bronchiectasis in COPD patients (16). And a study published recently identified that the number of fungal interactions is increased in COPD patients with very frequent exacerbations (exacerbate more than three times per year) (14). To some extent, these findings support our conclusion that Aspergillus causes a worse prognosis in COPD patients.
However, the molecular mechanism of the effects of Aspergillus on acute exacerbation remains unclear. The reason for no statistical difference in 1-year mortality is also waiting to explore. Would the intensity of AH fluctuate over time and result in the difference between short-term and long-term outcomes? Is it possible to determine which COPD patients are susceptible to AH based on specific characteristics? Is it able to help with treatment strategies by measuring total-IgE or specific-IgE? These are the keys to future study.
Also, we found that the eosinophil count and eosinophils% on admission in Aspergillus positive group were lower than those in the control group, which might be explained by the bacterial infection which served as the trigger of exacerbations.
The Risk Factors of Aspergillus Colonization
We observed that Aspergillus was more likely to colonize in patients treated with inhaled corticosteroids during the stable stage. This observation is consistent with the study of Tong et al. (6), who found that the proportion of patients who inhale high doses of corticosteroids during the stable stage in the Aspergillus colonization group was higher than that in the control group. Another study also indicated that patients with Aspergillus cultured positive had a higher inhaled corticosteroid dose during the stable stage compared with those cultured negative (12). The use of corticosteroids reduces the anti-fungal capacity of macrophages in the airways, inhibits the production of proinflammatory cytokine and chemokine (17, 18). In general, corticosteroids inhibit the ability of immune systems to eliminate Aspergillus conidia and promote the growth of Aspergillus (19). So, the use of inhaled corticosteroids is responsible for Aspergillus colonization.
The study of Tong et al. suggested that smoking history should be a risk factor for Aspergillus colonization (6). Chronic tobacco smoke can damage the function of cilia, reduce the removal rate of Aspergillus in the airways, and adjust the microbial environment to be suitable for Aspergillus's growth. In our baseline data before matching, the lower percentage of smokers in the Aspergillus positive group should be due to selection bias.
The genetic factor is also a possible risk factor for Aspergillus colonization. It is showed that genetic mutations could affect protein levels in the extracellular matrix and promote the adhesion of Aspergillus conidia. Moreover, affected by genetic factors, some patients might show a stronger inflammatory response to the conidia of Aspergillus (20).
Strengths and Limitations
This study was the first retrospective multicenter study on the impact of LRT Aspergillus colonization on the early relapse long-term prognosis of AECOPD. Another strength is propensity score matching. By matching control age, sex, smoking history, and severity of symptoms before acute exacerbation, this study minimized the individual differences in patients and obtained the data of impacts of Aspergillus colonization on the prognosis.
However, our study had several limitations. Firstly, this was a retrospective study. We could not confirm whether Aspergillus colonization was acquired during the stable stage or after the onset of the exacerbation. We only proved the relationship between the LRT Aspergillus colonization and outcome variables, like other available studies on this topic (6). Secondly, the positivity rate of Aspergillus culture was quite low (6%, 31/490), as we excluded the patients who received antifungal therapy, who should have positive results of Aspergillus culture. We didn't process the sputum samples to obtain homogenized sputum or sputum plugs, and we didn't use high-volume culture or quantitative real-time polymerase chain reaction to detect Aspergillus, which might be responsible for the low positive rate (21–23). We plan to perform a prospective study and adopt more sensitive methods to detect Aspergillus. Thirdly, data collected from medical records could not help us screen out the patients with AH through hypersensitivity skin testing or IgE determination. We intend to verify the causal relationship and explore the impacts of AH in the prospective study. Besides, we do not have a comprehensive microbiological test result, which may have some influence on the conclusion. It is reported that changes in the microbiome of COPD patients' airways may be related to the risk of exacerbations (14, 24). After various stimulations induce an inflammatory response, the airway environment would be conducive to the growth of certain microorganisms. This leads to the disturbance and imbalance of the airway microbiome homeostasis, causing further airway inflammation and forming a vicious circle (25, 26). For instance, several studies have confirmed that COPD patients who are positive for Pseudomonas aeruginosa culture have a higher risk of acute exacerbations and higher mortality (27, 28). More comprehensive detection of the microbiome in the airway will be taken in our future prospective study.
In conclusion, we presented that Aspergillus colonization would lead to the relapse of exacerbation in hospitalized AECOPD patients in a short period. We should pay attention to the Aspergillus cultured in LRT samples. But the mechanisms and the adjustment of clinical strategies still require further studies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongshan Hospital of Fudan University (Approval notice number: B2015-119R2017-108). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Y-xW collected and analyzed the data and wrote the manuscript. Y-hZ screened the patients and checked the data. Q-jC, YH, Z-yB, X-yJ, X-wG, C-lT, W-pH, J-qH, W-qW, and F-yZ contributed to the data collection. JZ designed, supervised the study, and revised the manuscript. All authors contributed to the manuscript, read, and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (grant No. 2017YFC1309300 and 2017YFC1309303), and the Shanghai Top-Priority Clinical Key Disciplines Construction Project (2017ZZ02013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all the patients who cooperated with the collection of follow-up information and all the staff in the centers who helped with the study.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2021 report). (2020). Available online at: https://goldcopd.org/2021-gold-reports/ (accessed November 24, 2018).
2. Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2017) 195:324–30. doi: 10.1164/rccm.201605-1014OC
3. Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. (2003) 124:459–67. doi: 10.1378/chest.124.2.459
4. Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. (2018) 52:1801261. doi: 10.1183/13993003.01261-2018
5. Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. (2007) 30:782–800. doi: 10.1183/09031936.00062206
6. Tong X, Cheng A, Xu H, Jin J, Yang Y, Zhu S, et al. Aspergillus fumigatus during COPD exacerbation: a pair-matched retrospective study. BMC Pulm Med. (2018) 18:55. doi: 10.1186/s12890-018-0611-y
7. Huerta A, Soler N, Esperatti M, Guerrero M, Menendez R, Gimeno A, et al. Importance of Aspergillus spp. isolation in acute exacerbations of severe COPD: prevalence, factors and follow-up: the FUNGI-COPD study. Respir Res. (2014) 15:17. doi: 10.1186/1465-9921-15-17
8. Connell D, Shah A. The contribution of Aspergillus fumigatus to COPD exacerbations: a “sensitive” topic. Eur Respir J. (2020) 56:2002223. doi: 10.1183/13993003.02223-2020
9. Guinea J, Torres-Narbona M, Gijón P, Muñoz P, Pozo F, Peláez T, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clin Microbiol Infect. (2010) 16:870–7. doi: 10.1111/j.1469-0691.2009.03015.x
10. Agarwal R, Hazarika B, Gupta D, Aggarwal AN, Chakrabarti A, Jindal SK. Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med Mycol. (2010) 48:988–94. doi: 10.3109/13693781003743148
11. Jin J, Liu X, Sun Y. The prevalence of increased serum IgE and Aspergillus sensitization in patients with COPD and their association with symptoms and lung function. Respir Res. (2014) 15:130. doi: 10.1186/s12931-014-0130-1
12. Bafadhel M, McKenna S, Agbetile J, Fairs A, Desai D, Mistry V, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J. (2014) 43:64–71. doi: 10.1183/09031936.00162912
13. Tiew PY, Ko FWS, Pang SL, Matta SA, Sio YY, Poh ME, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J. (2020) 56:2000418. doi: 10.1183/13993003.00418-2020
14. Tiew PY, Dicker AJ, Keir HR, Poh ME, Pang SL, Mac Aogáin M, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J. (2020) 57:2002050. doi: 10.1183/13993003.02050-2020
15. Fairs A, Agbetile J, Hargadon B, Bourne M, Monteiro WR, Brightling CE, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. (2010) 182:1362–8. doi: 10.1164/rccm.201001-0087OC
16. Everaerts S, Lagrou K, Dubbeldam A, Lorent N, Vermeersch K, Hoeyveld EV, et al. Sensitization to Aspergillus fumigatus as a risk factor for bronchiectasis in COPD. Int J Chron Obstruct Pulmon Dis. (2017) 12:2629–38. doi: 10.2147/COPD.S141695
17. Brummer E, Kamberi M, Stevens DA. Regulation by granulocyte-macrophage colony-stimulating factor and/or steroids given in vivo of proinflammatory cytokine and chemokine production by bronchoalveolar macrophages in response to Aspergillus conidia. J Infect Dis. (2003) 187:705–9. doi: 10.1086/368383
18. Duong M, Ouellet N, Simard M, Bergeron Y, Olivier M, Bergeron MG. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J Infect Dis. (1998) 178:1472–82. doi: 10.1086/314425
19. Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology (Reading). (1994) 140:2475–9. doi: 10.1099/13500872-140-9-2475
20. Gago S, Overton NLD, Ben-ghazzi N, Novak-Frazer L, Read ND, Denning DW, et al. Lung colonization by Aspergillus fumigatus is controlled by ZNF77. Nat Commun. (2018) 9:3835. doi: 10.1038/s41467-018-06148-7
21. Fraczek MG, Kirwan MB, Moore CB, Morris J, Denning DW, Richardson MD. Volume dependency for culture of fungi from respiratory secretions and increased sensitivity of Aspergillus quantitative PCR. Mycoses. (2014) 57:69–78. doi: 10.1111/myc.12103
22. Pashley CH, Fairs A, Morley JP, Tailor S, Agbetile J, Bafadhel M, et al. Routine processing procedures for isolating filamentous fungi from respiratory sputum samples may underestimate fungal prevalence. Med Mycol. (2012) 50:433–8. doi: 10.3109/13693786.2011.615762
23. Vergidis P, Moore CB, Novak-Frazer L, Rautemaa-Richardson R, Walker A, Denning DW, et al. High-volume culture and quantitative real-time PCR for the detection of Aspergillus in sputum. Clin Microbiol Infect. (2020) 26:935–40. doi: 10.1016/j.cmi.2019.11.019
24. Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, Cookson WO, et al. Significance of the microbiome in obstructive lung disease. Thorax. (2012) 67:456–63. doi: 10.1136/thoraxjnl-2011-201183
25. Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. (2014) 384:691–702. doi: 10.1016/S0140-6736(14)61136-3
26. Dima E, Kyriakoudi A, Kaponi M, Vasileiadis I, Stamou P, Koutsoukou A, et al. The lung microbiome dynamics between stability and exacerbation in chronic obstructive pulmonary disease (COPD): current perspectives. Respir Med. (2019) 157:1–6. doi: 10.1016/j.rmed.2019.08.012
27. Jacobs DM, Ochs-Balcom HM, Noyes K, Zhao J, Leung WY, Pu CY, et al. Impact of Pseudomonas aeruginosa isolation on mortality and outcomes in an outpatient chronic obstructive pulmonary disease cohort. Open Forum Infect Dis. (2020) 7:ofz546. doi: 10.1093/ofid/ofz546
28. Eklöf J, Sørensen R, Ingebrigtsen TS, Sivapalan P, Achir I, Boel JB, et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: an observational cohort study of 22 053 patients. Clin Microbiol Infect. (2020) 26:227–34. doi: 10.1016/j.cmi.2019.06.011
Keywords: chronic obstructive pulmonary disease, acute exacerbation, Aspergillus, prognosis, recurrence of AECOPD
Citation: Wu Y-x, Zuo Y-h, Cheng Q-j, Huang Y, Bao Z-y, Jin X-y, Gao X-w, Tu C-l, Hu W-p, Hang J-q, Wang W-q, Zhang F-y and Zhang J (2021) Respiratory Aspergillus Colonization Was Associated With Relapse of Acute Exacerbation in Patients With Chronic Obstructive Pulmonary Disease: Analysis of Data From A Retrospective Cohort Study. Front. Med. 8:640289. doi: 10.3389/fmed.2021.640289
Received: 11 December 2020; Accepted: 06 April 2021;
Published: 04 May 2021.
Edited by:
Jin-fu Xu, Tongji University, ChinaReviewed by:
David Denning, The University of Manchester, United KingdomXin Su, Nanjing General Hospital of Nanjing Military Command, China
Copyright © 2021 Wu, Zuo, Cheng, Huang, Bao, Jin, Gao, Tu, Hu, Hang, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhang, aHV4aXpoYW5namluZ0Bmb3htYWlsLmNvbQ==
†These authors share first authorship
 Yi-xing Wu
Yi-xing Wu Yi-hui Zuo1†
Yi-hui Zuo1† Qi-jian Cheng
Qi-jian Cheng Jing Zhang
Jing Zhang