- 1Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Medicine, University of Miami, Miami, FL, United States
- 3Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Faculty of Paramedical Sciences, Mazandaran University of Medical Sciences, Sari, Iran
- 5Division of Pulmonary and Critical Care, University of Miami, Miami, FL, United States
Background: Non-tuberculous mycobacteria (NTM), specifically Mycobacterium avium complex (MAC), is an increasingly prevalent cause of pulmonary dysfunction. Clofazimine has been shown to be effective for the treatment of M. avium complex, but there were no published large-scale analyses comparing clofazimine to non-clofazimine regimens in MAC treatment. The objective of this large-scale meta-analysis was to evaluate patient characteristics and treatment outcomes of individuals diagnosed with MAC and treated with a clofazimine-based regimen.
Methods: We used Pubmed/Medline, Embase, Web of Science, and the Cochrane Library to search for studies published from January 1, 1990 to February 9, 2020. Two reviewers (SSH and NY) extracted the data from all eligible studies and differences were resolved by consensus. Statistical analyses were performed with STATA (version 14, IC; Stata Corporation, College Station, TX, USA).
Results: The pooled success treatment rate with 95% confidence intervals (CI) was assessed using random effect model. The estimated pooled treatment success rates were 56.8% in clofazimine and 67.9% in non-clofazimine groups. Notably, success rates were higher (58.7%) in treatment of HIV patients with disseminated infection.
Conclusions: Treatment was more successful in the non-clofazimine group overall. However, HIV patients with disseminated infection had higher treatment response rates than non-HIV patients within the clofazimine group.
Introduction
Non-tuberculous Mycobacteria
Non-tuberculous mycobacteria (NTM) are found ubiquitously in the environment and serve as a common cause of pulmonary infection associated with increasing prevalence and significantly impaired health-related quality of life (HRQL). Symptoms of pulmonary NTM (PNTM) are non-specific (cough, fever, malaise) and severity is dependent on presence of baseline lung comorbidities (1). The majority (80%) of PNTM infections are caused by Myobacterium avium complex (MAC) (2–5).
Current Treatment Approaches
There are limited data to guide the treatment of pulmonary non-tuberculous mycobacterial infection in patients without HIV. Current strategies involve multimodal drug therapy, drug susceptibility testing, and extended courses of antimicrobials which can often be unsuccessful. Extended courses of targeted drug therapy for slow verses rapid growing NTM are selected with the assistance of drug susceptibility testing (DST) (4). The American Thoracic Society/Infectious Diseases Society of America guidelines recommend a three-drug macrolide combination therapy containing rifamycin (rifampin, rifapentine, or rifabutin), ethambutol, with a macrolide (clarithromycin or azithromycin) for at least 12 months after culture conversion. The addition of aminoglycoside therapy (amikacin or streptomycin) is recommended in the first 3–6 months of therapy for severe disease (6).
Role of Clofazimine
Continued discovery is crucial to streamline a treatment regimen for PNTM in attempt to lower costs, target treatment-resistant isolates, and increase health-related quality of life for patients. Clofazimine is a lipophilic antibiotic FDA approved for the treatment of Mycobacterium leprae, the bacteria causing Hansen's disease. Clofazimine inhibits mycobacterial respiratory chain and ion transporters in the outer membrane; the phenazine molecule acts as an artificial electron acceptor. Clofazimine is oxidized in place of NADH, leading to reduced cellular ATP and presence of damaging reactive oxygen species (7). The role of clofazimine in the treatment of MAC has not been elucidated. Its efficacy has been shown in several studies, but a comprehensive analysis has not been published. The objective of this large-scale meta-analysis was to evaluate patient characteristics and treatment outcomes of individuals diagnosed with MAC who were treated with a clofazimine based regimen.
Experimental Section
Search Strategy
We searched Pubmed/Medline, Embase, Web of science and the Cochrane Library for studies published from January 1, 1990 to February 9, 2020. The search strategy was based on the following key words: M. avium complex, Mycobacterium avium-intracellulare complex, MAC, macrolides, aminoglycosides, and clofazimine. Lists of references of selected articles and relevant review articles were hand-searched to identify further studies. Only studies written in English were selected. This study was conducted and reported according to the PRISMA guidelines (8).
Study Selection
The records found through database searching were merged and the duplicates were removed using EndNote X7 (Thomson Reuters, New York, NY, USA). Two reviewers (SSH and NY) independently screened the records by title and abstract to exclude those not related to the current study. The full-text of potentially eligible records was retrieved and evaluated by a third reviewer (MJN). Included studies met the following inclusion criteria: (i) patients were diagnosed with MAC using the criteria suggested by ATS/ IDSA; (ii) all study patients were treated with clofazimine or macrolide and/or aminoglycoside-containing regimens, with companion drugs; and (iii) the treatment outcomes were addressed. We defined treatment success as achievement of culture conversion and completion of the planned treatment without relapse while on treatment. Studies with insufficient information about patients' characteristics and treatment outcomes were excluded. Conference abstracts, editorials, and reviews were also excluded.
Data Extraction and Quality Assessment
A data extraction form was designed by two reviewers (SSH and NY). These reviewers extracted the data from all eligible studies and differences were resolved by consensus. The following data were extracted: first author name; year of publication; study duration, type of study, country/ies where the study was conducted; number of patients with MAC; age; HIV/AIDS status; treatment protocols (treatment regimens and duration of treatment), and treatment outcome. The methodological quality of the eligible studies was assessed according to the Cochrane-based criteria (9).
Data Synthesis and Analysis
Statistical analyses were performed with STATA (version 14, IC; Stata Corporation, College Station, TX, USA). The pooled success treatment rate with 95% confidence intervals (CI) was assessed using random effect model. The between-study heterogeneity was assessed by Cochran's Q and the I2 statistic. Publication bias was assessed statistically by using Begg's and Egger’s-tests (p < 0.05) was considered indicative of statistically significant publication bias). To explore sources of studies’ heterogeneity, sensitivity analyses were carried out with meta-regression and subgroup analysis.
Results
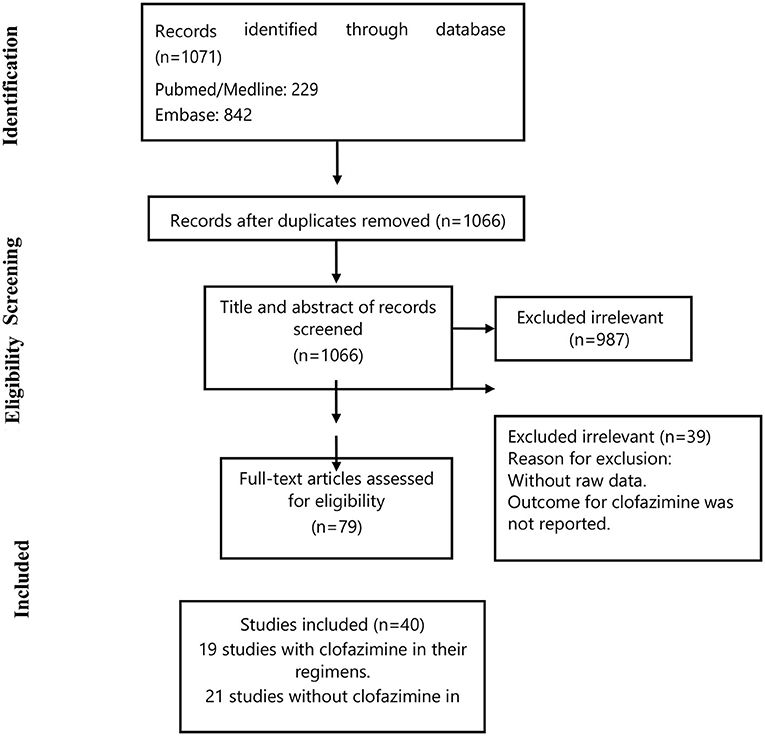
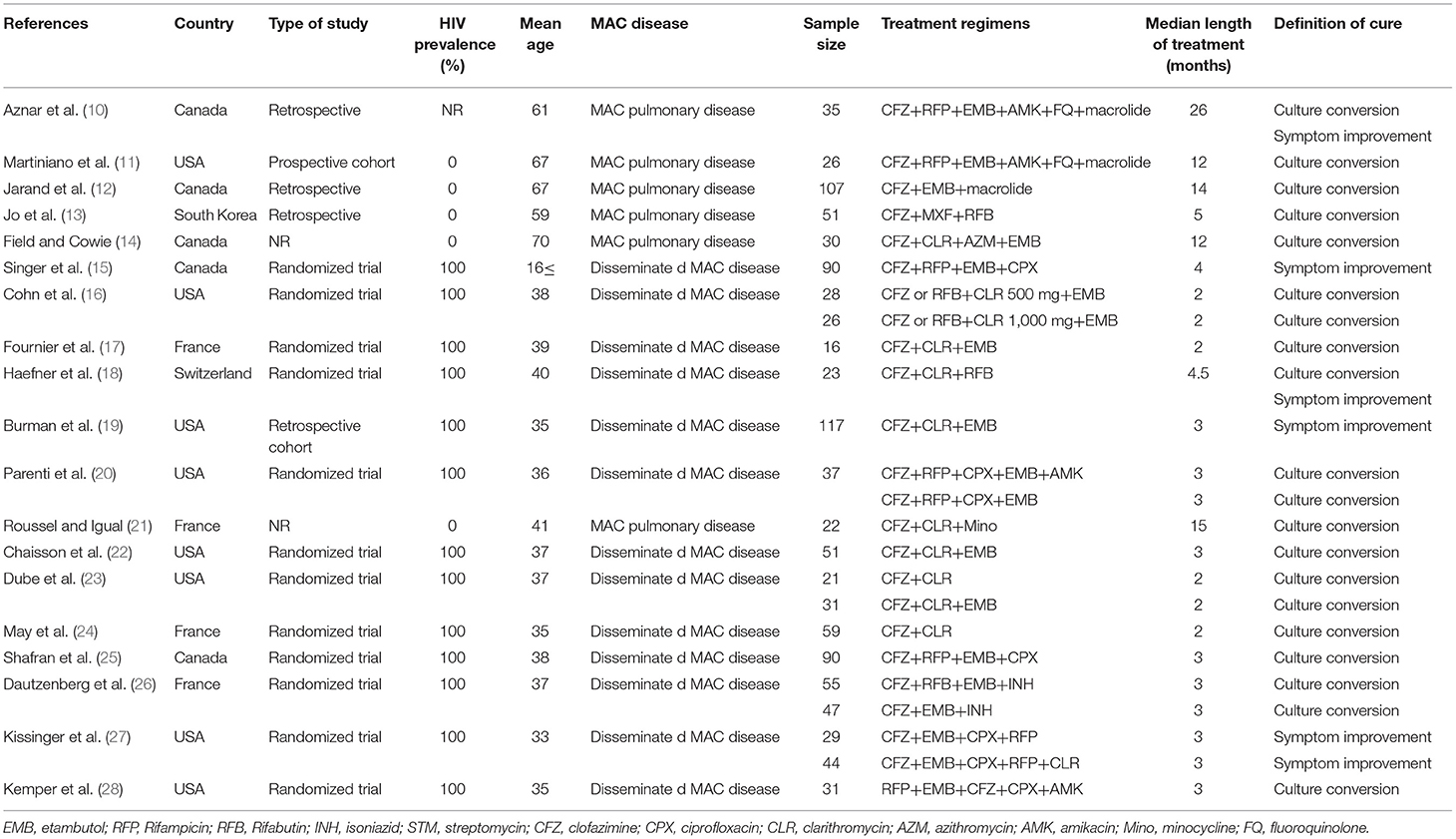
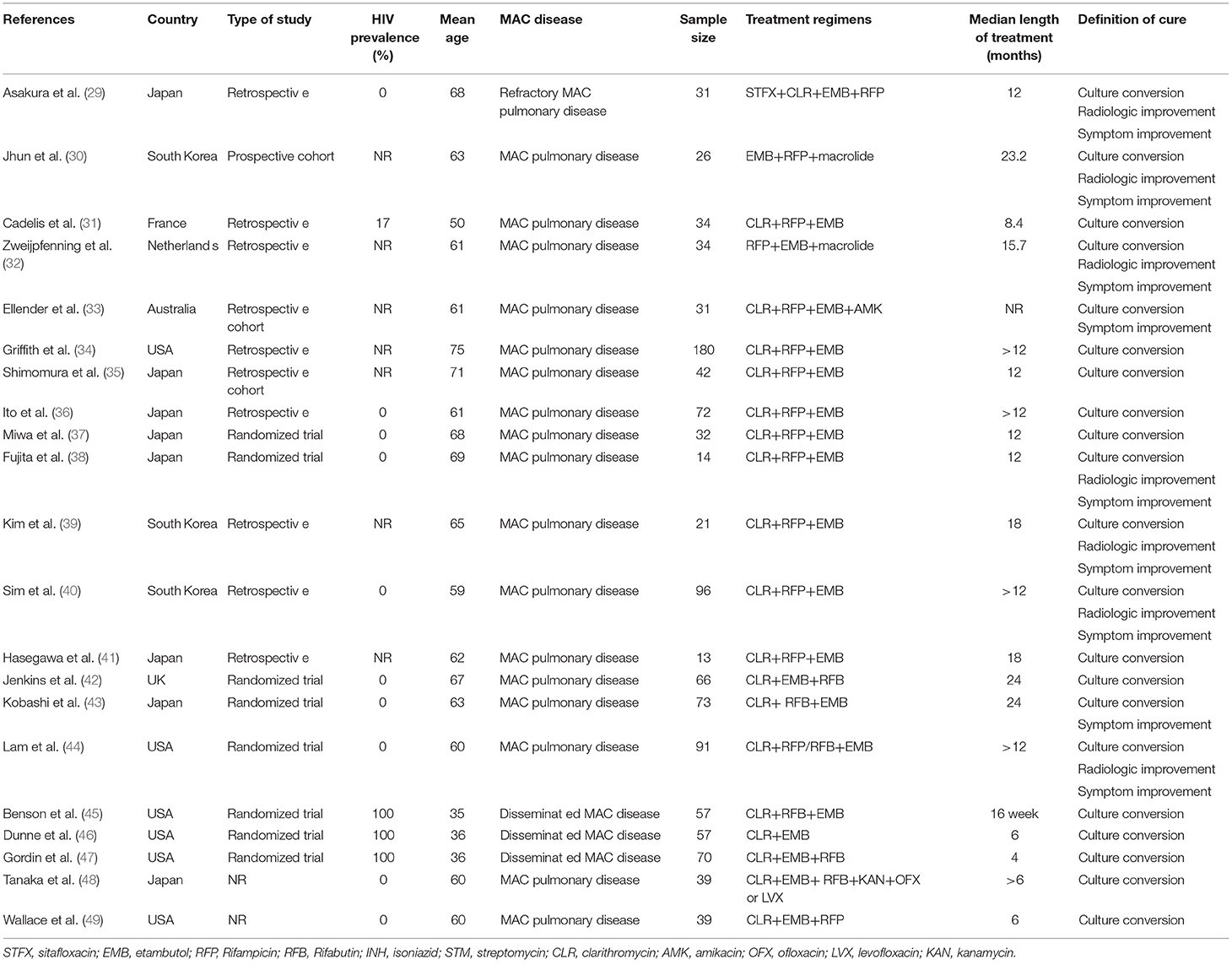
Figure 1 summarizes the study selection process. Briefly, we retrieved data from 40 selected articles comprising data for 19 studies with clofazimine in their regimens (clofazimine group) and 21 studies without clofazimine in their regimens (Non-clofazimine group). Characteristics of the included studies are described in Tables 1, 2.
Quality Assessment
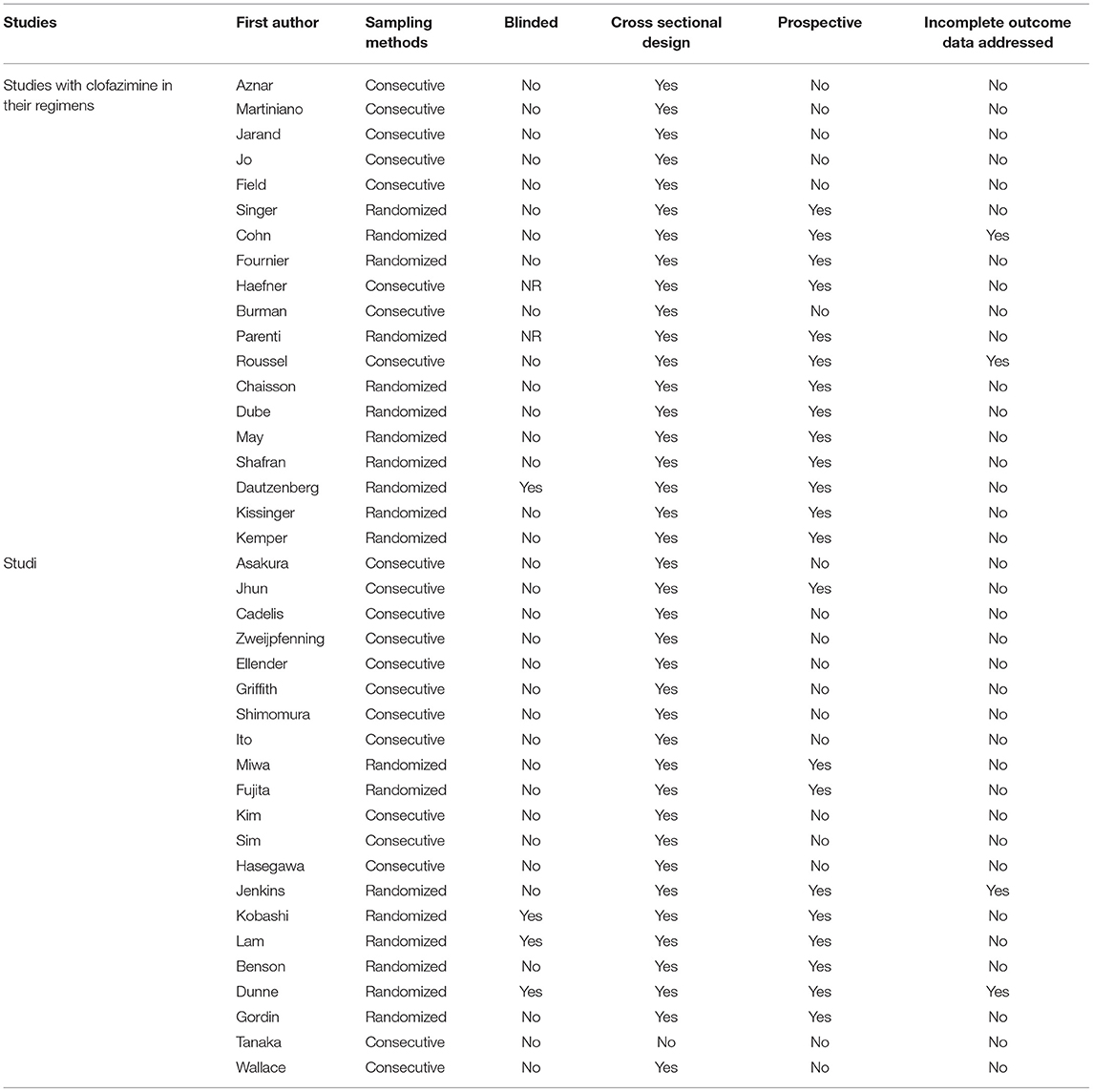
Based on Cochrane tool (Table 3), the included studies had a low risk of bias. In clofazimine group, 12 studies were randomized controlled trials and the rest were non-randomized controlled trials (i.e., cohort or retrospective observational studies). In this group, the statistical analysis methodology was well-described in 17 studies but was not reported in the other two studies.
Treatment Success
The estimated pooled treatment success rates were found to be 56.8% (95% CI 47.0–66.5%) and 67.9% (95% CI 62.0–73.8%) in clofazimine and non-clofazimine groups, respectively (Figures 2, 3). The heterogeneity in the study characteristics led to significant variation in the reported treatment outcomes. Varying treatment success rates caused heterogeneity in the pooled results. Thus, we ran a meta-regression to understand the source of heterogeneity. Based on meta-regression, different treatment success rates resulted as a significant source of heterogeneity (P-value = 0.000) in both clofazimine and non-clofazimine groups. In clofazimine group, there was some evidence of publication bias (Begg’s and tests P-value was 0.01).
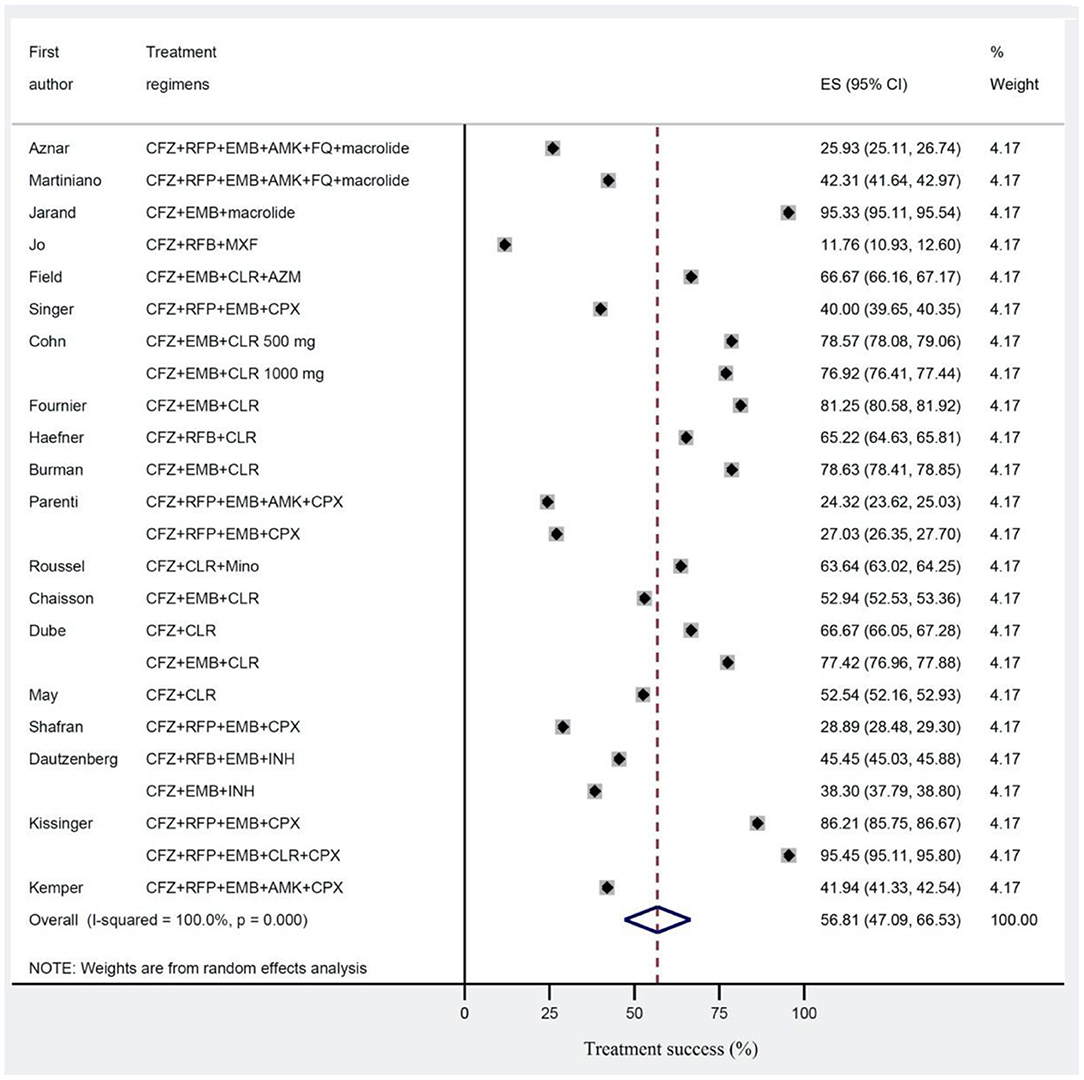
Figure 2. Treatment success for Mycobacterium avium complex (MAC) disease in studies with clofazimine in their regimens. Treatment effects and summaries were calculated using a random-effects model weighted by study population.
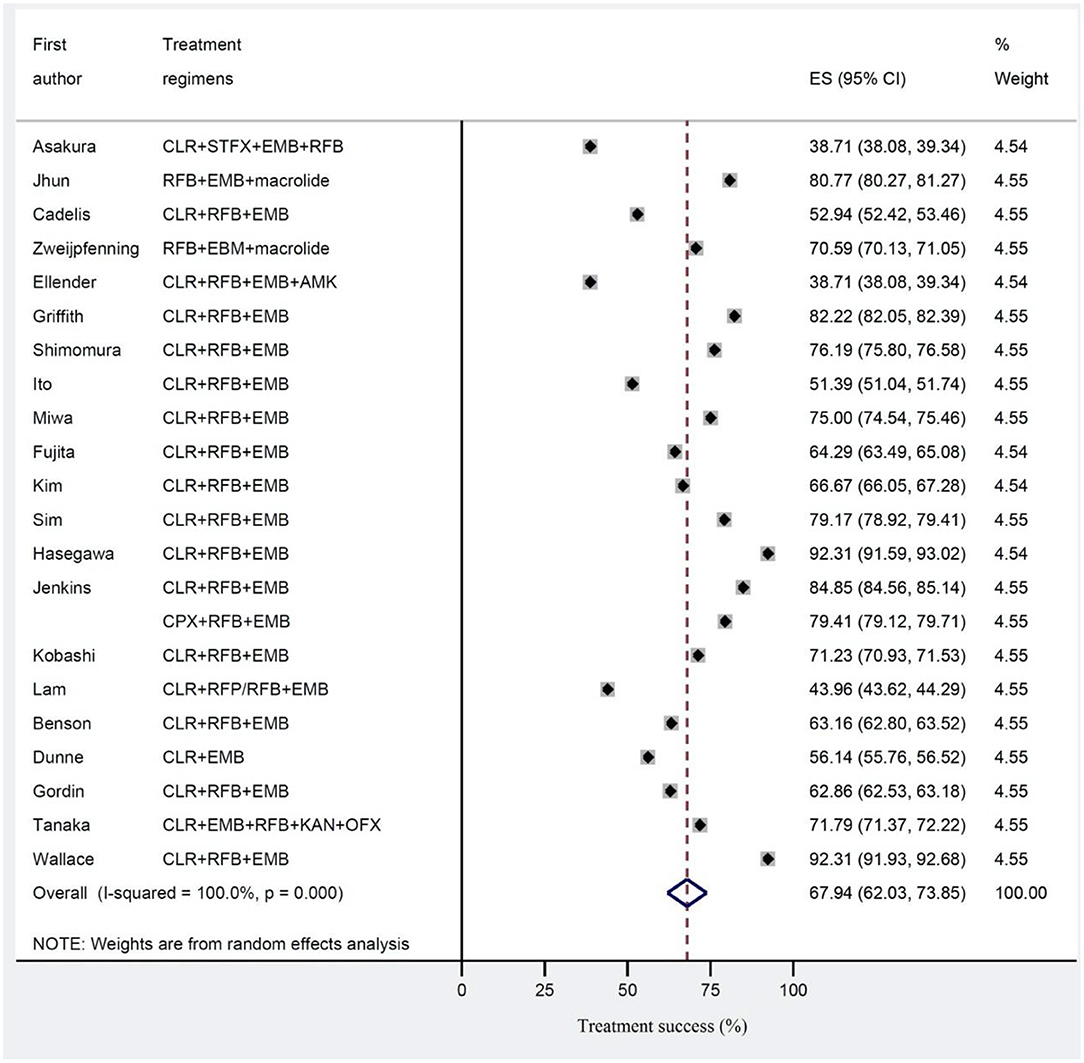
Figure 3. Treatment success for Mycobacterium avium complex (MAC) disease in studies without clofazimine in their regimen.
Subgroup Analysis
Table 4 shows the subgroup analysis of the studies based on treatment regimens, length of treatment, type of patients, number of drugs used, definition of cure, type of study and year of publication.
Discussion
Summary
This study found that the estimated pooled treatment success rates were 56.8% in clofazimine and 67.9% in non-clofazimine groups. The duration of treatment above 1 year after did not show any improvement in success rates. The success rate was higher (58.7%) in treatment of HIV patients with disseminated MAC compared to treatment of Non-HIV patients with MAC pulmonary disease (51.0%). Counterintuitively, treatment regimens containing more than three drugs were less successful (47.6% compared to 64.5%). The success rates were higher in studies which defined cure by symptomatic improvement rather than culture conversion.
Clofazimine
The need for novel therapies to combat MAC infection is high due to drug resistance, disease recurrence, and current suboptimal efficacy. Even though there is a lack of robust data supporting its efficacy, clofazimine has been used in combination therapies for the treatment of MAC. In this study we found lower treatment success rates when using clofazimine-based regimens, especially for the treatment of non-HIV related MAC pulmonary disease.
Resistance to clofazimine may have contributed to lower treatment success rates. In vitro isolates of NTM have been shown to be susceptible to clofazimine. Luo et al. tested 209 isolates containing rapid and slow growing NTM for in vitro susceptibility to clofazimine. Most slow growing clinical isolates were sensitive to clofazimine with MICs <1 μg/ml and 17 out of 30 rapid growing clinical isolates showed sensitivity with MICs below <1 μg/ml (50). However, Chen et al. found mutations in genes coding for transcriptional regulatory proteins of Mycobacterium which confer resistance to clofazimine (51).
In addition to its antimicrobial activity, clofazimine displays immune modulating effects which may alter patient response to therapy. Clofazimine increases humoral immune response by increasing major histocompatibility complex class II expression in monocytes and decreasing suppressor T-cell activity. However, it negatively modulates innate immune activity by inducing apoptosis in macrophages (52). It is possible that the clofazimine decreases cure rates through death of macrophages, however this decrease may be offset by the beneficial T-cell modulation in immunocompromised HIV patients.
Lower treatment success rates in the clofazimine group could be attributed to a clinically significant drug interaction with rifampin. Pooled treatment success rates were lower in regimens containing more than three drugs compared to regimens containing three or less drugs. Interestingly, every clofazimine study using a greater than three-drug regimen contained rifampin as part its regimen. Whereas, rifampin was only used in a minority of drug regimens containing ≤3 drugs (2/11). Rifamycin antimicrobials induce many hepatic cytochrome P450 enzymes as well as glucuronidation pathways. Clofazimine undergoes glucuronidation prior to excretion as it transitions out of its pharmacologically active state. Clinically noticeable drug-interactions have been reported between clofazimine and rifampin (53). This finding could be secondary to rifampin-induced glucuronidation and subsequent excretion of clofazimine.
Previous Data
Clofazimine based treatment regimens have been shown to be efficacious for the treatment on MAC in several previous studies, but data is scant, and treatments were never compared prospectively in a head-to-head fashion. Field et al. conducted a single-arm prospective study looking at the efficacy of macrolide/ethambutol/clofazimine regimen in MAC lung disease in 33 patients. Treatment for an average of 10 months converted sputum findings to negative in 87% of patients. However, relapse occurs in 19% of patients (14). Jarand et al. retrospectively reviewed patients with MAC lung disease being treated with regimens including clofazimine or rifampin and found a higher culture conversion rate in the clofazimine group rifampin (100 vs. 71%; P = 0.0002). However, relapse and re-treatment rates did not differ between groups (12). Martiniano et al. retrospectively found 50% of patients with pulmonary disease converted to negative cultures with the treatment of clofazimine regimens.
However, only 48% of patient had M. avium complex, 21% of patients were diagnosed with cystic fibrosis, and most patients (78%) had failed previous treatment attempts (11).
Strengths/Limitations
This study was the first comprehensive review comparing clofazimine and non-clofazimine treatment regimens for the treatment of MAC. Our sample size of studies (19) and controls (21) is robust. External validity of this paper is strong; we were able to include studies treating HIV related disseminated MAC and non-HIV related pulmonary MAC. However, there are some limitations to address. Our study did not characterize adverse effects and treatment adherence to clofazimine regimens. Adherence and associated adverse effects may have contributed to outcomes. Also, based on meta-regression, different treatment success rates resulted as a significant source of heterogeneity (P-value = 0.000) in both clofazimine and non-clofazimine groups. In the clofazimine group, there was some evidence of publication bias (Begg’s and tests P-value was 0.01). Interestingly, publication later than 2,000 showed lower success rates overall which could be explained by publication bias. However, subgroup analysis was conducted for the clofazimine treated group to compare heterogenous of the studies features.
Future Directions
Our findings will help in assigning a role to clofazimine in the treatment of MAC. Based on our results, clofazimine should be considered a last-line agent. It is possible that its only role is in the treated of disseminated HIV disease. Future clinical trials need to be done to assess the efficacy in disseminated MAC. Furthermore, we need novel therapeutic agents to target non-HIV pulmonary MAC as current therapies are long in duration and often result in relapse of disease process (54).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MN performed the literature review, conducted data analysis, and manuscript preparation. TC performed the literature review and manuscript preparation. SH, AH, NN, and MK-Y helped in the literature review and data analysis. MM conducted literature review, designed the study, and performed data analysis and manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
MN was supported by Research Department of the School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant Number: 26692).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med. (2011) 105:1718–25. doi: 10.1016/j.rmed.2011.08.004
2. Cayrou C, Turenne C, Behr MA, Drancourt M. Genotyping of Mycobacterium avium complex organisms using multispacer sequence typing. Microbiology. (2010) 156:687–94. doi: 10.1099/mic.0.033522-0
3. Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. (2010) 182:970–6. doi: 10.1164/rccm.201002-0310OC
4. Kim CJ, Kim NH, Song KH, Choe PG, Kim ES, Park SW, et al. Differentiating rapid- and slow-growing mycobacteria by difference in time to growth detection in liquid media. Diagn Microbiol Infect Dis. (2013) 75:73–6. doi: 10.1016/j.diagmicrobio.2012.09.019
5. Machado D, Ramos J, Couto I, Cadir N, Narciso I, Coelho E, et al. Assessment of the BD MGIT TBc identification test for the detection of Mycobacterium tuberculosis complex in a network of mycobacteriology laboratories. Biomed Res Int. (2014) 2014:398108. doi: 10.1155/2014/398108
6. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. (2007) 175:367–416. doi: 10.1164/rccm.200604-571ST
7. Mirnejad R, Asadi A, Khoshnood S, Mirzaei H, Heidary M, Fattorini L, et al. Clofazimine: a useful antibiotic for drug-resistant tuberculosis. Biomed Pharmacother. (2018) 105:1353–9. doi: 10.1016/j.biopha.2018.06.023
8. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
9. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken NJ: John Wiley and Sons (2011).
10. Aznar M, Brode S, Mehrabi M, Marras T. Safety and effectiveness of clofazimine in nontuberculous mycobacterial lung disease. Can J Resp Cri Care Sleep Med. (2018) 2:72–7. doi: 10.1080/24745332.2017.1410455
11. Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest. (2017) 152:800–9. doi: 10.1016/j.chest.2017.04.175
12. Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest. (2016) 149:1285–93. doi: 10.1378/chest.15-0543
13. Jo K-W, Kim S, Lee JY, Lee S-D, Kim WS, Kim DS, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother. (2014) 20:602–6. doi: 10.1016/j.jiac.2014.05.010
14. Field SK, Cowie RL. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest. (2003) 124:1482–6. doi: 10.1378/chest.124.4.1482
15. Singer J, Thorne A, Khorasheh S, Raboud JM, Wu AW, Salit I, et al. Symptomatic and health status outcomes in the Canadian randomized MAC treatment trial (CTN010). Int J STD and AIDS. (2000) 11:212–9. doi: 10.1258/0956462001915732
16. Cohn DL, Fisher EJ, Peng GT, Hodges JS, Chesnut J, Child CC, et al. A prospective randomized trial of four three-drug regimens in the treatment of disseminated Mycobacterium avium complex disease in AIDS patients: excess mortality associated with high-dose clarithromycin. Clin Infect Dis. (1999) 29:125–33. doi: 10.1086/520141
17. Fournier S, Burguiere A, Flahault A, Vincent V, Treilhou M, Eliaszewicz M. Effect of adding clofazimine to combined clarithromycin-ethambutol therapy for Mycobacterium avium complex septicemia in AIDS patients. Eur J Clin Microbiol Infect Dis. (1999) 18:16–22. doi: 10.1007/s100960050220
18. Haefner M, Funke-Kissling P, Pfyffer GE, Lüthy R, Opravil M. Clarithromycin, rifabutin and clofazimine for treatment of disseminated Mycobacterium avium complex disease in AIDS patients. Clin Drug Invest. (1999) 17:171–8. doi: 10.2165/00044011-199917030-00001
19. Burman WJ, Stone BL, Rietmeijer CA, Maslow J, Cohn DL, Reves RR. Long-term outcomes of treatment of Mycobacterium avium complex bacteremia using a clarithromycin-containing regimen. Aids. (1998) 12:1309–15. doi: 10.1097/00002030-199811000-00012
20. Parenti DM, Williams PL, Hafner R, Jacobs MR, Hojczyk P, Hooton TM, et al. A phase II/III trial of antimicrobial therapy with or without amikacin in the treatment of disseminated Mycobacterium avium infection in HIV-infected individuals. Aids. (1998) 12:2439–46. doi: 10.1097/00002030-199818000-00013
21. Roussel G, Igual J. Clarithromycin with minocycline and clofazimine for Mycobacterium avium intracellulare complex lung disease in patients without the acquired immune deficiency syndrome. Int J Tuberc Lung Dis. (1998) 462–470.
22. Chaisson RE, Keiser P, Pierce M, Fessel WJ, Ruskin J, Lahart C, et al. Clarithromycin and ethambutol with or without clofazimine for the treatment of bacteremic: Mycobacterium avium complex disease in patients with HIV infection. Aids. (1997) 11:311–7. doi: 10.1097/00002030-199703110-00008
23. Dubé MP, Sattler FR, Torriani FJ, See D, Havlir DV, Kemper CA, et al. A randomized evaluation of ethambutol for prevention of relapse and drug resistance during treatment of Mycobacterium avium complex bacteremia with clarithromycin-based combination therapy. J Infect Dis. (1997) 176:1225–32. doi: 10.1086/514116
24. May T, Brel F, Beuscart C, Vincent V, Perronne C, Doco-Lecompte T, et al. Comparison of combination therapy regimens for treatment of human immunodeficiency virus-infected patients with disseminated bacteremia due to Mycobacterium avium. Clin Infect Dis. (1997) 25:621–9. doi: 10.1086/513753
25. Shafran SD, Singer J, Zarowny DP, Phillips P, Salit I, Walmsley SL, et al. A comparison of two regimens for the treatment of Mycobacterium avium complex bacteremia in AIDS: rifabutin, ethambutol, and clarithromycin versus rifampin, ethambutol, clofazimine, and ciprofloxacin. N Engl J Med. (1996) 335:377–84. doi: 10.1056/NEJM199608083350602
26. Dautzenberg B, Olliaro P, Ruf B, Esposito R, Opravil M, Hoy J, et al. Rifabutin versus placebo in combination with three drugs in the treatment of nontuberculous mycobacterial infection in patients with AIDS. Clin Infect Dis. (1996) 22:705–8. doi: 10.1093/clinids/22.4.705
27. Kissinger P, Clark R, Morse A, Brandon W. Comparison of multiple drug therapy regimens for HIV-related disseminated Mycobacterium avium complex disease. J Acquired Immune Defic Syndr Human Retrovirol. (1995) 9:133–7. doi: 10.1097/00042560-199506000-00005
28. Kemper CA, Havlir D, Bartok AE, Kane C, Camp B, Lane N, et al. Transient bacteremia due to Mycobacterium avium complex in patients with AIDS. J Infect Dis. (1994) 170:488–93. doi: 10.1093/infdis/170.2.488
29. Asakura T, Suzuki S, Fukano H, Okamori S, Kusumoto T, Uwamino Y, et al. Sitafloxacin-containing regimen for the treatment of refractory Mycobacterium avium complex lung disease. Open Forum Infect Dis. (2019) 6:ofz108. doi: 10.1093/ofid/ofz108
30. Jhun BW, Moon SM, Kim SY, Park HY, Jeon K, Kwon OJ, et al. Intermittent antibiotic therapy for recurrent nodular bronchiectatic Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. (2018) 62. doi: 10.1128/AAC.01812-17
31. Cadelis G, Ducrot R, Bourdin A, Rastogi N. Predictive factors for a one-year improvement in nontuberculous mycobacterial pulmonary disease: an 11-year retrospective and multicenter study. PLoS Negl Trop Dis. (2017) 11:e0005841. doi: 10.1371/journal.pntd.0005841
32. Zweijpfenning S, Kops S, Magis-Escurra C, Boeree MJ, Van Ingen J, Hoefsloot W. Treatment and outcome of non-tuberculous mycobacterial pulmonary disease in a predominantly fibro-cavitary disease cohort. Respir Med. (2017) 131:220–4. doi: 10.1016/j.rmed.2017.08.031
33. Ellender CM, Law DB, Thomson RM, Eather GW. Safety of IV amikacin in the treatment of pulmonary non-tuberculous mycobacterial disease. Respirology. (2016) 21:357–62. doi: 10.1111/resp.12676
34. Griffith DE, Adjemian J, Brown-Elliott BA, Philley JV, Prevots DR, Gaston C, et al. Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. (2015) 192:754–60. doi: 10.1164/rccm.201503-0444OC
35. Shimomura H, Ono A, Imanaka K, Majima T, Masuyama H, Sato T, et al. Retrospective investigation of combination therapy with clarithromycin and levofloxacin for pulmonary Mycobacterium avium complex disease. J Pharm Health Care Sci. (2015) 1:24. doi: 10.1186/s40780-015-0025-4
36. Ito Y, Hirai T, Fujita K, Kubo T, Maekawa K, Ichiyama S, et al. The influence of environmental exposure on the response to antimicrobial treatment in pulmonary Mycobacterial avium complex disease. BMC Infect Dis. (2014) 14:522. doi: 10.1186/1471-2334-14-522
37. Miwa S, Shirai M, Toyoshima M, Shirai T, Yasuda K, Yokomura K, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A Prelim Study Ann Am Thorac Soc. (2014) 11:23–9. doi: 10.1513/AnnalsATS.201308-266OC
38. Fujita M, Kajiki A, Tao Y, Miyazaki M, Ouchi H, Harada E, et al. The clinical efficacy and safety of a fluoroquinolone-containing regimen for pulmonary MAC disease. J Infect Chemother. (2012) 18:146–51. doi: 10.1007/s10156-011-0303-5
39. Kim EY, Chi SY, Oh IJ, Kim KS, Kim YI, Lim SC, et al. Treatment outcome of combination therapy including clarithromycin for Mycobacterium avium complex pulmonary disease. Korean J Intern Med. (2011) 26:54–9. doi: 10.3904/kjim.2011.26.1.54
40. Sim YS, Park HY, Jeon K, Suh GY, Kwon OJ, Koh WJ. Standardized combination antibiotic treatment of Mycobacterium avium complex lung disease. Yonsei Med J. (2010) 51:888–94. doi: 10.3349/ymj.2010.51.6.888
41. Hasegawa N, Nishimura T, Ohtani S, Takeshita K, Fukunaga K, Tasaka S, et al. Therapeutic effects of various initial combinations of chemotherapy including clarithromycin against Mycobacterium avium complex pulmonary disease. Chest. (2009) 136:1569–75. doi: 10.1378/chest.08-2567
42. Jenkins PA, Campbell IA, Banks J, Gelder CM, Prescott RJ, Smith AP. Clarithromycin vs. ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax. (2008) 63:627–34. doi: 10.1136/thx.2007.087999
43. Kobashi Y, Matsushima T, Oka M. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med. (2007) 101:130–8. doi: 10.1016/j.rmed.2006.04.002
44. Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. (2006) 173:1283–9. doi: 10.1164/rccm.200509-1531OC
45. Benson CA, Williams PL, Currier JS, Holland F, Mahon LF, Macgregor RR, et al. A prospective, randomized trial examining the efficacy and safety of clarithromycin in combination with ethambutol, rifabutin, or both for the treatment of disseminated Mycobacterium avium complex disease in persons with acquired immunodeficiency syndrome. Clin Infect Dis. (2003) 37:1234–43. doi: 10.1086/378807
46. Dunne M, Fessel J, Kumar P, Dickenson G, Keiser P, Boulos M, et al. A randomized, double-blind trial comparing azithromycin and clarithromycin in the treatment of disseminated Mycobacterium avium infection in patients with human immunodeficiency virus. Clin Infect Dis. (2000) 31:1245–52. doi: 10.1086/317468
47. Gordin FM, Sullam PM, Shafran SD, Cohn DL, Wynne B, Paxton L, et al. A randomized, placebo-controlled study of rifabutin added to a regimen of clarithromycin and ethambutol for treatment of disseminated infection with Mycobacterium avium complex. Clin Infect Dis. (1999) 28:1080–5. doi: 10.1086/514748
48. Tanaka E, Kimoto T, Tsuyuguchi K, Watanabe I, Matsumoto H, Niimi A, et al. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. (1999) 160:866–72. doi: 10.1164/ajrccm.160.3.9811086
49. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. (1996) 153:1766–72. doi: 10.1164/ajrccm.153.6.8665032
50. Luo J, Yu X, Jiang G, Fu Y, Huo F, Ma Y, et al. In vitro activity of clofazimine against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob Agents Chemother. (2018) 62:e00072–18. doi: 10.1128/AAC.00072-18
51. Chen Y, Chen J, Zhang S, Shi W, Zhang W, Zhu M, et al. Novel mutations associated with clofazimine resistance in Mycobacterium abscessus. Antimicrob Agents Chemother. (2018) 62:e00544–18. doi: 10.1128/AAC.00544-18
52. Fukutomi Y, Maeda Y, Makino M. Apoptosis-inducing activity of clofazimine in macrophages. Antimicrob Agents Chemother. (2011) 55:4000–5. doi: 10.1128/AAC.00434-11
53. Holdiness MR. Clinical pharmacokinetics of clofazimine. A Rev Clin Pharmacokinet. (1989) 16:74–85. doi: 10.2165/00003088-198916020-00002
Keywords: clofazimine, Mycobacterium avium complex, pulmonary disease, mycobacteria, MAC
Citation: Nasiri MJ, Calcagno T, Hosseini SS, Hematian A, Nojookambari NY, Karimi-Yazdi M and Mirsaeidi M (2021) Role of Clofazimine in Treatment of Mycobacterium avium Complex. Front. Med. 8:638306. doi: 10.3389/fmed.2021.638306
Received: 07 December 2020; Accepted: 15 March 2021;
Published: 15 April 2021.
Edited by:
Marwan Osman, Lebanese University, LebanonCopyright © 2021 Nasiri, Calcagno, Hosseini, Hematian, Nojookambari, Karimi-Yazdi and Mirsaeidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Mirsaeidi, bXNtMjQ5QG1lZC5taWFtaS5lZHU=
 Mohammad Javad Nasiri
Mohammad Javad Nasiri Tess Calcagno
Tess Calcagno Sareh Sadat Hosseini3
Sareh Sadat Hosseini3 Mehdi Mirsaeidi
Mehdi Mirsaeidi