- 1Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
- 2Department of Critical Care Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 3Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 4Department of Pediatric, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 5School of Public Health, Lanzhou University, Lanzhou, China
- 6Institute of Health Data Science, Lanzhou University, Lanzhou, China
- 7World Health Organization Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China
- 8Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province, Lanzhou University, Lanzhou, China
Objective: The aims of this systematic review and meta-analysis were to summarize the current existing evidence on the outcome of critically ill patients with COVID-19 as well as to evaluate the effectiveness of clinical interventions.
Data Sources: We searched MEDLINE, the Cochrane library, Web of Science, the China Biology Medicine disc, China National Knowledge Infrastructure, and Wanfang Data from their inception to May 15, 2021. The search strings consisted of various search terms related to the concepts of mortality of critically ill patients and clinical interventions.
Study Selection: After eliminating duplicates, two reviewers independently screened all titles and abstracts first, and then the full texts of potentially relevant articles were reviewed to identify cohort studies and case series that focus on the mortality of critically ill patients and clinical interventions.
Main Outcomes and Measures: The primary outcome was the mortality of critically ill patients with COVID-19. The secondary outcomes included all sorts of supportive care.
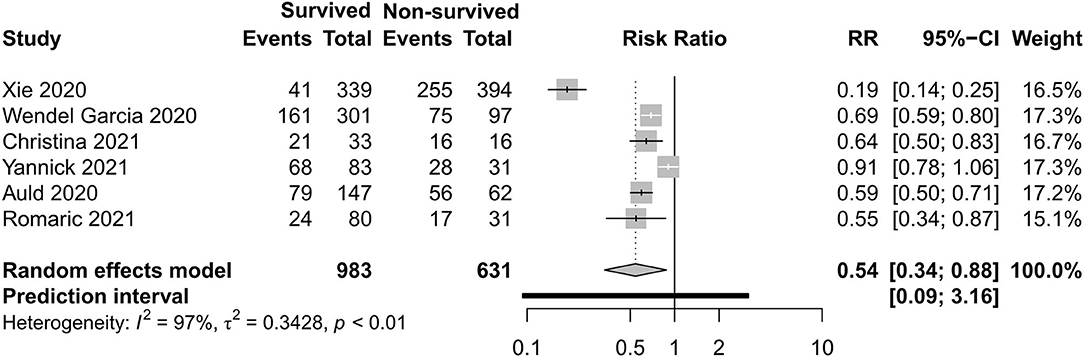
Results: There were 27 cohort studies and six case series involving 42,219 participants that met our inclusion criteria. All-cause mortality in the intensive care unit (ICU) was 35% and mortality in hospital was 32% in critically ill patients with COVID-19 for the year 2020, with very high between-study heterogeneity (I2 = 97%; p < 0.01). In a subgroup analysis, the mortality during ICU hospitalization in China was 39%, in Asia—except for China—it was 48%, in Europe it was 34%, in America it was 15%, and in the Middle East it was 39%. Non-surviving patients who had an older age [−8.10, 95% CI (−9.31 to −6.90)], a higher APACHE II score [−4.90, 95% CI (−6.54 to −3.27)], a higher SOFA score [−2.27, 95% CI (−2.95 to −1.59)], and a lower PaO2/FiO2 ratio [34.77, 95% CI (14.68 to 54.85)] than those who survived. Among clinical interventions, invasive mechanical ventilation [risk ratio (RR) 0.49, 95% CI (0.39–0.61)], kidney replacement therapy [RR 0.34, 95% CI (0.26–0.43)], and vasopressor [RR 0.54, 95% CI (0.34–0.88)] were used more in surviving patients.
Conclusions: Mortality was high in critically ill patients with COVID-19 based on low-quality evidence and regional difference that existed. The early identification of critical characteristics and the use of support care help to indicate the outcome of critically ill patients.
Introduction
With the rapid spread of coronavirus disease 2019 (COVID-19) globally, as of June 2, 2021, a total of 171,222,477 confirmed cases had been reported in 215 countries, areas, or territories, and COVID-19 has been responsiblefor at least 3,686,142 deaths (1). Critically ill patients are always companied by a high risk of lives, which may be complicated by an uncontrolled systemic inflammatory response leading to acute respiratory distress syndrome (ARDS) and multiple organ dysfunction. Patients with ARDS and requirement for respiratory support need urgently to be transferred to the intensive care unit (ICU). It is reported that nasal cannula or mask, high-flow nasal cannula, non-invasive ventilation (NIV), invasive mechanical ventilation (IMV), and veno-venous extracorporeal membrane oxygenation (VV-ECMO) were widely used in COVID-19 according to the severity of respiratory dysfunction (2–4). Cardiac injury is common in COVID-19, with an incidence of 36% and closely related to a higher risk of mortality (5). It is reported that, in a systematic review and meta-analysis, the pooled incidence of acute kidney injury (AKI) was 28.6% among hospitalized COVID-19 patients from the USA and Europe and 5.5% among patients from China. Kidney replacement therapy (KRT) was used in 20.6% of patients admitted to the intensive care unit (6).
As is universally known, the mortality of critically ill patients is higher than that of ordinary patients. A systematic review reported that the summary estimate for all-cause mortality was 10% for adult patients with COVID-19 and 34% for critically ill patients within minor countries (7). In order to gain a clearer picture of the mortality of critically ill patients within major countries and clinical interventions or supportive care for organ dysfunction in the ICU, we meta-analyzed the relevant literature. The results may provide a narrative for the mortality of critically ill patients with COVID-19 as well as the effect of clinical characteristics and interventions between surviving and non-surviving patient groups.
Methods
This systematic review was performed in compliance with the Centre of Reviews and Dissemination guidelines (8) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (9). In order to complete the systematic review and provide some references for clinical intervention during COVID-19 as soon as possible, this review was not registered.
Eligibility Criteria
We included studies that focused on the mortality of critically ill patients with laboratory-confirmed COVID-19, clinical characteristics, and interventions or supportive care of organ dysfunction.
We included original studies that fulfill the following criteria: (1) the type of study was cohort, case–control, or case–series designs, (2) the study topic was related to the mortality, clinical characteristics, and interventions or supportive care of critically ill patients with COVID-19, which is defined as a positive result of a real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs (10), and (3) the study was published or posted in English or Chinese. We excluded duplicates, conference abstracts, letters, and studies for which we could not access the full text and missing data of outcomes. In order to avoid a small size, only studies of more than 50 patients were included. If there were two or more studies that included the same population, only the study with the largest sample size was chosen.
In this review, the primary outcome was the mortality of critically ill patients with COVID-19. The secondary outcomes included all sorts of supportive care, including non-invasive respiratory support, IMV, KRT, and vasopressor. Critically or severely ill patients were defined as those patients who were admitted to the ICU or required respiratory support. Surviving patients were defined as those discharged from the ICU or hospital or who remained hospitalized. Non-surviving patients were defined as those who died in the ICU or hospital. Immunoregulation therapy includes corticosteroids, interferon, and intravenous immunoglobulin G.
Search of Studies
Two reviewers (ZQ and SL) carried out the search independently in the following six electronic databases from their inception to May 15, 2021: MEDLINE (via PubMed), the Cochrane library, Web of Science, China Biology Medicine disc, China National Knowledge Infrastructure, and Wanfang Data. The main terms were “mortality,” “critically ill patient,” “severely ill patient,” “novel coronavirus,” “2019-novel coronavirus,” “Novel CoV,” “SARS-CoV-2,” “COVID-19,” “2019-CoV,” “invasive mechanical ventilation,” “high flow nasal cannula,” “non-invasive ventilation,” “extracorporeal membrane oxygenation,” “renal replacement therapy,” “kidney replacement therapy,” “vasopressor,” and so on (the details of the search strategy can be found in Supplementary File 1). Moreover, we also searched the clinical trial registry platforms, the Google Scholar, the reference lists of the identified reviews, and the preprint platforms [including SSRN (https://www.ssrn.com/index.cfm/en/), medRxiv (https://www.medrxiv.org/), and bioRxiv (https://www.biorxiv.org/)] for further potential studies.
Selection of Studies
After eliminating duplicates by using EndNote X9.3.2 software, two reviewers independently screened all titles and abstracts first, and then the full texts of potentially relevant articles were reviewed to identify the final inclusion. Discrepancies were settled by discussion or consultation with a third reviewer. All reasons for exclusion of ineligible studies were recorded, and the process of study selection was documented using a PRISMA flow diagram (11).
Data Extraction
Two reviewers (ZQ and SL) extracted data independently with a standard data collection form. Any disagreements were resolved by consensus, and a third reviewer (XL) checked the consistency and accuracy of all data. The following data and information were extracted for each included study: basic information (title, first author, publication year, funding, and study design), information on the participants (sample size, age, and inclusion/exclusion criteria of participants), details of the intervention and control conditions, outcome information [for dichotomous data, we abstracted the number of events and total participants per group; for continuous data, we abstracted the means, standard deviations (SD), and number of total participants per group].
Risk of Bias in Individual Studies
Two reviewers (ZQ and SL) assessed the potential risk of bias of each included study independently. Discrepancies were resolved by discussion and consensus with a third researcher (XL). We assessed the risk of bias in cohort studies using Newcastle–Ottawa Scale (12), which contains eight domains: representativeness of exposure cohorts, selection of non-exposure cohorts, determination of exposure, outcome events that did not occur before study initiation, comparability of cohort based on design or analysis, assessment of outcome events, adequacy of follow-up time, and completeness of follow-up. For case series, we used the Joanna Briggs Institute critical appraisal checklist for case series (13), which consists of 10 domains. Each domain was graded as one sore if reported.
Statistical Analysis
All statistical analyses were performed using RStudio, version 1.3.1056. Comparable data from studies with one outcome were pooled using forest plots according to the Cochrane Handbook by using random-effects model separately (14). Mortality in the ICU and in hospital was used for a detailed description. A subgroup analysis was performed according to different regions. For dichotomous outcomes, we calculated the risk ratios (RR) and the corresponding 95% confidence intervals (CI) and P-values. For continuous outcomes, we calculated the standardized mean difference and its corresponding 95% CI if means and SD were reported. Furthermore, 95% prediction interval (PI) was used to evaluate the range that, we assert with 95% certainty, will fall into during a future validation test. We reported the effect size with 95% CI by using random-effects models. Two-sided P < 0.05 were considered statistically significant. Heterogeneity was defined as P < 0.10 and I2 >50%.When effect sizes could not be pooled due to only one study for a comparison, we reported the study findings narratively. We used sensitivity analyses to evaluate the stability of mortality outcomes of the included studies. For a result that included more than 10 studies, publication bias was tested by visual funnel plots.
Quality of the Evidence
The quality of evidence for each outcome was assessed by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The judgments of quality for specific outcomes were based on five main factors: study design and execution limitations, inconsistency, indirectness, imprecision of results (random-effects model), and publication bias across all studies (15, 16). The quality of evidence for each outcome was graded as high, moderate, low, or very low (17) and presented in “GRADE Evidence Profiles” (18).
Results
Search Results
The literature search retrieved 9,362 records through database searching and 51 additional records through other sources, which included 36 from the Google Scholar and 15 from preprint platforms. After removing duplicates, we screened the titles and abstracts of 5,138 records and reviewed the full text of 101 articles. Finally, we included 33 studies (cohort studies and case–series) (19–51) that reported either the mortality of critically ill patients or the clinical interventions between surviving and non-surviving patients with COVID-19 (Figure 1). All of them were published in English.
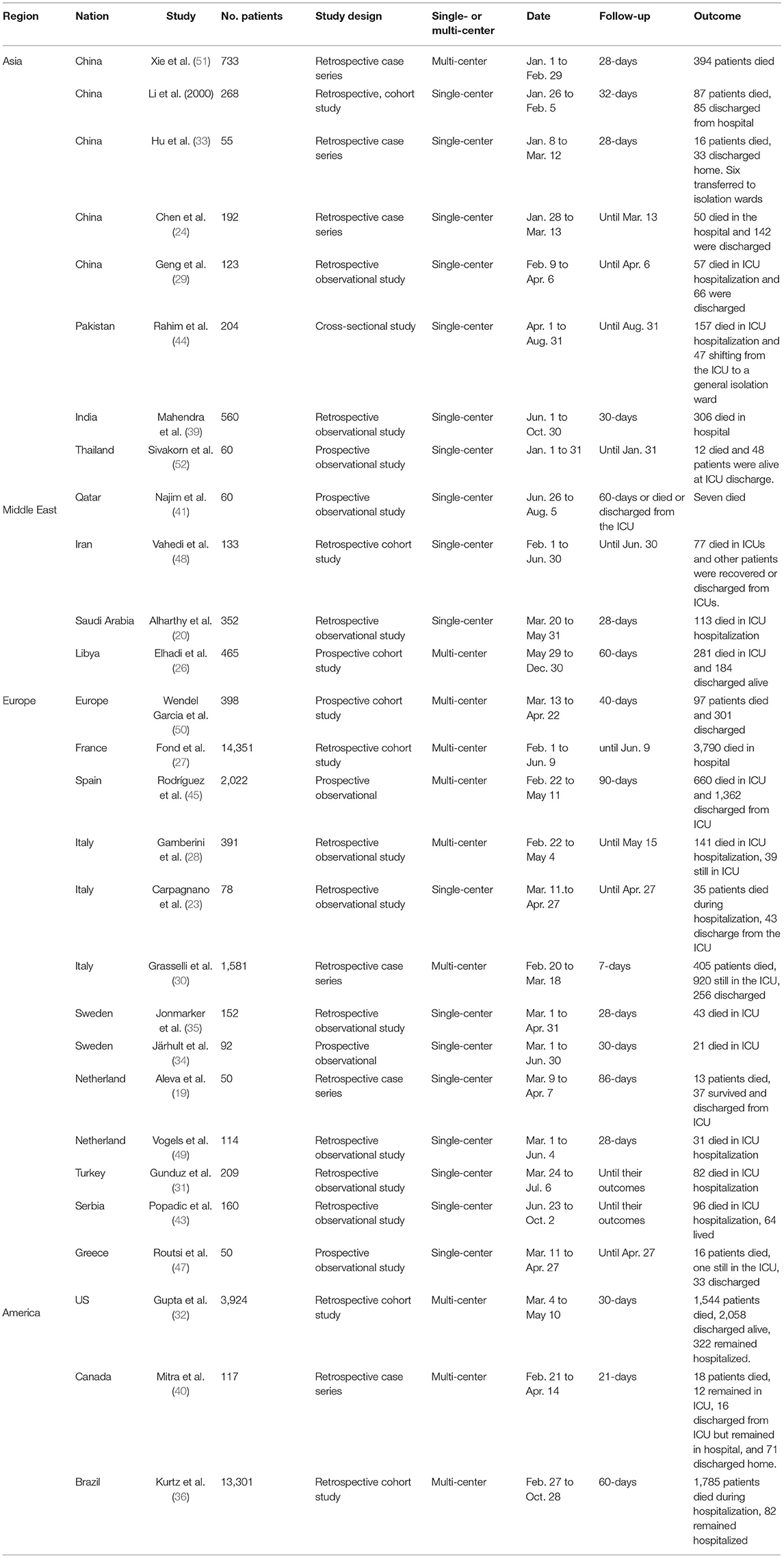
The Characteristics of the Included Studies
The basic characteristics of the included studies of the mortality of critically ill patients are summarized in Table 1 (Supplementary File 2). These 28 studies involving 40,195 participants were admitted between January 1 and December 30, 2020, which covered Asia, Europe, and America. Of the 28 studies, 19 were single-center studies and nine were multi-center studies in design. Mortality was demonstrated and concluded with a follow-up of more than 7 days and expressed as mortality in the ICU or in hospital. Among 33 studies, 17 studies (22, 25, 50, 51) with 6,414 participants compared clinical interventions between surviving and non-surviving patients. All studies assessed the risk of bias with scores of 3–9, indicating low to high quality (Supplementary File 3). A visual analysis of the funnel plot indicated that no publication bias was suspected in the results of age and mortality in the ICU. The results of IMV, PaO2/FiO2 ratio, and SOFA source were suggestive of publication bias (Supplementary File 4).
Clinical Outcome of Critically Ill Patients
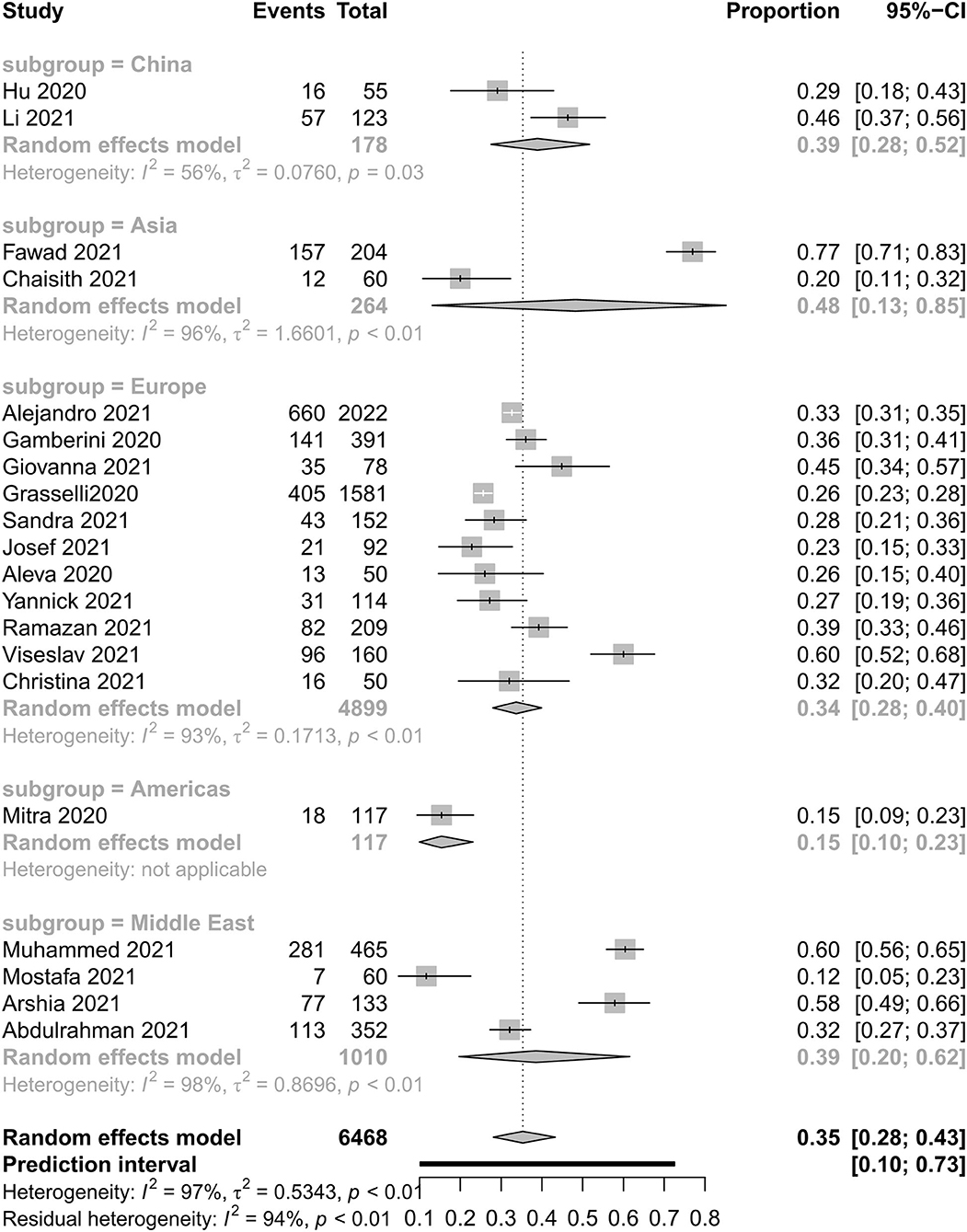
Figures 2, 3 show all-cause mortality in the ICU and in hospital as per peer-reviewed studies from countries around the world. In the present study, all-cause mortality in the ICU was 35% in critically ill patients (95% PI, 10–73%) with very high between-study heterogeneity. In a subgroup analysis, the mortality in China was 39%, in Asia—except for China—it was 48%, in Europe it was 34%, in America it was 15%, and in the Middle East it was 39%. For mortality in hospital, all-cause mortality was 32% (95% PI, 8–72%) with very high between-study heterogeneity. In a subgroup analysis, the mortality in China was 37%, in Asia—except for China—it was 55%, in Europe it was 26%, and in America it was 24%.
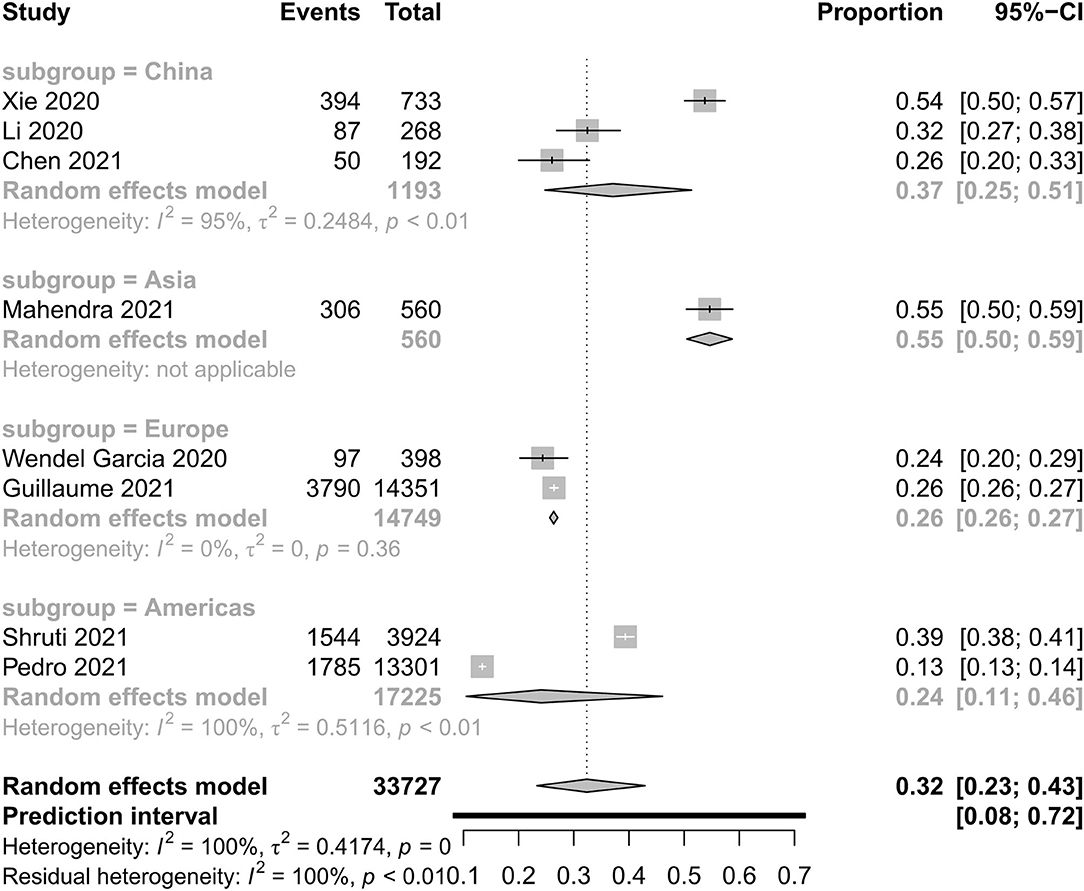
Basic Clinical Characteristics Between Two Different Outcome Groups
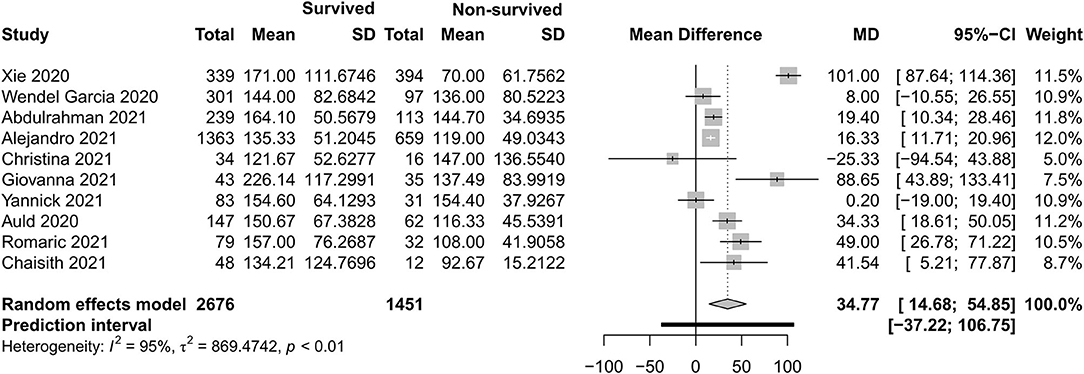
Figures 4–7 show the basic clinical characteristics including age, acute physiological and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA) score, and PaO2/FiO2 ratio between surviving and non-surviving patients. Patients who did not survive had an older age [−8.10, 95% CI (−9.31 to −6.90)], a higher APACHE II score [−4.90, 95% CI (−6.54 to −3.27)], a higher SOFA score [−2.27, 95% CI (−2.95 to −1.59)], and a lower PaO2/FiO2 ratio [34.77, 95% CI (14.68 to 54.85)] than those who survived.

Figure 5. Mean difference of Acute Physiological and Chronic Health Evaluation II score between survived and non-survived patients.

Figure 6. Mean difference of Sequential Organ Failure Assessment score between survived and non-survived patients.
Respiratory Support Care
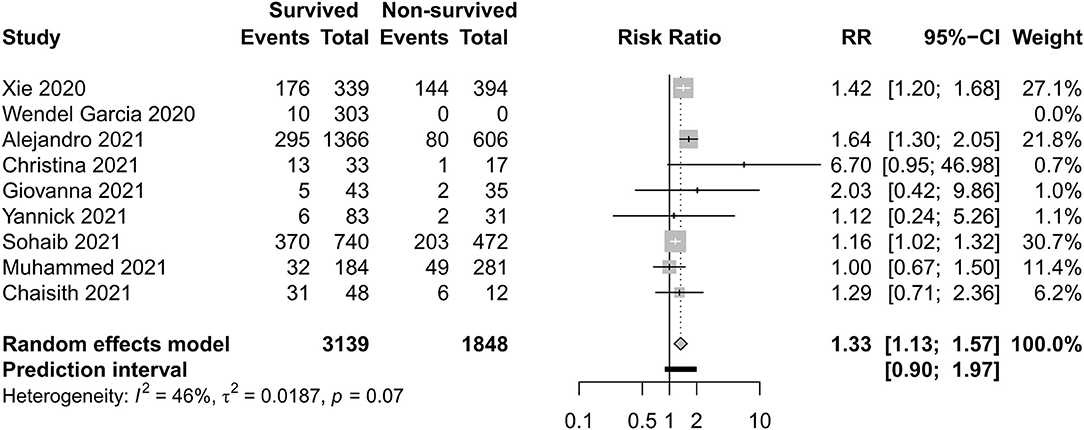
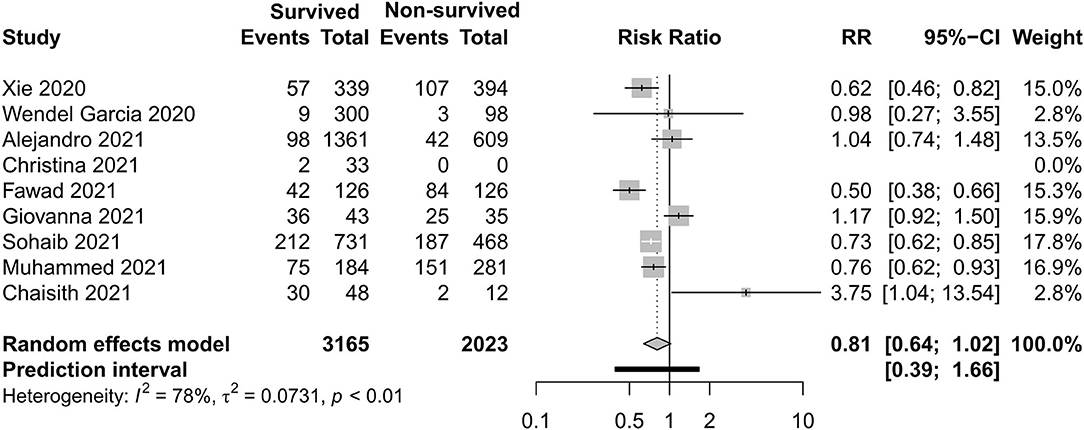
Figures 8–11 display different ways of respiratory support care during ICU hospitalization between surviving and non-surviving patients. High-flow nasal oxygenation (HFNO) was more commonly used in non-surviving patients [with RR 1.33, 95% CI (1.13–1.57)], and IMV was more commonly used in surviving patients [with RR 0.49, 95% CI (0.39–0.61)]. There was no statistically significant difference in NIV [RR 0.81, 95% CI (0.64–1.02)] and ECMO [RR 0.78, 95% CI (0.49–1.22)] between the two groups.
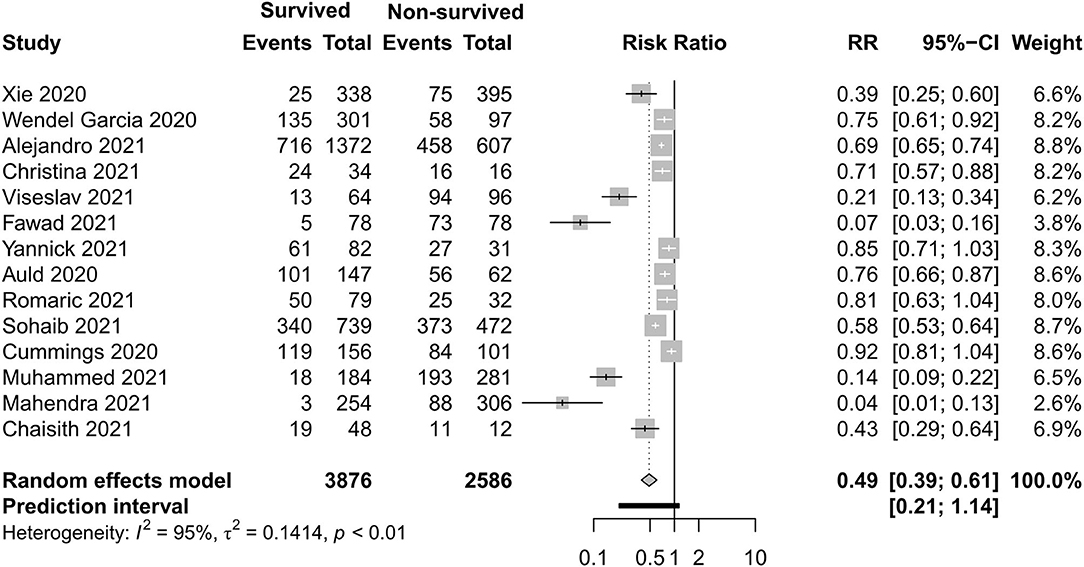
Figure 10. Risk ratio of invasive mechanical ventilation between survived and non-survived patients.
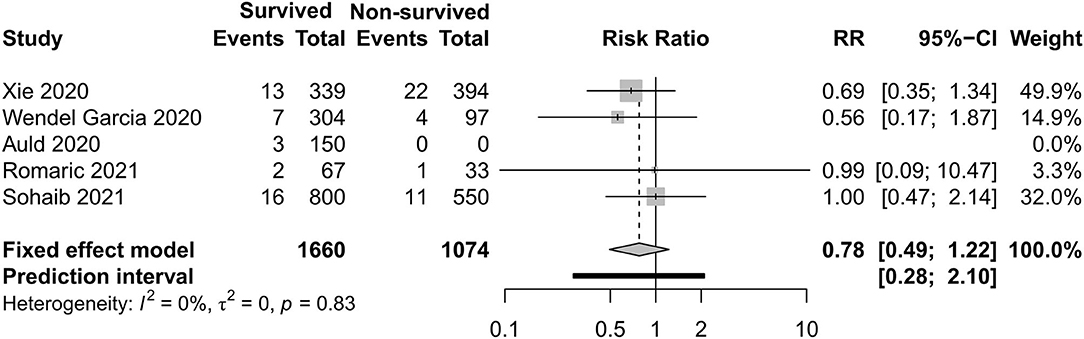
Figure 11. Risk ratio of extracorporeal membrane oxygenation between survived and non-survived patients.
Renal and Cardiac Support Care
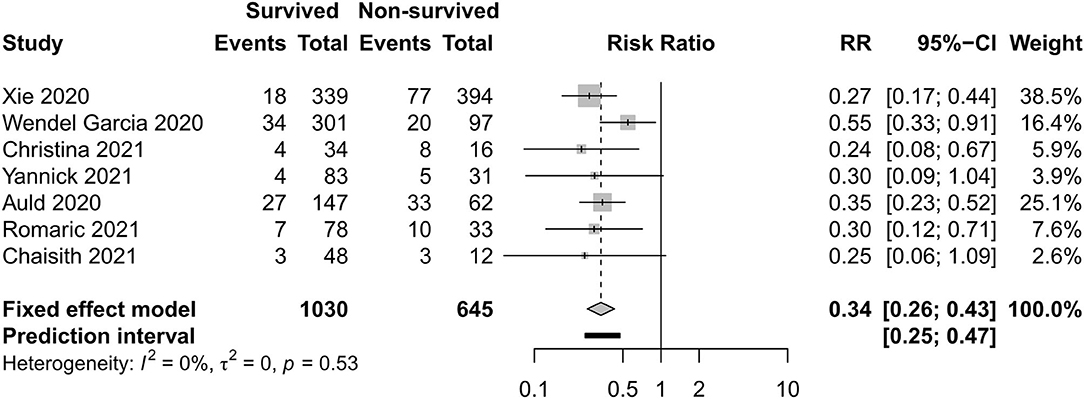
Figures 12, 13 exhibit the surviving patients who received more KRT [RR 0.34, 95% CI (0.26–0.43)] and vasopressor [RR 0.54, 95% CI (0.34–0.88)].
Quality of Evidence
We evaluated the quality of evidence for 11 outcomes. Among them, two outcomes (18%) were graded as of moderate quality, four outcomes (36%) were graded as of low quality, and five (45%) outcomes were graded as of very low quality. We produced “GRADE evidence profiles,” and the details of GRADE can be found in Supplementary File 5.
Sensitivity Analysis
We conducted a sensitivity analysis on each result by omitting one study at a time. No study had a significant impact on the results of the meta-analysis (Supplementary File 6). A sensitivity analysis showed that all studies had little or acceptable effect on the total combined effect and that the results were stable.
Discussion
The epidemic of COVID-19 is not stopping yet, especially in western countries. In previous reports, the incidence of mortality associated with critically ill patients remains poorly characterized. The novel findings in this study include the mortality of critically ill patients with laboratory-confirmed COVID-19 worldwide and the clinical interventions between surviving and non-surviving patients. The results show that all-cause mortality in ICU was 35% and mortality in hospital was 32% around the world for the year 2020. Differences were distinct between regions. The incidence of mortality that occurred in Southeast Asia was as high as 48%, followed by 39% in China and the Middle East. The lowest incidence occurred in America, which is 15%. The plausible explanations for the high mortality in China and other Asia countries are that the arrival and peak of the COVID-19 pandemic in Asia were earlier than in any region, and there was a shortage of ICU resources and experience. Moreover, data may be subject to patient selection for ICU admission, and some nations adopted a stringent strategy (19). In addition, mortality also relates to the time of follow-up. Some of the participants remained in the hospital in mechanical ventilation even at the end of follow-up. A recent meta-analysis reported that all-cause mortality associated with COVID-19 was 10% overall and 34% in patients admitted to the ICU (7), but most of their participants were from China; in this part, we had a close result. This new meta-analysis included more participants and covered much wider regions.
Early identification and prompt organ function support care would provide relief in critical cases (53). Among the included studies, five identified independent risk factors were associated with ICU mortality from laboratory parameters to clinical intervention, but the results are not the same (22, 25, 38, 50, 51, 54). We compared the baseline clinical characteristics between surviving and non-surviving patients. What we found based on the univariate analysis was that old age, APACHEII score, and SOFA score displayed consistency with multivariate Cox regression analysis in these five studies. Besides these, the PaO2/FiO2 ratio is an important index to reflect the severity of respiratory failure. Our results also showed that the PaO2/FiO2 ratio is helpful to predict the outcome.
With regard to the outcome of the clinical interventions of this meta-analysis, respiratory support is the most important part of life sustaining treatments. According to this study, HFNO during ICU hospitalization was more often used in non-surviving patients, and IMV was more often used in surviving patients. In previous studies, Auld and Capone (22, 54) reported that receipt of IMV was associated with a decreased likelihood of survival. When we discuss the difference of respiratory support, respiratory support as rescue therapy and the different severity levels of the two groups should not be ignored. HFNO and NIV can be safely used in COVID-19-related mild–moderate ARDS. In the study of non-COVID-19, HFNO has been associated with lower mortality in hypoxemic respiratory failure (55), but in some moderate–severe ARDS patients, HFNO or NIV should be used cautiously due to rapid progression to severe type and a high risk of treatment failure. According to Mukhtar et al. (56), the use of NIV with a predefined algorithm in subjects with moderate–severe COVID-19 ARDS was successful in 77% of the subjects. IMV is the most widely used therapy of severe hypoxemia. The population with IMV was larger than with non-invasive support in this study. The need of endotracheal intubation and invasive mechanical ventilation was eight times that of non-invasive ventilation in a previous study (30). Although the timing of IMV is disputed, as evidenced in a recent publication, a meta-analysis reported that early intubation was not associated with improved survival (57). A latest meta-analysis (42) reported that the timing of intubation may have not influenced the mortality of critically ill patients with COVID-19. ECMO can be taken into consideration if the respiratory dysfunction of patients develop into severe ARDS, which cannot sustain with IMV, but this salvage treatment did not have a statistically significant difference between the two groups. In a study with a small sample (3), two of five patients survived by the support of ECMO. The appropriate time and eligible patients need to be evaluated.
In a previous research, as high as 31% of patients in a cohort developed severe acute kidney injury requiring renal replacement therapy during hospitalization (25). High creatinine level, AKI, and receipt of RRT were independent risk factors for the in-hospital mortality of patients (22, 51, 58). Similarly, high high-sensitivity cardiac troponin I level, ischemic heart disease, cardiac injury, and vasopressor support were associated with death in patients (22, 38, 50, 51, 54). In the present study, the result shows that vasopressors and RRT were more often used in the surviving group.
There were some limitations in the current study that must be acknowledged. First is the high level of heterogeneity in the study. Plausible explanations for the heterogeneous risks of mortality include differences in age, nation and race, disease severity, and insufficient length of follow-up. It was difficult for us to control for the effects of these confounding factors. The heterogeneity in the component studies was addressed with random-effects models. Second, as for the secondary outcomes, is that this part of the clinical interventions was derived from an observational cohort, not a randomized controlled trial, so these results should be treated cautiously. The key purpose of this study is to describe the effect of the actual use of various clinical interventions in the surviving group and non-surviving group rather than the impact of individual measures on the prognosis. Third is that most studies were retrospective and recall bias might have occurred.
Conclusions
Mortality was high in critically ill patients with COVID-19 based on low-quality evidence, and intercontinental differences existed. The early identification of critical characteristics and the use of support care help to indicate the outcome of critically ill patients.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
LL, YC, and ZQ: concept and design. LL: administrative, technical, or material support. ZQ and SL: statistical analysis and drafting of the manuscript. LL and YC: supervision. All authors critical revision of the manuscript for important intellectual content, acquisition, analysis, or interpretation of data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.635560/full#supplementary-material
References
1. WHO Coronavirus Disease (COVID19) Dashboard. Available online at: https://covid19.who.int (accessed September 15, 2020).
2. Franco C, Facciolongo N, Tonelli R, Dongilli R, Vianello A, Pisani L, et al. Feasibility and clinical impact of out-of-ICU non-invasive respiratory support in patients with COVID-19 related pneumonia. Europ Resp J. (2020) 56:2002130. doi: 10.1183/13993003.02130-2020
3. Xuan W, Chen C, Jiang X, Zhang X, Zhu H, Zhang S, et al. Clinical characteristics and outcomes of five critical COVID-19 patients treated with extracorporeal membrane oxygenation in Leishenshan Hospital in Wuhan. J Clin Anesth. (2020) 67:110033. doi: 10.1016/j.jclinane.2020.110033
4. Zhang Q, Shen J, Chen L, Li S, Zhang W, Jiang C, et al. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. (2020) 89:1092–8. doi: 10.1097/TA.0000000000002939
5. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Card. (2020) 76:533–46. doi: 10.1016/j.jacc.2020.06.007
6. Fu EL, Janse RJ, de Jong Y, van der Endt VHW, Milders J, van der Willik EM, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kid J. (2020) 13:550–63. doi: 10.1093/ckj/sfaa160
7. Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. (2020) 24:389. doi: 10.1186/s13054-020-03022-1
8. Tacconelli E. CRD's guidance for undertaking reviews in health care. Lancet Infect Dis. (2010) 10:226. doi: 10.1016/S1473-3099(10)70065-7
9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Inter Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
10. WHO. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance. (2020). (accessed March 30 2020).
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidem. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Europ J Epidem. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
13. Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI. (2019) 18:2127–33. doi: 10.11124/JBISRIR-D-19-00099
14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
15. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
16. Schunemann H BJ, Guyatt G. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendation (2010). Available online at: https://gradepro.org/
17. Norris SL, Meerpohl JJ, Akl EA, Schünemann HJ, Gartlehner G, Chen Y, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidem. (2016) 79:150–8. doi: 10.1016/j.jclinepi.2016.07.001
18. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epid. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
19. Aleva FE, van Mourik L, Broeders M, Paling AJ, de Jager CPC. COVID-19 in critically ill patients in North Brabant, the Netherlands: patient characteristics and outcomes. J Crit Care. (2020) 60:111–5. doi: 10.1016/j.jcrc.2020.08.001
20. Alharthy A, Aletreby W, Faqihi F, Balhamar A, Alaklobi F, Alanezi K, et al. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidem Global Health. (2021) 11:98–104. doi: 10.2991/jegh.k.200928.001
21. Alser O, Mokhtari A, Naar L, Langeveld K, Breen KA, El Moheb M, et al. Multisystem outcomes and predictors of mortality in critically ill patients with COVID-19: demographics and disease acuity matter more than comorbidities or treatment modalities. J Trauma Acute Care Surg. (2021) 90:880–90. doi: 10.1097/TA.0000000000003085
22. Auld S, Caridi-Scheible M, Blum JM, Robichaux CJ, Kraft CS, Jacob JT, et al. ICU and ventilator mortality among critically ill adults with COVID-19. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.23.20076737
23. Carpagnano GE, Buonamico E, Migliore G, Resta E, Di Lecce V, de Candia ML, et al. Bilevel and continuous positive airway pressure and factors linked to all-cause mortality in COVID-19 patients in an intermediate respiratory intensive care unit in Italy. Expert Rev Resp Med. (2020) 15:835–7. doi: 10.1080/17476348.2021.1866546
24. Chen Y, Linli Z, Lei Y, Yang Y, Liu Z, Xia Y, et al. Risk factors for mortality in critically ill patients with COVID-19 in Huanggang, China: a single-center multivariate pattern analysis. J Med Virol. (2021) 93:2046–55. doi: 10.1002/jmv.26572
25. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
26. Elhadi M, Alsoufi A, Abusalama A, Alkaseek A, Abdeewi S, Yahya M, et al. Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: a prospective multi-center cohort study. PLoS ONE. (2021) 16:e0251085. doi: 10.1371/journal.pone.0251085
27. Fond G, Pauly V, Leone M, Llorca PM, Orleans V, Loundou A, et al. Disparities in intensive care unit admission and mortality among patients with schizophrenia and COVID-19: a national cohort study. Schizoph Bull. (2021) 47:624–634. doi: 10.1093/schbul/sbaa158
28. Gamberini L, Tonetti T, Spadaro S, Zani G, Mazzoli CA, Capozzi C, et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care. (2020) 8:80–92. doi: 10.1186/s40560-020-00514-8
29. Geng L, He C, Kan H, Zhang K, Mao A, Zhang C, et al. The association between blood pressure levels and mortality in critically ill patients with COVID-19 in Wuhan, China: a case-series report. Hypert Res. (2021) 44:368–370. doi: 10.1038/s41440-020-00594-x
30. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591. Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
31. Gunduz R, Yildiz BS, Ozdemir IH, Cetin N, Ozen MB, Bakir EO, et al. CHA2DS2-VASc score and modified CHA2DS2-VASc score can predict mortality and intensive care unit hospitalization in COVID-19 patients. J Thromb Thromb. (2021) 2021:1–11. doi: 10.1007/s11239-021-02427-1
32. Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Internal Med. (2021) 181:41–51. doi: 10.1001/jamainternmed.2020.6252
33. Hu HT, Xu S, Wang J, Rao X. Respiratory support in severely or critically ill ICU patients with COVID-19 in Wuhan, China. Curr Med Sci. (2020) 40:636–41. doi: 10.1007/s11596-020-2227-8
34. Järhult JD, Hultström M, Bergqvist A, Frithiof R, Lipcsey M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Scient Rep. (2021) 11:7163. doi: 10.1038/s41598-021-86500-y
35. Jonmarker S, Hollenberg J, Dahlberg M, Stackelberg O, Litorell J, Everhov Å H, et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Criti Care. (2020) 24:653. doi: 10.1186/s13054-020-03375-7
36. Kurtz P, Bastos LSL, Dantas LF, Zampieri FG, Soares M, Hamacher S, et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intens Care Med. (2021) 47:538–548. doi: 10.1007/s00134-021-06388-0
37. Larcher R, Besnard N, Akouz A, Rabier E, Teule L, Vandercamere T, et al. Admission high-sensitive cardiac troponin t level increase is independently associated with higher mortality in critically ill patients with COVID-19: a multicenter study. J Clin Med. (2021) 10:81656. doi: 10.3390/jcm10081656
38. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. (2020) 146:110–8. doi: 10.1016/j.jaci.2020.04.006
39. Mahendra M, Nuchin A, Kumar R, Shreedhar S, Mahesh PA. Predictors of mortality in patients with severe COVID-19 pneumonia - a retrospective study. Adv Resp Med. (2021) 89:135–44. doi: 10.5603/ARM.a2021.0036
40. Mitra AR, Fergusson NA, Lloyd-Smith E, Wormsbecker A, Foster D, Karpov A, et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ. (2020) 192:E694–e701. doi: 10.1503/cmaj.200794
41. Najim M, Rahhal A, Khir F, Aljundi AH, Abu Yousef S, Ibrahim F, et al. Prevalence and clinical significance of antiphospholipid antibodies in patients with coronavirus disease 2019 admitted to intensive care units: a prospective observational study. Rheumatol Int. (2021) 41:1243–52. doi: 10.1007/s00296-021-04875-7
42. Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, et al. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. (2021) 25:121. doi: 10.1186/s13054-021-03540-6
43. Popadic V, Klasnja S, Milic N, Rajovic N, Aleksic A, Milenkovic M, et al. Predictors of mortality in critically ill COVID-19 patients demanding high oxygen flow: a thin line between inflammation, cytokine storm, and coagulopathy. Oxidat Med Cell Long. (2021) 2021:6648199. doi: 10.1155/2021/6648199
44. Rahim F, Amin S, Noor M, Bahadur S, Gul H, Mahmood A, et al. Mortality of patients with severe COVID-19 in the intensive care unit: an observational study from a major COVID-19 receiving hospital. Cureus. (2020) 12:e10906. doi: 10.7759/cureus.10906
45. Rodríguez A, Ruiz-Botella M, Martín-Loeches I, Jimenez Herrera M, Solé-Violan J, Gómez J, et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit Care. (2021) 25:63. doi: 10.1186/s13054-021-03487-8
46. Roomi S, Shah SO, Ullah W, Abedin SU, Butler K, Schiers K, et al. Declining intensive care unit mortality of COVID-19: a multi-center study. J Clin Med Res. (2021) 13:184–90. doi: 10.14740/jocmr4452
47. Routsi C, Magira E, Kokkoris S, Siembos I, Vrettou C, Zervakis D, et al. Hospital resources may be an important aspect of mortality rate among critically ill patients with COVID-19: the paradigm of Greece. J Clin Med. (2020) 9:730. doi: 10.3390/jcm9113730
48. Vahedi A, Tabasi F, Monjazebi F, Hashemian SMR, Tabarsi P, Farzanegan B, et al. Clinical features and outcomes of ICU patients with COVID-19 Infection in Tehran, Iran: a single-centered retrospective cohort study. Tanaffos. (2020) 19:300–11.
49. Vogels Y, Pouwels S, van Oers J, Ramnarain D. Characteristics and risk factors associated with mortality in critically ill patients with COVID-19. Cureus. (2021) 13:e14442. doi: 10.7759/cureus.14442
50. Wendel Garcia PD, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, Roche-Campo F, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. (2020) 25:100449. doi: 10.1016/j.eclinm.2020.100449
51. Xie J, Wu W, Li S, Hu Y, Hu M, Li J, et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Inten Care Med. (2020) 2020:1–10. doi: 10.1007/s00134-020-06211-2
52. Sivakorn C, Dechsanga J, Jamjumrus L, Boonnak K, Schultz MJ, Dorndorp AM, et al. High Mobility Group Box 1 and Interleukin 6 at Intensive Care Unit Admission as Biomarkers in Critically Ill COVID-19 Patients. Am J Trop Med Hygiene. (2021) 2021:tpmd210165. doi: 10.4269/ajtmh.21-0165
53. Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intens Care Med. (2020) 46:357–60. doi: 10.1007/s00134-020-05954-2
54. Capone S, Abramyan S, Ross B, Rosenberg J, Zeibeq J, Vasudevan V, et al. Characterization of critically ill COVID-19 patients at a brooklyn safety-net hospital. Cureus. (2020) 12:e9809. doi: 10.7759/cureus.9809
55. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. (2015) 372:2185–96. doi: 10.1056/NEJMoa1503326
56. Mukhtar A, Lotfy A, Hasanin A, El-Hefnawy I, El Adawy A. Outcome of non-invasive ventilation in COVID-19 critically ill patients: a retrospective observational Study. Anaesth Crit Care Pain Med. (2020) 9:579–80. doi: 10.1016/j.accpm.2020.07.012
57. Lee YH, Choi KJ, Choi SH, Lee SY, Kim KC, Kim EJ, et al. Clinical significance of timing of intubation in critically ill patients with COVID-19: a multi-center retrospective study. J Clin Med. (2020) 9:92847. doi: 10.3390/jcm9092847
Keywords: mortality, critically ill patients, COVID-19, clinical interventions, supportive care
Citation: Qian Z, Lu S, Luo X, Chen Y and Liu L (2021) Mortality and Clinical Interventions in Critically ill Patient With Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Front. Med. 8:635560. doi: 10.3389/fmed.2021.635560
Received: 30 November 2020; Accepted: 10 June 2021;
Published: 23 July 2021.
Edited by:
Xiangdong Chen, Union Hospital of Tongji Medical College of Huazhong University of Science and Technology, ChinaReviewed by:
Nanyang Liu, China Academy of Chinese Medical Sciences, ChinaChenyu Sun, AMITA Health St Joseph Hospital, United States
Copyright © 2021 Qian, Lu, Luo, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaolong Chen, Y2hlbnlhb2xvbmdAbHp1LmVkdS5jbg==; Ling Liu, bGl1bGluZ2RvY3RvckAxMjYuY29t
†These authors have contributed equally to this work
 Zhicheng Qian1,2†
Zhicheng Qian1,2† Xufei Luo
Xufei Luo Yaolong Chen
Yaolong Chen