- 1A. Cardiothoracic Department, Respiratory and Critical Care Unit Bari Policlinic University Hospital, B. Section of Respiratory Diseases, Department of Basic Medical Science Neuroscience and Sense Organs, University of Bari ‘Aldo Moro’, Bari, Italy, University of Bari, Bari, Italy
- 2Istituti Clinici Scientifici Maugeri Istituto di Ricovero e Cura a Carattere Scientifico Pneumologia Riabilitativa, Istituto di Montescano, Montescano, Italy
- 3Department of Emergency and Organ Transplantation, University of Bari, Bari, Italy
Background: The COVID-19 pandemic has led to new approaches to manage patients outside the ICU, including prone positioning in non-intubated patients.
Objectives: To report the use of prolonged active prone positioning in spontaneously breathing patients with COVID-19-associated acute respiratory failure. Spontaneously breathing vs non-invasive respiratory support for COVID19 associated acute respiratory failure.
Methods: Patients with PaO2/FiO2 > 150, with lung posterior consolidations as assessed by means of lung ultrasound, and chest x-ray were studied. Under continuous pulse oximetry (SpO2) monitoring, patients maintained active prone position. A PaO2/FiO2 < 150 was considered as treatment failure and patients had to be switched to non-invasive respiratory support. Retrospectively, data of 16 patients undergoing who refused proning and underwent non-invasive respiratory support were used as controls. The primary outcome was the proportion of patients maintaining prolonged prone position and discharged home. Secondary outcomes included improvement in oxygenation, hospital length of stay, and 6-month survival.
Results: Three out of 16 (18.7%) patients did not tolerate the procedure. Three more patients showed a worsening in PaO2/FiO2 to <150 and required non-invasive support, two of whom finally needing endotracheal intubation. After 72 h, 10 out of 16 (62.5%) patients improved oxygenation [PaO2/FiO2: from 194.6 (42.1) to 304.7 (79.3.2) (p < 0.001)] and were discharged home. In the control group, three out of 16 failed, required invasive ventilatory support, and died within 1 month in ICU. Thirteen were successful and discharged home.
Conclusion: In non-intubated spontaneously breathing COVID-19 patients with PaO2/FiO2 >150, active prolonged prone positioning was feasible and tolerated with significant improvement in oxygenation.
Introduction
The Coronavirus disease 19 (COVID-19) pandemic has led to more than 1 million casualties worldwide (1). COVID-19 severely hit Italy with an increasingly number of patients admitted to hospitals with hypoxemic acute respiratory failure (ARF) needing intensive care unit (ICU) admission, often exceeding the availability of ICU beds. Due to ICU bed and ventilator shortage, critical care resources were saved to care for patients with ARF also outside the ICU (2, 3).
Mechanical ventilation in the prone position (PP) is a validated strategy in the treatment of acute respiratory distress syndrome (ARDS) (4) with several beneficial effects on pulmonary physiology. In supine position, pulmonary edema accumulates in basal regions, leading to increased air volume delivered to apical and anterior lung units, which are also the regions receiving less pulmonary circulation. Prone positioning leads to more homogeneous distribution of ventilation, thus decreasing the shunt fraction and improving matching of ventilation and perfusion (4). Recently, studies have reported improvement in oxygenation during PP in non-intubated awake patients with COVID-19 during spontaneous and non-invasively assisted breathing (5–8, 8–11). However, those were mainly physiologic studies of active PP for a few daily hours. We aimed to report the feasibility and the tolerability of prolonged active PP in non-intubated, spontaneously breathing patients with arterial oxygen tension to inspiratory oxygen fraction ratio (PaO2/FiO2) > 150 in a respiratory intensive care unit (RICU). Secondary outcomes were PaO2/FiO2 before and after the trial and during PP, the length of stay (LoS) in hospital, and 1- and 6-month survival rates.
Methods
This observational, prospective, single-center study was approved by the Bari Policlinico ethic committee (study number 6363), and written or verbal informed consent was obtained from all patients.
Patients
From March 11, to April 30, 2020, all consecutive patients with confirmed COVID-19 without indication for immediate non-invasive respiratory support (NRS) were considered eligible if they had (1) PaO2/FiO2 > 150 and (2) lung ultrasound (LUS) and chest x-ray signs of basal and posterior consolidations. Under continuous pulse oximetry (SpO2) and EKG monitoring and oxygen supplementation to maintain SpO2 > 96%, spontaneously breathing patients were asked to actively maintain PP (Study Group). Active PP had to be maintained as longest as possible with intervals for meals and other personal care.
Patients who refused to undergo PP were initiated to NRS either with high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), or non-invasive ventilation (NIV). These patients with the same clinical and physiological characteristics as the study group were retrospectively considered as controls.
One or more of the following drugs were administered: hydrosi-chloroquine, macrolides, steroids, anticoagulants, tocilizumab, N-acetyl-cysteine, vitamin D, and vitamin C (12), and the rest of patients' comorbidities treatment.
Measurements
Demographics, clinical characteristics, comorbidities, the sequential organ failure assessment (SOFA), and 1- and 6-month survival were recorded. Chest x-ray and LUS were assessed at admission and at discharge. The CT chest was not promptly available in the same location; therefore, considering the severity of the hypoxic ARF, it was not performed in all patients.
The study group underwent arterial blood gas analysis at the 6th, 24th, 48th, and 72nd hour during PP and in supine position before and after the trial. Controls underwent arterial blood gas analysis at the start of the NRS and at discharge from the RICU.
In patients of the study group, the ventilatory ratio (VR), an index of respiratory effort, was calculated according to Morales-Quinteros et al. (13).
Worsening in PaO2/FiO2 to <150 was considered as treatment failure. Patients in the PP group who dropped out or who were failing treatment were switched to NRS. Control patients failing NRS were transferred to the ICU for invasive ventilator support.
Procedure
The nurse-to-patient ratio in the RICU was 1:4 to 1: 5. Patients enrolled maintained PP with the Venturi Oxygen mask under non-invasive monitoring of EKG, SpO2, and arterial blood pressure. During PP, EKG patches were moved from the front to the back of the patients' chest. All healthcare providers wore personal protective equipment, whereas all patients were wearing a surgical mask on top of the Venturi mask (14, 15) (Figure 1).
Figure 1. Cartoon demonstrating continuous prone positioning with oxygen supplementation only via Venturi Mask demonstrated under continuous monitoring.
Control patients continuously performed NRS in semi-recumbent supine position, with only short intervals for eating and personal care. Patients on HFNC were wearing a surgical mask on top of nasal cannula, whereas those on CPAP and NIV used masks closed without expiratory holes and an antiviral filter was inserted between the mask and the circuit to filter all patients' exhaled air.
Statistical Analysis
Statistical analysis was performed using the R statistical environment (16). For each variable, Shapiro–Wilk test and graphical evaluations (Q–Q plots) were applied to assess the correspondence with the normal distribution. Descriptive statistics are shown as mean ± standard deviation (SD) and/or median and interquartile range [IQR] for normally distributed and non-normally distributed continuous variables, respectively, whereas categorical variables were indicated with absolute frequency (%). Student's t-test and Mann–Whitney U-test were performed to assess between group comparisons, as appropriate. In particular, to evaluate differences between PaO2/FiO2 at admission and discharge, a paired t-test was used. Differences for categorical variables between groups were assessed by a Pearson χ2 test and or Fisher's exact test. Then, multivariate ANOVA and ANCOVA analyses were performed to compare PaO2/FiO2 at discharge between the two experimental groups, namely, PaO2/FiO2 at admission as a covariate. p-values lower than 0.05 were regarded as significant. Plots and graphs were realized using the Excel graph tool.
Results
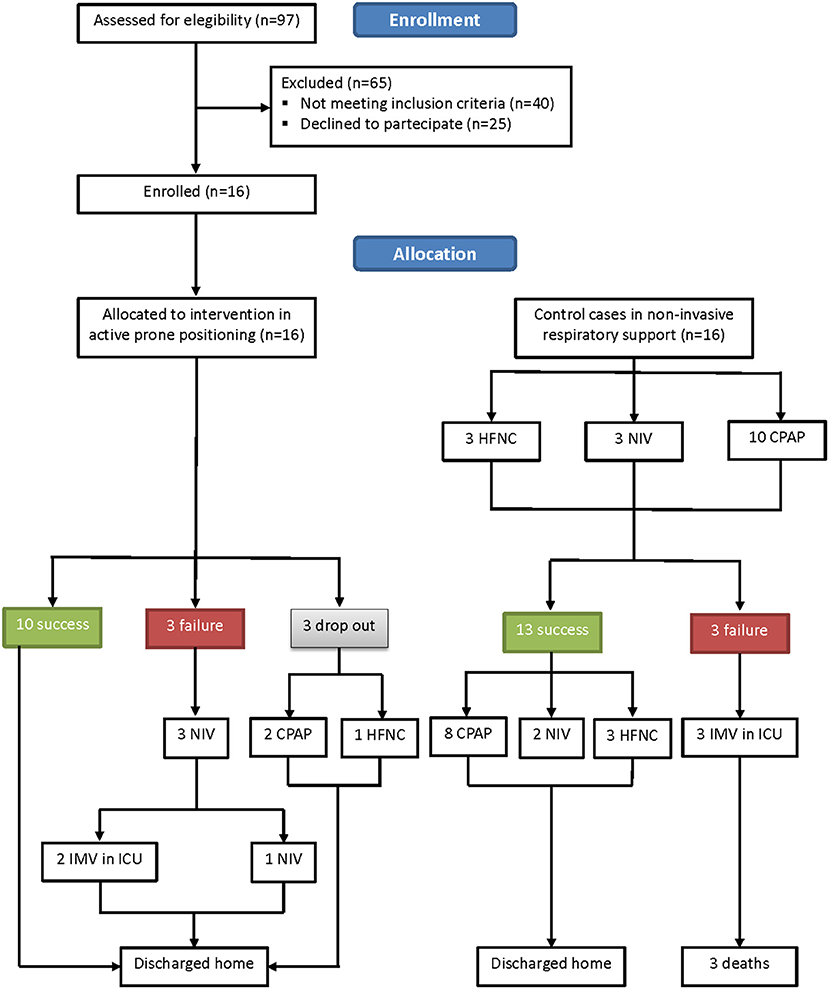
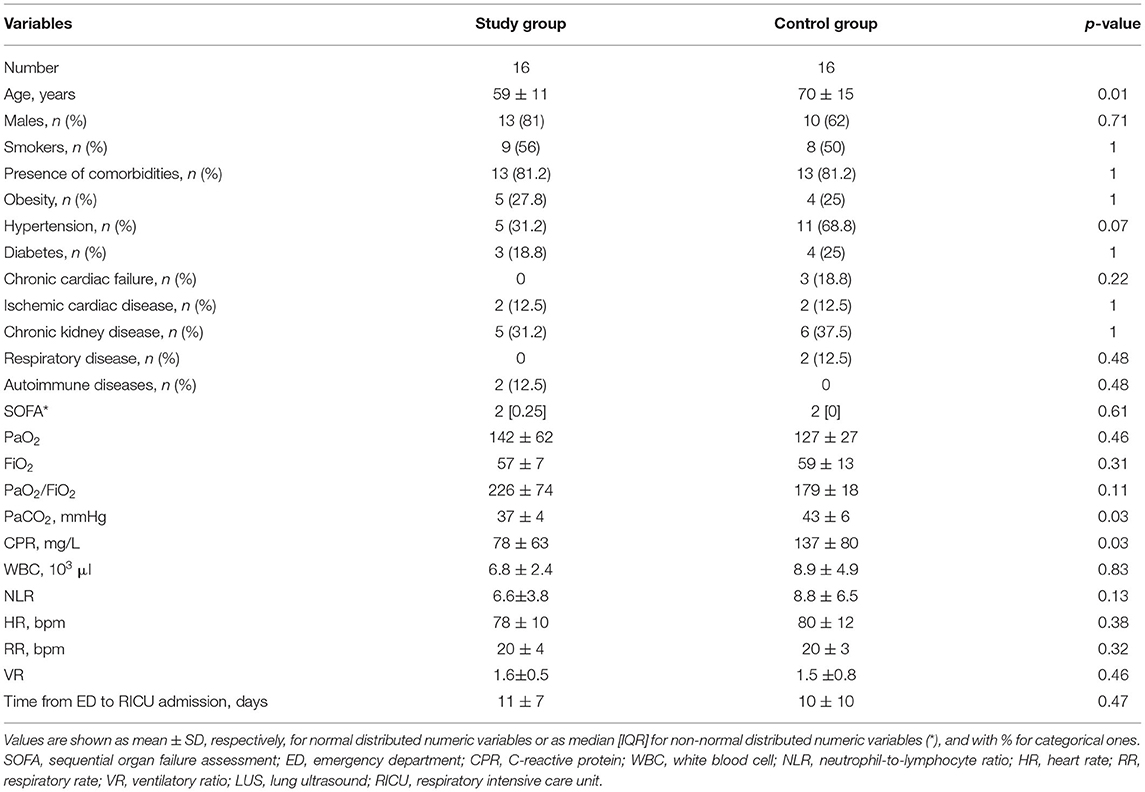
Sixteen patients showed eligible criteria and agreed to be enrolled in the study. The other 16 patients were controls. The flow diagram of the study is shown in Figure 2. The demographic, anthropometric, and clinical characteristics of patients at admission are shown in Table 1. The baseline characteristics were similar between the two groups, with the exception of age, CRP, and PaCO2, with the controls being older, being more hypercapnic, and having a higher CRP.
Three out of 16 (18.7%) patients did not tolerate PP for chronic osteoarticular pain related to knee and spine degeneration; therefore, they received oxygen therapy in supine position. However, within 24 h from the discontinuation of PP, they all experienced worsening of their gas exchange. In more detail, one of them was supported via HFNC with FiO2 0.75 and two were supported via helmet CPAP with FiO2 0.70. None of them required intubation (Figure 2). Three more patients showed worsening in PaO2/FiO2 to <150 and underwent NIV; two of them finally needed endotracheal intubation. Ten out of 16 patients of the study group showed a significant improvement in oxygenation [PaO2/FiO2 from 194.6 (42.1) before to 304.7 (79.32) after 72 h of PP via ANCOVA test (p < 0.001)]. Figure 3 shows the individual and mean PaO2/FiO2 values during the trial in the successful patients of study group. The mean PaO2/FiO2 before discharge from the RICU was 330 ± 89.1.
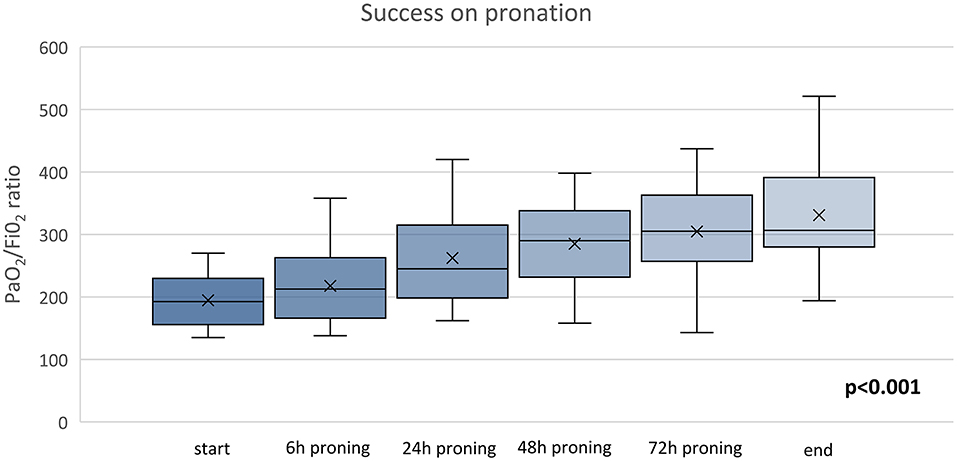
Figure 3. Time course of PaO2/FiO2 of PP group ANOVA, p = 0.001. The x refers to the average value and the continuous line refers to the median value.
As shown in Table 2, the baseline clinical characteristics of successful patients were not significantly different between groups with the exception of the PaCO2 being higher in controls. However, only two patients were affected by chronic respiratory failure in the control group before admission.
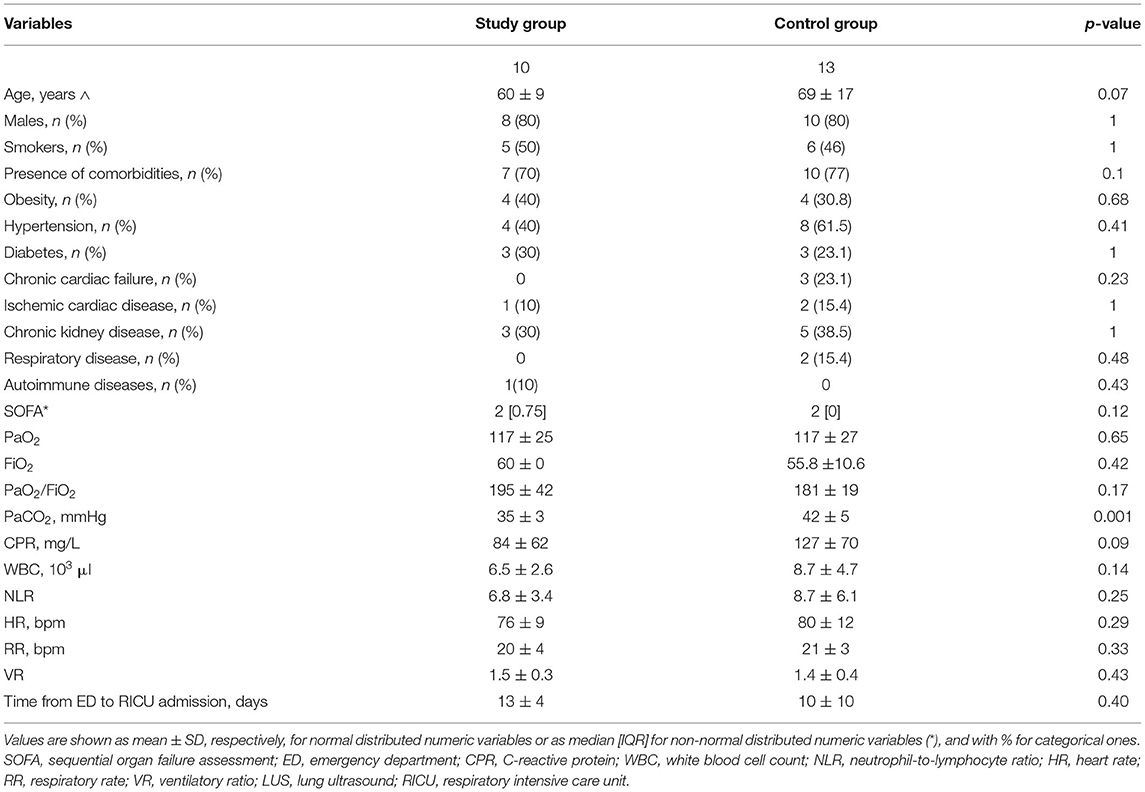
Table 2. Demographic, physiological, and clinical characteristics of successful patients at admission.
The baseline VR was not different between the two groups. The LUS scores of the 10 successful patients changed from 13.0 [IQR 6.0–16.5] before, to 5.0 [IQR 2.5–12.0] at the end of PP (p = 0.0364), indicating a significant improvement in lung consolidations. All 10 patients were discharged home after a mean LoS of 21.0 (7.0) days (Figure 2). Patients undergoing active PP <12 days from hospital admission had a significantly shorter LoS as compared to the others [17.0 (5.0) vs. 25.0 (6.0) days, p = 0.04]. No patient who had undergone PP had died at 1- and 6-month follow-up.
As also shown in Figures 2, 4 out of 16 controls showed a worsening in PaO2/FiO2 to <150, requiring admission to ICU and endotracheal intubation. All three patients died in ICU within 1 month. The 13 successful controls showed a significant improvement in oxygenation from admission to discharge [PaO2/FiO2 from 181.2 (19.1) at the start to 290.5 (81.4) at discharge from RICU (p < 0.001)]. There was no significant difference in discharge to admission changes in PaO2/FiO2 between the successful patients of the study group or control group (p = 0.3156).
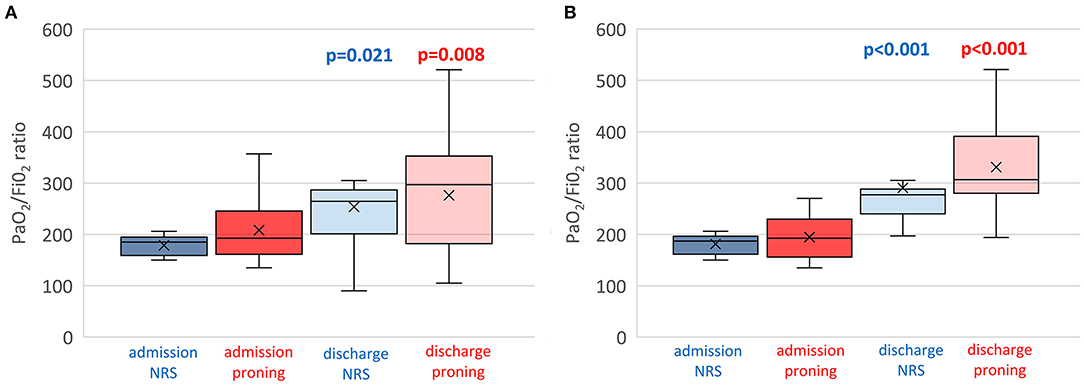
Figure 4. Individual (box plots) and time course of PaO2/FiO2 of both proning and control group on non-invasive respiratory support. (A) Total patients enrolled including dropouts and failures. (B) Only successful patients. Assessments at RICU admission and at RICU discharge. ANOVA, p = 0.001. The x refers to the average value and the continuous line refers to the median value.
Figure 4A shows the individual and mean PaO2/FiO2 values at admission and discharge of all patients and controls while Figure 4B shows only successful patients including study group and controls.
Only one healthcare provider suffered from COVID-19 infection during the study period.
Discussion
In this small single-center cohort study, we found that the use of PP in awake, spontaneously breathing patients with COVID-19-induced hypoxemic ARF with PaO2/FiO2 > 150 was feasible, tolerated for a prolonged time (three consecutive days), with similar results to patients undergoing NRS. The PP was associated with improved oxygenation and lung consolidations. Patients did not require ventilatory support in 62.5% of cases with good 1- and 6-month outcome.
Before the COVID-19 pandemic, there was limited published research on PP in non-intubated patients. The COVID-19 pandemic has led to a dramatic increase in patients requiring critical care, pushing clinicians to use new approaches to save resources for mechanical ventilation, including awake proning. A few physiological and clinical studies have evaluated the effects of PP in non-intubated patients with COVID-19 under NRS or spontaneously breathing (6–8, 8–10), and early helmet CPAP with moderate pressure and PP has been suggested (11).
This study enrolled unsupported breathing patients, and the active PP was tolerated all day long, with just short intervals for meals and personal care, for 3 consecutive days: to the best of our knowledge, this is the longest reported period: in literature, up to 8 daily hours of active PP are reported (9).
During the PP, the expansion of the anterior chest wall is limited, resulting in more homogeneous chest wall compliance and gravitational forces on lung parenchyma. This enables greater recruitment of the posterior zones, recruiting a larger proportion of alveoli clusters to participate in gas exchange. The diaphragm also greatly contributes to improve stress forces during PP, which may help to reduce lung injury during spontaneous breathing (17, 18). Indeed, the PP enhances the inferior movement of the diaphragm, which relieves compression on posterior lung zones that are usually atelectatic during supine positioning, thus improving their recruitment (19, 20).
The Ventilatory ratio (VR) is considered an index of respiratory effort, and it has been found to be independently associated with mortality in non-COVID-19 ARDS patients (21). In our patients, the mean VR was similar to survivors of non-COVID ARDS (13). Further studies will be required to confirm the VR ranges within COVID-19 patients.
Active PP may result in good physiological and clinical results, avoiding contacts and waste of human, economic, therapeutic resources and personal protective equipment (12, 14, 15). These patients were able to maintain active PP, and the only additional nursing workload was to move the EKG patches from the front to the back of the patients' chest.
The two groups of patients analyzed were similar but showed one significant difference in terms of age with the control group being older. Furthermore, the control group showed higher CRP and PaCO2 compared to the other. This, on one hand, would potentially expose the control group to a better response to NRS; on the other hand, both the SOFA score and the P/F ratio did not differ between the two groups, showing a substantial similar prediction of mortaility and grade of severity of hypoxic respiratory failure.
The setting of the study allows its repeatability in a clinical unit with patients under 24 h monitoring and adequate nurse support and supervision. Although promising, our case series should be interpreted with caution. In this selected group (PaO2/FiO2 > 150), three patients did not tolerate the PP, and two patients required intubation. The RICU is the right location for these unstable patients where adequately trained personnel may promptly recognize the need for treatment escalation and switch to NIV or IMV, respectively (22). Although improved oxygenation with the PP is important, hypoxemia has not been a reliable surrogate biomarker for mortality in clinical trials of ARDS (23).
This study has limitations. The sample size of patients was small (16 patients vs. 16 controls), and the data of controls were retrospectively collected. The performance of a chest CT scan in all admitted patients with hypoxic ARF would have added important information on the status of the lung parenchyma. However, due to the large number of patients to manage at that time, this was not promptly available for all admitted patients.
A control population of consecutively admitted patients only on low flow oxygen not undergoing NRS would have been more suitable for adequate comparison; however, at the time of the study, patients with PaO2/FiO2 > 150 used to be initiated to NRS.
The choice of SpO2/FiO2 would have been useful as a surrogate evaluation of time points in the initial 24 h; however, this parameter, although monitored, was not recorded.
On the other hand, our study highlights the feasibility, tolerability, and effectiveness of prolonged PP. Indeed, the shorter LoS of patients undergoing earlier PP suggests greater effectiveness in early application. Within the 6-month follow-up, no deaths occurred in the group that underwent PP. Furthermore, in consideration of the high shortage of ICU beds, the PP may represent a first-line treatment outside the ICU.
Spontaneously breathing patients with hypoxemic ARF may generate relatively large tidal volumes with potential self-inflicted lung injury (SILI) (24). Therefore, continuous monitoring of paradoxical breathing pattern and vital parameters should be warranted in these patients as they may deteriorate very fast. These concerns should be balanced with the lack of ICU bed availability and the risks of mechanical ventilation, including the need for prolonged sedation and the risk of ventilator-associated pneumonia.
Conclusion
In conclusion, in the majority of the studied non-intubated spontaneously breathing series of patients with COVID-19-associated hypoxemic ARF with PaO2/FiO2 ratio >150 prolonged active PP was feasible and well-tolerated and associated to improvement in PaO2/FiO2. This approach may be confirmed by larger randomized controlled studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Bari Policlinico Ethic committee. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
PP, VD, and GC made substantial contribution to the conception and design of the work. MD, EB, and AP: data acquisition. SB, NA, and GC: analysis. NA and OR: interpretation. PP, VD, MD, SB, EB, and AP helped in drafting the article. NA, GC, and OR revised it critically for important intellectual content. All authors gave the final approval of the version to be published and agreed for the accuracy or integrity of any part of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Mrs. Barione, Dr. Buonamico, Dr. Carrassi, Dr. Diaferia, Dr. Di Gioia, Dr. Labate, Dr.Majorano, Dr. Palumbo, Dr. Santomasi, Dr. Valerio, and all the RICU team for their contribution in the study. We also thank Dr. D Portacci for the Figure 1 cartoon design.
References
1. Johns Hopkins University. Covid-19 Dashboard by the Centre for Systems Science and Engineering (CSSE) at Johns Hopkins University. Available online at: https://coronavirus.jhu.edu/map.html (accessed October 30, 2020).
2. Franco C, Facciolongo N, Tonelli R, Dongilli R, Vianello A, Pisani L, et al. Feasibility and clinical impact of out-of-ICU non-invasive respiratory support in patients with COVID-19 related pneumonia. Eur Respir J. (2020) 3:2002130. doi: 10.1183/13993003.02130-2020
3. Di Lecce V, Carpagnano GE, Pierucci P, Quaranta VN, Barratta F, Zito A, et al. Baseline characteristics and outcomes of COVID-19 patients admitted to a Respiratory Intensive Care Unit (RICU) in Southern Italy. Multidiscip Respir Med. (2020) 15:704. doi: 10.4081/mrm.2020.704
4. Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. (2013) 188:1286–93. doi: 10.1164/rccm.201308-1532CI
5. Ding L, Wang L, Ma W, He H, Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Critical Care. (2020) 24:28. doi: 10.1186/s13054-020-2738-5
6. Elharrar X, Trigui Y, Dols A-M, Touchon F, Martinez S, Prud'homme E, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. J Am Med Assoc. (2020) 323:2336–8. doi: 10.1001/jama.2020.8255
7. Sartini C, Tresoldi M, Scarpellini P, Tettamanti A, Carcò F, Landoni G, et al. Respiratory parameters in patients with COVID-19 after using non-invasive ventilation in the prone position outside the intensive care unit. J Am Med Assoc. (2020) 323:2338–40. doi: 10.1001/jama.2020.7861
8. Paul V, Patel V, Royse M, Odish M, Malhotra A, Koenig S. Proning in non-intubated (PINI) in times of COVID-19: case series and a review. J Intensive Care Med. (2020) 35:818–24. doi: 10.1177/0885066620934801
9. Coppo A, Bellani G, Winterton D, DI Pierro M, Soria A, Faverio P, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. (2020) 8:765–74. doi: 10.1016/S2213-2600(20)30268-X
10. Scaravilli V, Grasselli G, Castagna L, Zanella A, Isgro' S, Lucchini A, et al. Prone positioning improves oxygenation in spontaneously breathing non-intubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. (2015) 30:1390–4. doi: 10.1016/j.jcrc.2015.07.008
11. Longhini F, Bruni A, Garofalo E, Navalesi P, Grasselli G, Cosentini R, et al. Helmet Continuous Positive Airway Pressure and prone positioning: a proposal for an early management of COVID-19 patients. Pulmonology. (2020) 26:186–91. doi: 10.1016/j.pulmoe.2020.04.014
12. World Health Organization. Severe Acute Respiratory Infections Treatment Centre Practical Manual to Set Up and Manage a SARI Treatment Centre and a SARI Screening Facility in Health Care Facilities. (2020).
13. Morales-Quinteros L, Schultz MJ, Bringué J, Calfee CS, Camprubí M, Cremer OL, et al. Estimated dead space fraction and the ventilatory ratio are associated with mortality in early ARDS. Ann Intensive Care. (2019) 9:128. doi: 10.1186/s13613-019-0601-0
14. Ippolito M, Vitale F, Accurso G, Ozzo P, Gregoretti C, Giarratano A, et al. Medical masks and respirators for the protection of healthcare workers from COVID-19 and other viruses. Pulmonology. (2020) 26:204–12. doi: 10.1016/j.pulmoe.2020.04.009
15. NIH. Covid-19 Treatment Guidelines. Available online at: https://www.covid19treatmentguidelines.nih.gov/whats-new/ (accessed May 30, 2021).
16. RCore Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020). Available online at: https://www.r-project.org/ (accessed June 19, 2021).
17. Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest. (2017) 151:215–24. doi: 10.1016/j.chest.2016.06.032
18. Pappert D, Rossaint R, Slama K, Grüning T, Falkeet KJ. Influence of positioning on ventilation–perfusion relationships in severe adult respiratory distress syndrome. Chest. (1994) 106:1511–6. doi: 10.1378/chest.106.5.1511
19. Telias I, Katira BH, Brochard L. Is the prone position helpful during spontaneous breathing in patients with COVID-19? J Am Med Assoc. (2020) 323:2265–7. doi: 10.1001/jama.2020.8539
20. Slessarev M, Cheng J, Ondrejicka M, Arntfield R. Patient self-proning with high-flow nasal cannula improves oxygenation in COVID-19 pneumonia. Can J Anaesth. (2020) 67:1288–90. doi: 10.1007/s12630-020-01661-0
21. Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. (2019) 199:333–41. doi: 10.1164/rccm.201804-0692OC
22. Karim HMR, Burns KEA, Ciobanu LD, El-Khatib M, Nicolini A, Vargas N, et al. Noninvasive ventilation: education and training. A narrative analysis and an international consensus document. Adv Respir Med. (2019) 87:36–45. doi: 10.5603/ARM.a2019.0006
23. Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. doi: 10.1056/NEJM200005043421801
Keywords: COVID-19, prone position, non-intubated, spontaneously breathing, hypoxic respiratory failure
Citation: Pierucci P, Ambrosino N, Di Lecce V, Dimitri M, Battaglia S, Boniello E, Portacci A, Resta O and Carpagnano GE (2021) Prolonged Active Prone Positioning in Spontaneously Breathing Non-intubated Patients With COVID-19-Associated Hypoxemic Acute Respiratory Failure With PaO2/FiO2 >150. Front. Med. 8:626321. doi: 10.3389/fmed.2021.626321
Received: 05 November 2020; Accepted: 09 June 2021;
Published: 21 July 2021.
Edited by:
Reza Lashgari, Shahid Beheshti University, IranReviewed by:
Eduardo Luis De Vito, University of Buenos Aires, ArgentinaPedro David Wendel Garcia, University Hospital Zürich, Switzerland
Copyright © 2021 Pierucci, Ambrosino, Di Lecce, Dimitri, Battaglia, Boniello, Portacci, Resta and Carpagnano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Pierucci, cGFvbGEucGllcnVjY2lAcG9saWNsaW5pY28uYmEuaXQ=
 Paola Pierucci
Paola Pierucci Nicolino Ambrosino2
Nicolino Ambrosino2 Valentina Di Lecce
Valentina Di Lecce Esterina Boniello
Esterina Boniello Onofrio Resta
Onofrio Resta Giovanna Elisiana Carpagnano
Giovanna Elisiana Carpagnano