
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 02 March 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.622015
This article is part of the Research Topic Infectious Disease Surveillance: Applying Cooperative Research to Recent Outbreaks including COVID-19 View all 50 articles
Cooperative research that addresses infectious disease surveillance and outbreak investigations relies heavily on availability and effective use of appropriate diagnostic tools, including serological and molecular assays, as exemplified by the current COVID-19 pandemic. In this paper, we stress the importance of using these assays to support collaborative epidemiological studies to assess risk of rickettsial disease outbreaks among international partner countries. Workforce development, mentorship, and training are important components in building laboratory capability and capacity to assess risk of and mitigate emerging disease outbreaks. International partnerships that fund cooperative research through mentoring and on-the-job training are successful examples for enhancing infectious disease surveillance. Cooperative research studies between the Naval Medical Research Center's Rickettsial Diseases Research Program (RDRP) and 17 institutes from nine countries among five continents were conducted to address the presence of and the risk for endemic rickettsial diseases. To establish serological and molecular assays in the collaborative institutes, initial training and continued material, and technical support were provided by RDRP. The laboratory methods used in the research studies to detect and identify the rickettsial infections included (1) group-specific IgM and IgG serological assays and (2) molecular assays. Twenty-six cooperative research projects performed between 2008 and 2020 enhanced the capability and capacity of 17 research institutes to estimate risk of rickettsial diseases. These international collaborative studies have led to the recognition and/or confirmation of rickettsial diseases within each of the partner countries. In addition, with the identification of specific pathogen and non-pathogen Rickettsia species, a more accurate risk assessment could be made in surveillance studies using environmental samples. The discoveries from these projects reinforced international cooperation benefiting not only the partner countries but also the scientific community at large through presentations (n = 40) at international scientific meetings and peer-reviewed publications (n = 18). The cooperative research studies conducted in multiple international institutes led to the incorporation of new SOPs and trainings for laboratory procedures; biosafety, biosurety, and biosecurity methods; performance of rickettsia-specific assays; and the identification of known and unknown rickettsial agents through the introduction of new serologic and molecular assays that complemented traditional microbiology methods.
Rickettsial diseases are vector-borne diseases caused by agents of the genus Rickettsia (1, 2). However, the definition of rickettsial diseases can also be more inclusive to include diseases caused by agents that are genetically related to Rickettsia, such as Orientia species of the family Rickettsiaceae, and Anaplasma, Ehrlichia, and Neorickettsia species of the family Anaplasmataceae (3). Both families, Rickettsiaceae and Anaplasmataceae, are members of the order Rickettsiales within the class Alphaproteobacteria and phylum Proteobacteria. Lastly, there are some diseases such as Q fever and trench fever that are often associated with rickettsial diseases because the causative agents at one time were considered Rickettsia species (i.e., Coxiella burnetii–Rickettsia burnetii and Bartonella quintana–Rickettsia quintana, respectively) (3, 4). For the purposes of this report, rickettsial diseases will be limited to those diseases caused by Rickettsia and Orientia species.
Rickettsial diseases (and their causative agents) have been traditionally separated into three major groups based on their disease presentation, antigenicity, and vectors (Table 1). Those groups include the typhus group (epidemic typhus [Rickettsia prowazekii] and murine typhus [Rickettsia typhi]); spotted fever group (Rocky Mountain spotted fever (RMSF) [Rickettsia rickettsii], Mediterranean spotted fever (MSF) [Rickettsia conorii], African tick-bite fever [Rickettsia africae], flea-borne spotted fever [Rickettsia felis], Queensland tick typhus [Rickettsia australis], Japanese spotted fever [Rickettsia japonica], etc.), and scrub typhus group (scrub typhus [Orientia tsutsugamushi, Candidatus Orientia chuto, and Candidatus Orientia chiloensis]) (4–7) (Table 1). Genotyping of pathogenic and non-pathogenic rickettsial agents have led to over a dozen genogroups (8). These genogroups are not addressed herein.
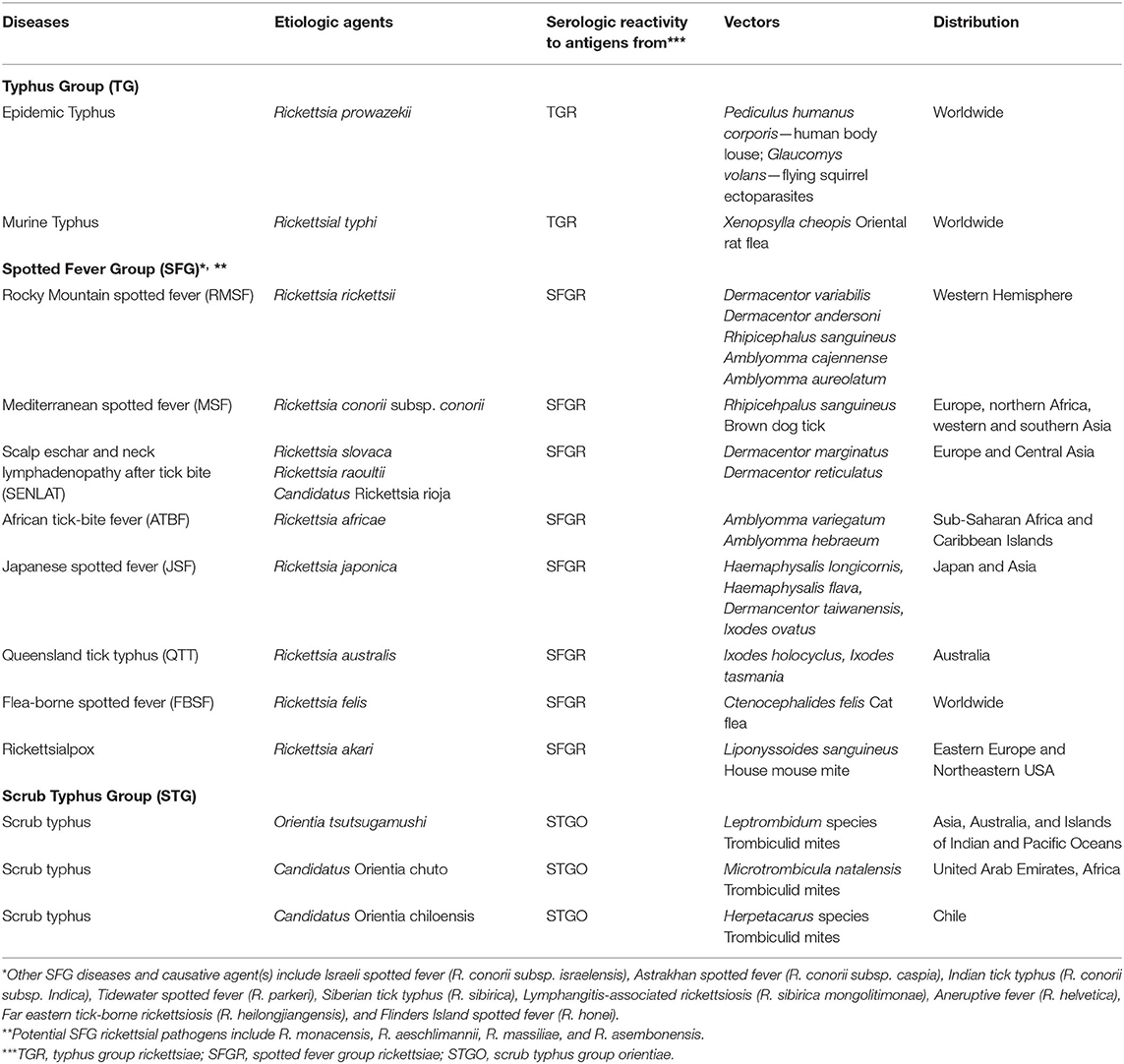
Table 1. Three major groups of rickettsial diseases exist based on their causative agents, host seroreactivity to group-specific antigens, arthropod vectors, and their distribution.
Rickettsial diseases are military and public health concerns because they are distributed widely throughout the world (9–14). Though many rickettsial diseases are mild and self-limiting, there are several of them such as epidemic typhus, RMSF, scrub typhus, murine typhus, and MSF that can be quite severe and life threatening (6, 14). Such rickettsial agents have the potential for use as biological weapon (BW) agents (15). Since the early 2000s, the United States (US) Department of Defense (DoD) has funded and implemented a Biological Threat Reduction Program (BTRP) through the Defense Threat Reduction Agency (DTRA). DTRA BTRP funded multiple cooperative biological research (CBR) multi-year and Threat Agent Detection and Response Activity Project (TAP) single-year biosurveillance studies in countries throughout the world (16). Moreover, the Global Emerging Infections Surveillance (GEIS) Branch of the Armed Forces Health Surveillance Division, responsible for identifying military health relevant threats to inform force health protection decision making, has supported infectious disease surveillance globally (17, 18). These two agencies are major sources of funding supporting the development of and provision of rickettsial assays and methodology by the Rickettsial Diseases Research Program (RDRP) at the Naval Medical Research Center (NMRC). With this support, RDRP participates in international cooperative research and herein describes collaborations with nine countries resulting in the development/support of rickettsial disease research that provided partner countries with the capacity and capability to conduct rickettsial diseases surveillance that informed medical and scientific leaders as to the risk of rickettsial outbreaks in their area of responsibility.
The distribution of rickettsial diseases is varied throughout all continents except Antarctica (5–7). The specific knowledge of the presence, identity, prevalence, and distribution of the rickettsioses and their causative rickettsial agents are only partially known and varies significantly from country to country. This lack of knowledge is often directly tied to limited laboratory diagnostic capability and access to rickettsial assays and therefore places many countries and regions at risk of underestimating the impact and risk of rickettsial diseases, both sporadic occurrences and outbreaks (6). To overcome the shortfall of rickettsiology in underserved countries/regions, we have conducted cooperative research to determine the risk of various rickettsial diseases. Our team provided the rickettsial reagents and assays needed to initiate this work and increased local laboratory capability and capacity through general and specific laboratory training, access to and training on rickettsial assays and reagents, assistance with the evaluation of results, and drawing proper conclusions to be shared with local public health leaders and the international scientific community. The cooperative research among participating institutions has led to enhanced laboratory capability and reinforced knowledge on the presence, identity, distribution, and prevalence of rickettsial agents and diseases within their sphere of responsibility. This enhanced capability has led to partner country scientists' capacity to determine the risk of rickettsial diseases, identify outbreaks, publish results for general observation, and submission of grant applications to further rickettsial disease research (16, 19).
The goal of this paper is to describe the particular components utilized and outcomes obtained during the development of international cooperative rickettsial diseases research to determine the risk of rickettsial disease outbreaks in nine countries from 2008 to 2020: Azerbaijan, Chile, Georgia, India, Kazakhstan, Madagascar, Thailand, Ukraine, and Vietnam. The narrative is divided into specific areas that address the (1) development of collaborations; (2) general description of 17 research institutes from nine countries; (3) overall goals of the research projects for each institute or combination of institutions; (4) serologic and molecular assays utilized to assess the presence, identity, distribution, and prevalence of rickettsial agents; (5) risk assessments made for rickettsial diseases; (6) training provided; and (7) results obtained by partner countries and the important knowledge gain from the studies making great contribution to rickettsiology, and enhancement of capacity and capability of the institutes. Lastly, there is a discussion of the importance of continuing these collaborations, especially in regard to the results obtained. The overall benefit of international collaborations is to improve partner countries assessment of infectious diseases by enhancing their ability to accurately assess the risk of endemic diseases and the potential of outbreaks of infectious diseases such as the recent COVID-19 pandemic.
Discussions with potential collaborators were initiated in-person at conferences or correspondence by email, teleconference, through colleague referrals, and funding agencies' annual meetings. The Research Topics addressed included specific rickettsial disease research (n = 15 projects) as well as rickettsial disease research included in febrile disease projects (n = 2) and arthropod-borne and/or zoonotic disease research projects (n = 9). The proposals for these projects were initiated by collaborators with mutual interests but were often augmented by additional collaborators and institutions to broaden the scope. The final proposals were subsequently submitted to institutions for approval prior to submission to funding agencies. The institutes received approval for research grants from one or more funding organizations. The projects that were performed and their funding sources are shown in Table 2. The funding organizations included DTRA (n = 16), GEIS (n = 9), Nacional de Desarrollo Cientifico y Technologico (FONDECYT) (n = 7), National Foundation for Science and Technology Development of Vietnam (NAFOSTED) (n = 3), Indian Council for Medical Research (ICMR) (n = 3), Institut Pasteur de Madagascar (IPM) (n = 1), and Khon Kaen University (KKU) (n = 1).
Seventeen research institutes from nine countries collaborated with the Rickettsial Diseases Research Program (RDRP) of the Naval Medical Research Center (NMRC), Silver Spring, Maryland, USA, including (1) Azerbaijan: Republican Antiplague Station, Baku; Republican Hygiene and Epidemiology Center, Baku; and Ministry of Defense (MoD) Laboratory, Baku; (2) Chile: School of Medicine, Pontifi via Universidad Católica de Chile, Santiago; Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia; Clínica Alemana de Santiago, Facultad de Medicina Clínica Alemana, Universidad del Desarrollo, Santiago; (3) Georgia: the National Center for Disease Control and Public Health (NCDC); and Laboratory of the Ministry of Agriculture, Tbilisi, Georgia; (4) India: Northeast Regional Medical Research Centre (NRMR), Dibrugarh, India; (5) Kazakhstan: M. Aikimbayev's National Scientific Center for Especially Dangerous Infections (NSCEDI), formerly named Kazakh Scientific Center of Quarantine and Zoonotic Diseases (KSCQZD) and the Scientific Practical Center for Sanitary Epidemiological Expertise and Monitoring (SPC-SEEM), Almaty, and Uralsk Anti-Plague Station (UAPS), Uralsk; (6) Madagascar: Institut Pasteur de Madagascar (IPM), Antananarivo; (7) Thailand: Khon Kaen University (KKU), Khon Kaen, Thailand; (8) Ukraine: Lviv Scientific Research Institute of Epidemiology and Hygiene (LSRIEH), Lviv; and 9) Vietnam: Hanoi Medical University (HMU) and the National Hospital for Tropical Diseases (NHTD), Hanoi, Vietnam.
The research goals of a single institute was often to investigate a newly described or recently rediscovered rickettsial disease(s) and/or agent(s) in a particular region in these countries: (1) India: “Identify previously recognized scrub typhus as well as determine the presence of other rickettsial diseases and/or agents in Northeast India” by RMRC, Dibrugarh, India; (2) Madagascar: “Identify flea-borne rickettsial agents near the capital city” by Institut Pasteur, Antananarivo, Madagascar; (3) Thailand: “Assess the role of cats and cat fleas in presence of spotted fever group rickettsiae in Northeast Thailand” by Khon Kaen University, Khon Kaen, Thailand; and (4) Ukraine: “Ascertain whether typhus group rickettsiae are still present and whether spotted fever group rickettsiae are present by assessing the seroprevalence of the agents infecting humans residing in Lviv Oblast,” by the LSRIEH, Lviv, Ukraine.
Unlike the above single-institute studies, many of the projects discussed below involved multiple institutes within a country, because investigating the presence and distribution of rickettsial diseases and/or agents was often a similar goal of multiple institutes due to the collaborative nature of the projects performed within the countries (e.g., Azerbaijan, Chile, Georgia, Kazakhstan, and Vietnam). These institutes worked together or independently on the following research goals within the countries: (1) Azerbaijan: Initially, there were three goals to assess the risk of rickettsial diseases in Azerbaijan, which involved multiple institutes: (a) “Determine presence of TGR and SFGR infections by seroprevalence study of rural populations in 3 regions of Azerbaijan”; (b) “Determine incidence and prevalence of rickettsial infections among a cohort of military individuals”; and (c) “Ascertain whether arthropods from rodents contained rickettsial agents.” These studies conducted independently were conducted by the Republican Antiplague Station, Baku; Republican Hygiene and Epidemiology Center, Baku; and Ministry of Defense (MoD) Laboratory, Baku, Azerbaijan. (2) Chile: Scrub typhus for hundreds of years was thought to be only found in the Asia–Australia region. So, when an individual presented to a clinic in 2006 in Chile with signs and symptoms of rickettsial disease, it was quite unexpected and even more unexpected that it was subsequently determined to be scrub typhus. This led to clinicians and researchers searching for further evidence of scrub typhus in Chile. The clinical and scientific investigations utilize various expertise of clinicians, scientists, and institutions. Thus, the overall goal in this country was to determine the clinical presentation, distribution, prevalence, incidence, vectors, reservoirs, and genetic characteristics of the disease and its agents. Thus, the multiple institutions worked well together on various aspects of the overall goal. The institutes included the following: School of Medicine, Pontifi via Universidad Católica de Chile, Santiago; Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia; Clínica Alemana de Santiago, Facultad de Medicina Clínica Alemana, Universidad del Desarrollo, Santiago, Chile. (3) Georgia: For the country of Georgia, a current assessment of rickettsial diseases/agents was needed as only limited knowledge of rickettsial diseases existed. Thus, the goal was to ascertain the presence of rickettsial infections and rickettsial agents in Georgia by (a) determining the role of rickettsial agents among febrile patients and (b) assessing ticks for the presence of rickettsial agents and specifically identifying them with new molecular assays. The institutes involved in these studies included the National Center for Disease Control and Public Health (NCDC); and Laboratory of the Ministry of Agriculture, Tbilisi, Georgia. (4) Kazakhstan: Tick-borne (Crimean-Congo hemorrhagic fever; tick-borne encephalitis) and flea-borne (plague) diseases are endemic to Kazakhstan. However, there was only limited knowledge of the presence of rickettsial diseases in Kazakhstan. Thus, the overall goal of the rickettsial disease studies was to augment the minimal knowledge of the presence of rickettsiae in Kazakhstan. Thus, various studies of rickettsial disease agents were investigated, especially those associated with ticks and fleas, including (a) “Identify Tick-borne Rickettsial Agents in Kazakhstan” and (b) “Determine the Presence and Distribution of Flea-borne Rickettsiae in Kazakhstan” conducted by NSCEDI and SPC-SEEM Almaty, and UAPS Uralsk, Kazakhstan; and (5) Vietnam: Similar to other countries, Vietnam had historical evidence of rickettsial diseases; however, for several decades, there was limited investigation into the presence, prevalence, and distribution of rickettsial diseases and agents. Thus, the overall goal for Vietnam in the past decade has been to rectify the deficiency by conducting seroprevalence, clinical, and environmental studies in various number of locations. Three studies addressed the limited knowledge of rickettsial infections in northern Vietnam: (a) “The presence and prevalence of rickettsial infections among humans in northern Vietnam”; (b) “Characterization of the clinical manifestations of rickettsial diseases and determine the applicability of molecular assays in rickettsial diagnosis”; and (c) “Ascertain the genetic makeup of Orientia tsutsugamushi causing scrub typhus in northern Vietnam.” The institutes involved in these studies included the National Hospital for Tropical Diseases and the Hanoi Medical University, Hanoi, Vietnam.
Commercially available and NMRC's serological assays that they developed in-house and not for commercial use were utilized. NMRC's serological assays with standard operating procedures (SOPs) included typhus group rickettsiae (TGR)-specific enzyme-linked immunosorbent assay (ELISA)-immunoglobulin gamma (IgG), spotted fever group rickettsiae (SFGR)-specific ELISA-IgG, and scrub typhus group orientiae-specific (STGO) ELISA-IgG (13, 20, 21). Positive controls (n = 1) and negative controls (n = 3) for each assay were provided to confirm that the assays were performing correctly (4). Subsequently, the institutions identified positive and negative control sera from their studies to use in these assays.
Commercial serological assays with instructions for ELISA and indirect immune fluorescence assay (IFA) were used according to manufacturers' instructions. These assays included InBios Scrub Typhus IgM ELISA (InBios International Inc., Seattle, WA) and Scrub Typhus IFA-IgG (Fuller Laboratories, Fullerton, CA).
Four types of polymerase chain reaction (PCR) were used: (1) standard PCR (sPCR), (2) nested PCR (nPCR) or hemi-nested PCR (hnPCR), and (3) quantitative real-time PCR (qPCR) assays.
The qPCR assays (genus-, group-, or species-specific) were either developed at NMRC or found in peer-reviewed publications, and were used to screen for and identify rickettsial and oriental agents (4). The primers, probes, and controls [positive controls included either plasmids containing the target gene fragment(s) or linear target gene fragment(s), and molecular-grade water served as the negative controls] were used as described (4). Either reagents for the qPCR assays were supplied; their product numbers were provided; the primers, probes, and linear positive control oligonucleotide sequences were provided; or a combination of reagents, oligonucleotide sequences, and product numbers were provided. With the provided information for the reagents, the institutes could subsequently obtain the reagents independently of NMRC.
The sPCR and nPCR/hnPCR were used to produce amplicons for specific gene fragment sequencing either for a single gene or multiple genes in multilocus sequence typing (MLST) to identify and characterize rickettsial agents (4). Multiple gene fragment sequences were used as described in the MLST scheme initially described by Fournier et al. (22) to identify known Rickettsia species, incompletely characterized Candidatus Rickettsia species, and not previously described rickettsial agents (4). The genes most commonly used in the described studies for the identification of rickettsiae included 17-kDa antigen gene, rrs, gltA, ompB, ompA, and sca4 (4). Identification of Orientia species by MLST utilized the following genes: rrs; 47-kDa antigen high-temperature requirement A protease gene (htrA); and the 56-kDa type-specific antigen gene (tsa56) (23).
The products from sPCR/nPCR for a single gene or for multiple genes to conduct MLST were sequenced in-house or using commercial companies as previously described (24). The sequencing data were assembled by Lasergene version 15.0 software (DNASTAR, Inc. Madison, WI, USA) or similar software, and sequences were compared with sequences available in GenBank (NCBI) using the BLAST search tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch).
Gene sequences, excluding the primer regions, were aligned by the ClustalW and phylogenetic analysis performed using MEGA X software (or similar software). The phylogenetic trees were constructed based on the alignment of the various gene fragment sequences (described above) obtained using the maximum likelihood method and Tamura-Nei model (25), and bootstrap analysis (1,000 reiterations) was carried out according to the Kimura 2-parameter method. All positions containing alignment gaps and missing data were eliminated.
Assessing the risk of endemic rickettsial diseases and the potential for outbreaks requires measuring epidemiological metrics such as determining the presence, spatial and temporal distribution, prevalence for individual samples or minimal infection rate for pooled samples, and incidence of rickettsial infections and their causative agents. The evidence of rickettsial infections (e.g., antibodies against group-specific rickettsial antigens and/or detection of rickettsial agents in clinical samples) clearly indicates the presence of rickettsial pathogens in a location/region/country. Subsequent studies to determine their prevalence, incidence, and distribution are required to better localize the risk of rickettsioses. Molecular studies utilizing assays such as qPCR provide the specificity required to identify Rickettsia pathogens; however, if only genus- or group-specific assays are used, non-pathogens within the genus or group may be detected and confused with pathogens. Therefore, more species-specific assays are needed in accurately determining rickettsial disease risk assessments.
NMRC staff scientists trained and mentored partner country scientists involved in the rickettsial diseases research projects prior to and during performance of laboratory work (Table 3). Training provided was project driven and included the following subjects: (1) conducting BSL-2 general laboratory procedures as they pertained to rickettsial studies; and (2) developing, updating, and/or augmenting SOPs to include rickettsial specific assays and general procedures for sequencing and MLST. New assays/procedures utilized in the laboratories were conducted with appropriate controls and standards to evaluate performance. Once partner country scientists were confident with the new procedures, they used the assays to assess environmental and/or clinical samples. Performance of laboratory assays was routinely assessed by comparing results of controls with the same controls performed at NMRC or described by manufacturers. The commercial and in-house assays and their sources that were utilized by the various laboratories are shown by country in Table 4.
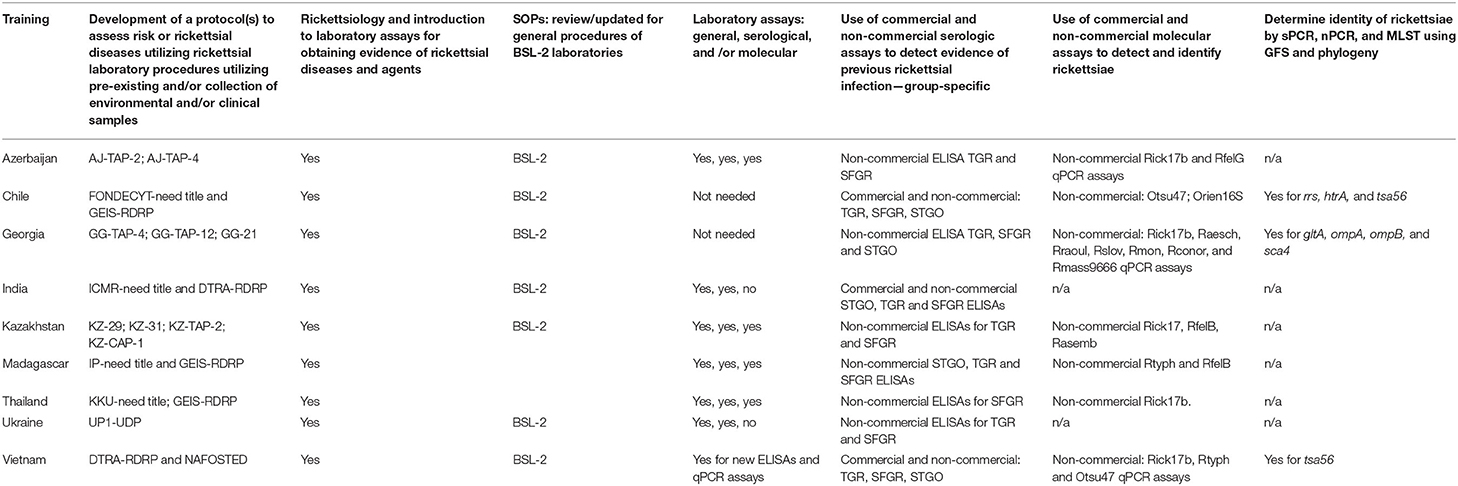
Table 3. Training utilized by research institutes to identify rickettsiae and rickettsial infections.
The results of the various rickettsial disease research projects (n = 26) were completed at 17 institutions from nine countries and are described in detail below by country. The findings are summarized and appropriate references provided in Table 2 and Supplementary Table 1.
Serological data showed the prevalence of IgG against SFGR (3.7–15.9%) and TGR (0–0.6%) among individuals in Azerbaijan and indicated a low to moderate exposure to SFGR and a very low risk of TGR infections (Supplementary Table 1). Antibodies against SFGR are lifelong and therefore the point prevalence studies do not allow for a strong evaluation of outbreak risk. Additional serosurveys throughout Azerbaijan need to be performed, as well as tick and flea field studies in locations positive and negative for evidence of rickettsia agents following results of serosurveys. Moreover, clinical studies should be carried out to assess incidence over time as well as during outbreaks to determine the risk levels of endemic rickettsioses spatially and temporally. Molecular evidence of R. felis group was found among a small number of ixodid ticks collected from rodents in the Lankaran district located in the southeast of Azerbaijan near the border with Iran (Supplementary Table 1). However, the total number of arthropods collected was low, and the assay used to identify R. felis in this study has subsequently been found to have low specificity, both of which decrease our ability to determine risk of human infection with R. felis group of rickettsiae in this district or Azerbaijan. More arthropod surveys, both ticks and fleas, are needed.
The first recognized human case of scrub typhus in 2006 in South America was confirmed by molecular and serological studies and reported in 2011 (26) (Supplementary Table 1). An additional three cases were subsequently identified and reported in 2016 (27). Since then, more than 40 cases have occurred throughout Chile (Supplementary Table 1). The disease has been characterized clinically, and serological studies of dogs (sentinel animals) and humans have shown the distribution both locally and nationally (Supplementary Table 1). In addition, human serological studies have identified spotted fever and typhus group rickettsial infections in Chile. Molecular characterization of the causative agent, Candidatus Orientia chiloensis, from human, rodent, and mite samples has shown that it is distinct from O. tsutsugamushi and Candidatus O. chuto (Supplementary Table 1). Additional studies are needed to show the transmission of the agent by mites to confirm the vector and hospital studies to better characterize the disease, incidence, and potential for scrub typhus outbreaks in Chile.
Initially, only a single rickettsial agent, Rickettsia conorii, was known to be endemic to Georgia (28). More recently, ixodid ticks acquired from livestock, trapped rodents, and tick drags in May 2008 (n = 653) and 2009 (n = 264) from areas in the districts of Akhaltsikhe, Aspindza, Gori, and Kaspi of the country of Georgia were found to contain three additional rickettsial pathogens R. aeschlimannii, R. roultii, and R. slovaca (Supplementary Table 1). Additional studies followed utilizing new molecular assays and MLST to characterize the rickettsiae that were unable to be identified by qPCR. In the end, a total of nine tick-borne rickettsiae, six pathogens, and three rickettsial agents of unknown pathogenicity were identified. A hospital-based fever study determined that among 655 fever patients, 10 (1.5%), 2 (0.3%), and 2 (0.3%) patients had evidence of a previous infection with SFGR, STGO, and TGR, respectively (Supplementary Table 1). These results suggest the presence of rickettsial diseases among Georgians but at a very low prevalence, suggesting that the risk of a rickettsial outbreak is low. These results need to be confirmed with additional nationwide hospital-based fever studies.
Four epidemiological studies of rickettsial infections were initiated after it was discovered that scrub typhus had returned to Northeast India after a gap of 67 years (29). In the first study, of 1,264 human serum samples assessed by ELISA-IgG 390 (30.8%), 175 (13.8%), and 53 (4.2%) individuals had antibodies against STGO, SFGR, and TGR, respectively. Molecular studies of the positive serum samples identified two individuals had O. tsutsugamushi DNA. Investigation of arthropods (ticks, fleas, and mites) from domestic animals and rodents of the study area found only fleas that were positive for rickettsiae by the Rick17b genus-specific qPCR assay (4 of 16 individuals) and sequencing determined that the agent was Candidatus R. senegalensis (Supplementary Table 1). This study showed conclusively the presence of rickettsial diseases in this underserved area of India. In the second study, to determine the genotype of O. tsutsugamushi causing scrub typhus in NE India, patients screened positive by InBios Scrub Typhus ELISA-IgM blood samples were assessed for the presence of tsa56. Those positive amplicons were sequenced and three distinct genotypes were identified (Supplementary Table 1). This knowledge can be used in planning control strategies and prophylactic measures and identifying outbreak sources. The third study, involving the investigation of serum samples (n = 317) from individuals in another scrub typhus endemic region of NE India, found seroprevalence against scrub typhus, typhus, and spotted fever of 35.6, 2.2, and 0%, respectively. DNA extraction of seven SFGR positive blood samples only obtained a single amplicon for the 17-kDa gene. Sequence typing identified R. felis, a flea-borne rickettsia associated worldwide with flea-borne spotted fever (Supplementary Table 1). Future studies are needed to look for R. felis infection in patients with fever of unidentified origin (FUO) to determine the epidemiology and to understand the complex paradigm of R. felis transmission in India. Lastly, a study involving 2,199 clinical patients (762 with acute encephalitis syndrome and 1,437 with FUO) and 40 (1.8%) samples were found positive for IgG against R. typhi (Supplementary Table 1). This prevalence among patients suggests that a low risk of murine typhus exists in NE India and thus additional epidemiological studies throughout NE India should be performed to assess the risk of murine typhus with special attention to urban settings.
To assess arthropod-borne rickettsial diseases in Kazakhstan, new molecular methods were introduced to identify tick and Rickettsia species via United States Army Medical Research Institute of Infectious Diseases and NMRC in collaboration with NSCEDI-KSCQZD, SPC-SEEM, and UAPS. In Kazakhstan, North Asian tick typhus, also known as Siberian tick typhus, caused by Rickettsia sibirica, is endemic. However, it was unclear if other tick-borne diseases due to rickettsial infections occurred. To address this issue, Rickettsia species were identified initially only at the genus level with the Rick17b qPCR assay. The preliminary results showed the presence of tick-borne rickettsiae in Kazakhstan and the utility of molecular assays in arthropod-borne rickettsial surveillance. The addition of three species-specific qPCR assays identified never before known endemic regions for R. aeschlimannii, R. raoultii, and R. slovaca. In addition, flea-borne rickettsiae were identified in southwestern Kazakhstan that included R. felis/Ca. R. senegalensis and R. asembonensis (Supplementary Table 1). These new rickettsial pathogens identified in Northern, Western, Southern, and Southwestern Kazakhstan were significant in identifying health issues and stimulation of additional surveillance studies and brought awareness of the potential for rickettsial outbreaks in addition to the recognition of potential endemic rickettsioses.
Due to the presence of flea-borne diseases such as plague in Madagascar, this study was conducted to determine if a risk to flea-borne rickettsial diseases also exists in Madagascar. Notably, a vector for both plague and murine typhus is the Oriental rat flea (Xenopsylla cheopis). Thus, a survey among humans and rodents was conducted to assess the risk of flea-borne rickettsial diseases. A seroprevalence study was performed among humans and peri-domestic small mammals. The seroprevalence of SFGR and TGR among humans was 34 and 39%, respectively. However, among the small mammals collected, only 4.4% were IgG positive against TGR R. typhi antigens and none of the animals had evidence of IgG against SFGR antigens. Interestingly, among the peri-domestic small mammals' fleas collected and assessed for rickettsiae, 24.3 and 1.9% of Oriental rat fleas (X. cheopis) were positive for R. typhi (a TGR) and R. felis (a SFGR), respectively, and 30.8% of P. irritans were positive for R. felis (Supplementary Table 1). These results showed that at least the two rickettsial agents identified in the rodent fleas assessed have possible roles in the TGR and SFGR infections among people in Madagascar. Additional studies to determine the spatial and temporal distribution of flea-borne rickettsiae are needed, in addition to hospital-based studies to determine incidence of these rickettsial diseases.
A study of the role of domestic cats in the presence of rickettsial disease in Northeast Thailand investigated cat sera (42 serum samples) for the presence of antibodies against SFGR and cat fleas (n = 23) for molecular evidence of rickettsiae. Two cats (4.8%) had antibodies against SFGR, and 21 cat fleas (91.3%) were positive for R. asembonensis DNA (Supplementary Table 1). Thus, for Northeast Thailand, cats and cat fleas show evidence of spotted fever group rickettsiae, and physicians and veterinarians should be aware of the risk for rickettsial disease. These preliminary results should be followed up with additional surveillance and hospital studies to more clearly determine the risk of rickettsial diseases among the animal and human populations in Thailand.
Seroprevalence of 1,000 non-febrile hospital patients in western Ukraine for TGR was determined to be 1.5%, and for SFGR, it was 5.1%, indicating a low prevalence among non-febrile patients from two hospitals for previous exposure to rickettsiae (Supplementary Table 1). Remarkably, seropositivity to TGR was only 1.5%, and the study population included people who lived in an area where epidemic typhus was previously endemic. The seroprevalence studies should be increased to take in all of Ukraine. Moreover, a hospital-based fever study should be conducted to assess the identities, incidence, and distribution of rickettsial diseases for Ukraine.
Historically, rickettsial disease has been understudied in Vietnam. The following three studies conducted in northern Vietnam have added significantly to the knowledge of the presence of various rickettsial diseases, including scrub typhus and murine typhus and potentially spotted fever. In a serological study, the seroprevalence was determined to be 6.5, 1.1, and 1.7% for TGR, SFGR, and STGO infections of healthy humans, indicating the presence of all three groups infecting people in northern Vietnam, but at low levels. A subsequent study of hospitalized patients at two referral hospitals in Hanoi found that 34.1 and 3.3% of patients suspected of rickettsial disease were confirmed by serological and molecular assays to have scrub typhus and murine typhus, respectively. To follow up on these results, a second hospital-based study was conducted to characterize the O. tsutsuagmushi causing scrub typhus in northern Vietnam. It was determined that three geno-groups (Karp, Kato, and Gilliam) predominated. This information is important in the development of laboratory assays and vaccine candidates. Studies on rickettsial diseases with an emphasis on scrub typhus and murine typhus, but also including spotted fever, should continue to precisely determine the risk of endemic disease and the possibility of outbreaks for all of Vietnam.
In addition to providing leaders of the partner countries, research institutions, funding organizations, scientists, and clinicians with important rickettsial disease surveillance information based on these findings, the research was also made available to the international scientific community by providing abstracts to presentations (n = 40) given at national and international conferences and the publication of peer-reviewed articles (n = 18) in international scientific journals (Table 5). The important contributions of the rickettsia surveillance conducted due to the collaborations described herein to the scientific community for each country is exemplified when comparing the number of publications of rickettsial research before and after the collaborations. By searching PubMed for rickettsia, and rickettsia associated with arthropod-borne diseases and zoonosis, we found the number of publications varied significantly by country, with the most articles (without counting those described herein) published by India (281), Thailand (165), Chile (18), Vietnam (12), Ukraine (5), Madagascar (5), and Kazakhstan (3). Azerbaijan and Georgia had no other rickettsia publications by the PubMed search within the last 10 years other than the two described herein (Table 5). Thus, with the exception of India and Thailand, the percentage of publications due to those reported herein from seven countries (mean, 47.6%; range, 0–100%) shows the important knowledge that collaborative research provides to the partner institutes and global health.
During the implementation of our cooperative research, each country and institute(s) faced similar and unique challenges. Some laboratories were able to quickly incorporate rickettsial disease research or augment what they had into a self-sustaining capability. The primary challenge shared by all of the laboratories was recognizing the limited knowledge on rickettsial disease presence, identity, distribution, and prevalence, within their area of responsibility. Moreover, clinical presentations of rickettsial diseases are not distinct and are easily confused with multiple other infectious diseases such as dengue, leptospirosis, flu, malaria, etc. (6, 14). This issue is compounded by the lack of access to reliable diagnostic tests, especially in resource-limited and middle-income countries (2). Certain issues could not be overcome, especially those political in nature that arose between collaborating countries.
NMRC staff scientists provided training as needed and scheduled to conduct serological and molecular assays utilized to identify rickettsia and rickettsial infections (4). Performance of general laboratory procedures was discussed early on to ensure that all involved had the same knowledge and perceptions of working in BSL-2 laboratories. Trainings for rickettsial assays were accomplished in a mentoring fashion that included didactic lectures, hands-on laboratory instruction, and overseeing supervised instruction. Positive and negative controls, initially provided by NMRC, were utilized to ensure that laboratory results were obtained similarly in the partner laboratory and NMRC laboratory. Follow-up training was conducted as new procedures were added to the institutes' portfolios. This additional training was performed in the partner countries during visits and at NMRC where scientists from Chile, Georgia, India, Kazakhstan, and Vietnam visited and worked side by side with members of RDRP.
NMRC staff scientists also provided guidance and training on data analysis especially in the proper evaluation of laboratory results, most importantly, data quality: when to accept and when not to accept the results. This required the evaluation of standard controls (commercial and non-commercial) as acceptable ranges established and could be used for reference and quality control. Understanding how to analyze results relative to the controls and to know when there is an issue with a control are vital for interpreting results. This allowed for the development of appropriate conclusions from data obtained (4, 131–133). Examples of these discussions often occurred during partner country visits, side meetings of conferences, and video conference calls.
When detection of rickettsial disease is limited to serological data, it is difficult to determine the causative agent responsible for antibody response in a patient. Thus, the knowledge necessary to assess the specific rickettsial risk(s) is relegated to the general assumptions as to the cause of the infection, being limited to group specificity. Therefore, it is important to obtain specific information associated with proposed agents such as the host(s), the hosts' natural settings, prevalence of infected hosts, and their distribution (4, 14, 133).
Limited molecular identification of rickettsiae to the genus or group level in the environment does not allow one to determine accurately the risk of particular rickettsial diseases. Detection of pathogenic rickettsiae must be accomplished by Rickettsia species identification, as sympatric non-pathogenic rickettsiae can be found among the same arthropod hosts (134). For example, one can commonly find sympatric pathogens with non-pathogenic Rickettsia species among the same arthropod species [e.g., Rickettsia rickettsii and Rickettsia montanensis in Dermacentor variabilis (135); Rickettsia parkeri and Rickettsia andeanae in Amblyomma maculatum (136, 137); Rickettsia felis and Rickettsia asembonensis in Ctenocephalides felis (24, 138)].
The commonly used method of preparing arthropod vectors is to employ pooling, which groups the same arthropod species from one location, the same host, or drag/flag sheet into one pool sample (94). The pool samples can be restricted to individuals of the same arthropod species and life stage. As indicated above, since more than one rickettsial agent may be found within a single species of tick or flea, then pooled samples may contain more than one Rickettsia species. In this situation, one of the Rickettsia species might be missed and therefore one cannot determine the prevalence of each rickettsia accurately. The minimum infection rate (MIR) equals the total number of positive pools divided by the total number of individual arthropods in all the pools assessed multiplied by 100. A positive pool with 5 individuals in it is not counted the same as a pool with 50 individuals; i.e., the prevalence of each pool would be considered 100%, whereas the MIR would be considered to be 20 and 5%, respectively. If a pool has more than one Rickettsia species in it, then it will be assumed that only one agent of each species is among the pool sample (78). For example, if a pool of 10 A. maculatum individuals had both qPCR assays for R. parkeri and R. andeanae as positive, then the MIR for both agents would be 10%. Therefore, the most accurate way, though more time-consuming and costly, is to determine the prevalence of rickettsiae and the agents' identity within their arthropod hosts based on individual arthropods, not pools. Lastly, if you have both individual and pooled samples, you will have to determine both prevalence and MIR for the respective sample types (78). By definition, a pool sample cannot contain a single arthropod. To address the issues above, a strategy of processing arthropod hosts individually, screening by pools, and testing each individual arthropod in a positive pool can be used.
Unlike the genus Rickettsia where there is much more information on the pathogenicity of the individual species, significantly less is known about Orientia species. Moreover, the rodent animal models for O. tsutsugamushi strains do not necessarily mimic the virulence of the same agents in humans and non-human primates, thus confounding the matter of identifying pathogens (139, 140). However, it has been known for a very long time that differences in pathogenicity exist among the large diversity of O. tsutsugamushi strains (141). The scrub typhus story becomes more compelling now that we know there are additional Orientia species found outside the endemic region of O. tsutsugamushi (known as the Tsutsugamushi Triangle incorporating lands in Asia, Australia, and islands in the Indian and Pacific oceans). Scrub typhus is now considered endemic for multiple areas throughout the world (7). With the recent added complexity of orientiae, there is need for more research to be performed to determine presence of pathogen and non-pathogens within Orientia. For the present, detection of Orientia species is considered evidence of a causative agent for and therefore used in the risk assessment of scrub typhus.
The projects described herein clearly demonstrate that the partner country laboratories benefited from enhanced training, capacity/capability, education (science, laboratory procedures, language, and risk assessment), and appropriate funding support. The ultimate consequences were not only the data collected, which, following analysis, allowed the institutes and partner countries to determine risk of rickettsial diseases for districts, regions, and/or countries and which can subsequently be used by medical leaders and policy makers to institute further education and projects and augment preventive medicine, diagnostic, and treatment modalities to enhance public health. The true success of these projects is best measured by the ability of the partner laboratories to continue research on their own in this area of research as well as research in other infectious diseases. Fortunately, as can be seen by this paper and others (16, 19), the collaborating governments, funding partners, and research institution involvements were extremely positive, resulting in host country institutions and personnel enhancement in conducting rickettsial disease research, which also enhanced scientific and medical knowledge worldwide. Ultimately, collaborative researchers are able to provide information to public health decision makers with advanced awareness on emerging infectious disease threats such as rickettsial diseases, and thereby promote the timely, science-based disease outbreak prevention, preparedness, and control-and-response actions necessary to improve global public health.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The work was supported by the Defense Threat Reduction Agency's BioThreat Reduction Program, work unit number: 6227878708J25GYP1FMTK and the Armed Forces Health Surveillance Division's Global Emerging Surveillance Branch, ProMIS ID P0071_19_NM_02, work unit number: A0074.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government or The Henry M. Jackson Foundation for the Advancement of Military medicine, Inc. The work was supported by work unit number: 6227878708J25GYP1FMTK and the Defense Threat Reduction Agency's Biological Threat Reduction Program (DTRA-BTRP), and the Armed Forces Health Surveillance Division, Global Emerging Infections Surveillance Branch (AFHSB-GEIS), ProMIS ID P0071_19_NM_02, NMRC work unit numberA0074. CF and AR are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a military Service member or employee of the U.S. Government as part of that person's official duties.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to recognize the following scientists involved in the aforementioned research projects:
-Azerbaijan: Matthew J Hepburn, Eric C Garges, Robert G Rivard, Danielle V Clark, Amanda K Lane, Martin Adams, Rita Ismayilova, Telman Ahmadkhanov, Namiq Huseynov, and Agil Seyidov
-Chile: Katia Abarca, Thomas Weitzel, Constanza Martínez-Valdebenito, and Gerardo Acosta-Jamett
School of Medicine, Pontificia Universidad Católica de Chile, Santiago; Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia; Clínica Alemana de Santiago, Facultad de Medicina Clínica Alemana, Universidad del Desarrollo, Santiago
-Georgia: Roena Sukhiashvili, Ekaterine Zhgenti, Tinatin Kuchuloria, Marina Donduashvili, Ekaterine Khmaladze, and Giorgi Chakhunashvili
National Center for Diseases Control and Prevention, Tbilisi; Laboratory of the Ministry of Agriculture, Tbilisi, Georgia
-Northeast India: Siraj A Khan and Trishna Bora
Regional Medical Research Centre, Dibrugarh
-Kazakhstan: Talgat Nurmakhanov, Zhanna Shapieva, Lyazzat Musralina, Aleksandr Grazhdanov, Yerlan Sansyzbayev, and Alexey Andryushenko
Uralsk Anti-plague Station, Uralsk; M. Aikimbayev's National Scientific Center for Especially Dangerous Infections (NSCEDI) formerly Kazakhstan Scientific Center of Quarantine and Zoonotic Diseases; and Scientific and Practical Center of Sanitary and Epidemiological Expertise and Monitoring, Almaty
-Madagascar: Rado J L Rakotonanahary and Minoarisoa Ragerison
Institut Pasteur de Madagascar, Antananarivo, Madagascar;
Northeast Thailand: Sirirat Phomjareet, Fanan Suksawat
Khon Kaen University, Khon Kaen, Thailand
-Ukraine: Oleksandra Tarasyuk, Iryna Kurhanova, Mary Guttieri, Karen Hite, Roman Woelfel, Gerhard Dobler, and William L Nicholson
Lviv Scientific Research Institute of Epidemiology and Hygiene, Lviv, Ukraine; Bundeswehr's Institute for Microbiology, Munich, Germany, Centers for Disease Control and Prevention, Atlanta, Georgia
-Vietnam: Hoi Le Thi and Trung Nguyen Vu
Hanoi Medical University and National Hospital for Tropical Diseases, Hanoi, Vietnam.
DTRA representative: Gavin Braunstein science lead EUCOM, CENTCOM; Marty Stokes DTRA science lead Asia; Brett Forshey GEIS representative.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.622015/full#supplementary-material
1. Walker DH. Ricketts creates rickettsiology, the study of vector-borne obligately intracellular bacterial. J Infect Dis. (2004) 189:938–55. doi: 10.1086/381710
2. Paris DH, Kelly DJ, Fuerst PA, Richards AL. A brief history of the major rickettsioses in the Asia-Australia-Pacific region: a capstone review for the special issue of TMID. Trop Med Infect Dis. (2020) 5:165. doi: 10.3390/tropicalmed5040165
3. Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equip and ‘HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. (2001) 51:2145–65. doi: 10.1099/00207713-51-6-2145
4. Luce-Fedrow A, Mullins K, Jiang J, Richards AL. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol. (2015) 10:537–64. doi: 10.2217/fmb.14.141
5. Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microiol Rev. (2013) 2:657–702. doi: 10.1128/CMR.00032-13
6. Abdad MY, Abou Abdallah R, Fournier PE, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol. (2018) 56:e01728–17. doi: 10.1128/JCM.01728-17
7. Richards AL, Jiang J. Scrub typhus: historic perspective and current status of the worldwide presence of Orientia species. Trop Med Infect Dis. (2020) 5:49. doi: 10.3390/tropicalmed5020049
8. Park E, Poulin R. Widespread Torix group Rickettsia in New Zealand amphipods and the use of blocking primers to rescue host COI sequences. Sci Rep. (2020) 10:16842. doi: 10.1038/s41598-020-73986-1
9. Kelly DJ, Richards AL, Temenak JJ, Strickman D, Dasch GA. (2002). The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 34(Suppl. 4):s145–69. doi: 10.1086/339908
10. Jiang J, Marienau KJ, May LA, Beecham HJ, Wilkinson R, Ching W-M, et al. Laboratory diagnosis of two scrub typhus outbreaks at Camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and Western blot assay using outer membrane 56 kDa recombinant proteins. Am J Trop Med Hyg. (2003) 69:60–6. doi: 10.4269/ajtmh.2003.69.60
11. Bavaro MF, Kelly DJ, Dasch GA, Hale BR, Olson P. History of U.S. military contributions to the study of rickettsial diseases. Mil Med. (2005) 170(4 Suppl.):49–60. doi: 10.7205/MILMED.170.4S.49
12. Frances SP. Rickettsial diseases of military importance: an Australian perspective. J Military Veterans Health. (2011) 19:26–31.
13. Jiang J, Myers TE, Rozmajzl PJ, Graf PC, Chretien JP, Gaydos JC, et al. Seroconversions to rickettsiae in US military personnel in South Korea. Emerg Infect Dis. (2015) 21:1073–4. doi: 10.3201/eid2106.141487
14. Fang R, Blanton LS, Walker DH. Rickettsiae as emerging infectious agents. Clin Lab Med. (2017) 37:383–400. doi: 10.1016/j.cll.2017.01.009
15. Azad AF. Pathogenic rickettsiae as bioterrorism agents. Clin Infect Dis. (2007) 45(Suppl. 1):S52–5. doi: 10.1086/518147
16. Yeh KB, Parekh FK, Musralina L, Sansyszbai A, Tabynov K, Shapieva Z, et al. A case history in cooperative biological research: compendium of studies and program analyses in Kazakhstan. Trop Med Infect Dis. (2019) 4:136. doi: 10.3390/tropicalmed4040136
17. Fukuda MM, Klein TA, Kochel T, Quandelacy TM, Smith BL, Villinski J, et al. Malaria and other vector-borne infection surveillance in the U.S. Department of Defense Armed Forces Health Surveillance Center-Global Emerging Infections Surveillance program: review of 2009 accomplishments. BMC Public Health. (2011) 11:S9. doi: 10.1186/1471-2458-11-S2-S9
18. Witt CJ, Richards AL, Masuoka PM, Folley DH, Buczak AL, Musila LA, et al. The AFHSC-Division of GEIS Operations Predictive Surveillance Program: a multidisciplinary approach for the early detection and response to disease outbreaks. BMC Public Health. (2011) 11(Suppl. 2):S10. doi: 10.1186/1471-2458-11-S2-S10
19. Hay J, Yeh KB, Dasgupta D, Shapieva Z, Omasheva G, Deryabin P, et al. Biosurveillance in Central Asia: successes and challenges of tick-borne disease research in Kazakhstan and Kyrgyzstan. Front Public Health. (2016) 4:4. doi: 10.3389/fpubh.2016.00004
20. Dasch GA, Halle S, Bourgeois AL. Sensitive microplate enzyme-linked immunosorbent assay for detection of antibodies against the scrub typhus rickettsia, Rickettsia tsutsugamushi. J Clin Microbiol. (1979) 9:38–48.
21. Halle S, Dasch GA. Use of a sensitive microplane enzyme-linked immunosorbent assay in a retrospective serological analysis of a laboratory population at risk of infection with typhus group rickettsiae. J Clin Microbiol. (1980) 12:343–50. doi: 10.1128/JCM.12.3.343-350.1980
22. Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov J Clin Microbiol. (2003) 41:5456–65. doi: 10.1128/JCM.41.12.5456-5465.2003
23. Abarca K, Martinez-Valdebenito C, Angulo J, Jiang J, Farris CM, Richards AL, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. (2020) 26:2148–56. doi: 10.3201/eid2609.200918
24. Jiang J, Maina AN, Knobel DL, Cleaveland S, Laudisoit A, Wamburu K, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. (2013) 13:550–8. doi: 10.1089/vbz.2012.1123
25. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. (2013) 28:2731–9. doi: 10.1093/molbev/mst197
26. Balcells ME, Rabagliati R, García P, Poggi H, Oddó D, Concha M, et al. Endemic scrub typhus-like illness, Chile. Emerging Infect Dis. (2011) 17:1659–63. doi: 10.3201/eid1709.100960
27. Weitzel T, Dittrich S, López J, Phuklia W, Martinez-Valdebenito C, Velásquez K, et al. Endemic Scrub Typhus in South America. N Engl J Med. (2016) 375:954–61. doi: 10.1056/NEJMoa1603657
28. Eremeeva ME, Balayeva NM, Ignatovich VF, Raoult D. Proteinic and genomic identification of spotted fever group rickettsiae isolated in the former USSR. J Clin Microbiol. (1993) 10:2625–33. doi: 10.1128/JCM.31.10.2625-2633.1993
29. Khan SA, Dutta P, Khan AM, Topno R, Borah J, Chowdhury P, et al. Re-emergence of scrub typhus in northeast India. Int J Infect Dis. (2012) 16:e889–90. doi: 10.1016/j.ijid.2012.05.1030
30. Clark DV, Ismayilov A, Seyidova E, Hajiyeva A, Bakhishova S, Hajiyev H, et al. Seroprevalence of select arthropod-borne and zoonotic infections in rural Azerbaijan. In: International Conference on Emerging Infectious Diseases. Atlanta, GA (2010).
31. Garges E, Richards A, Seyidov A, Nasirova E, Rivard R, Dyson H, et al. Seroprevalence of Rickettsia-specific antibodies in young male volunteers in Azerbaijan. In: International Conference on Emerging Infectious Diseases. Atlanta, GA (2012).
32. Huseynova E, Garges E, Seyidov A, Robert R, Richards A, Farris C, et al. Seroprevalence of specific antibodies to rickettsial pathogens in young male volunteers in Azerbaijan. In: International Meeting on Emerging Diseases and Surveillance. Vienna (2014).
33. Mammadov S, Garges E, Huseynov N, Ahmedkhanov T, Richards A, Farris C, et al. Seroprevalence and seroincidence indicators of the arthropodborne and zoonotic infections among male communities (aged 18-35) in the Republic of Azerbaijan. In: American Society of Tropical Medicine and Hygiene 67th Annual Meeting. Abst #LB-5131 Poster. New Orleans, LA (2018).
34. Jiang J, You BJ, Liu E, Apte A, Yarina TR, Myers TE, et al. Development of three quantitative real-time PCR assays for the detection of Rickettsia raoultii, Rickettsia slovaca and Rickettsia aeschlimannii and their validation with ticks collected from the country of Georgia and the Republic of Azerbaijan. Ticks Tick Borne Dis. (2012) 3:327–31. doi: 10.1016/j.ttbdis.2012.10.004
35. Weitzel T, Jiang J, Martinez-Valdebenito C, Lopez J, Richards A, Abarca K. Canine exposure to Orientia spp. in southern Chile – A house-hold-based cross-sectional serosurvey. In: One Health - 9th Tick and Tick-borne Pathogen Conference and the 1st Asia Pacific Rickettsia Conference. Oral Presentation, Abst # 284. Cairns, QLD (2017).
36. Jiang J, Martinez-Valdebenito C, Weitzel T, Abarca K, Richards AL. Development of an Orientia genus-specific quantitative real-time PCR assay and the detection of Orientia species in DNA preparations from O. tsutsugamushi, Candidatus Orientia chuto, and Orientia species from Chile. In: 29th Meeting of the American Society for Rickettsiology. Poster Abstract # 46. Milwaukee, WI (2018).
37. Weitzel T, Martinez-Valdebenito C, Acosta-Jamett G, Jiang J, Gambra MP, Bidart T, et al. South American scrub typhus: first case series from continental Chile. American Society of Tropical Medicine and Hygiene 67th Annual Meeting. Abst #LB-5478 Poster. New Orleans, LA (2018).
38. Martínez-Valdebenito C, Angulo J, Jiang J, Acosta-Jamett G, Richards A, Weitzel T, Abarca K. Molecular description of Candidatus Orientia chiloensis, a novel Orientia species causing scrub typhus in Chile. In: 2nd Asia Pacific Rickettsia Conference. Chiang Rai (2019).
39. Martínez-Valdebenito C, Carolina Silva-de la Fuente M, Acosta-Jamett G, Weitzel T, Jiang J, Richards A, et al. Molecular detection of Orientia spp. in trombiculid mites from rodents on Chiloé Island, Chile. In: 2nd Asia Pacific Rickettsia Conference. Chiang Rai (2019).
40. Kuijpers S, Martínez-Valdebenito C, Azócar T, Jiang J, Acosta-Jamett G, Richards A, et al. InBios Scrub Typhus Detect IgG and IgM ELISA kits for the diagnosis of scrub typhus acquired in Chile: proposed cut-off values. In: 2nd Asia Pacific Rickettsia Conference. Chiang Rai (2019).
41. Abello R, Acosta-Jamett G, Martínez-Valdebenito C, Jiang J, Richards A, et al. Molecular detection of Orientia spp. in wild rodents from Chiloé Island, southern Chile. In: 2nd Asia Pacific Rickettsia Conference. Chiang Rai (2019).
42. Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, Jiang J, Farris C, Richards A, et al. Seroprevalence to spotted fever group, typhus group, and scrub typhus group rickettsial antigens among healthy adults in five regions in Chile. In: 2nd Asia Pacific Rickettsia Conference. Chiang Rai (2019).
43. Weitzel T, Jiang J, Acosta-Jamett G, Martinez-Valdebenito C, Lopez J, Richards AL, et al. Canine seroprevalence to Orientia species in southern Chile: A cross–sectional survey on the Chiloé Island. PLoS ONE. (2018) 13:e0200362. doi: 10.1371/journal.pone.0200362
44. Weitzel T, Aylwin M, Martinez-Valdebenito C, Jiang J, Munita JM, Thompson L, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. (2018) 4:10. doi: 10.1186/s40794-018-0070-8
45. Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, Jiang J, Richards AL, Abarca K. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. (2019) 25:1214–7. doi: 10.3201/eid2506.181860
46. Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, Silva-de La Fuente MC, Jiang J, Richards AL, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species. PLoS Negl Trop Dis. (2020) 14:e0007619. doi: 10.1371/journal.pntd.0007619
47. Weitzel T, Acosta-Jamett G, Jiang J, Martínez-Valdebenito C, Farris CM, Richards AL, et al. Human seroepidemiology of Rickettsia and Orientia species in Chile – A cross-sectional study in five regions. Ticks Tick Borne Dis. (2020) 11:101503. doi: 10.1016/j.ttbdis.2020.101503
48. Abarca K, López J, Acosta-Jamett G, Lepe P, Soares JF, Labruna MB. A third Amblyomma species and the first tick-borne rickettsia in Chile. J Med Entomol. (2012) 49:219–22. doi: 10.1603/ME11147
49. Abarca K, López J, Acosta-Jamett G, Martinez-Valdebenito C. Identificación de Rickettsia andeanae en dos regiones de Chile [Detection of Rickettsia andeanae in two regions of Chile]. Rev Chilena Infectol. (2013) 30:388–94. doi: 10.4067/S0716-10182013000400006
50. Cabello J, Altet L, Napolitano C, Sastre N, Hidalgo E, Dávila JA, et al. Survey of infectious agents in the endangered Darwin's fox (Lycalopex fulvipes): high prevalence and diversity of hemotrophic mycoplasmas. Vet Microbiol. (2013) 167:448–54. doi: 10.1016/j.vetmic.2013.09.034
51. Muñoz-Leal S, Tarragona EL, Martins TF, Martín CM, Burgos-Gallardo F, Nava S, et al. Liolaemus lizards (Squamata: Liolaemidae) as hosts for the nymph of Amblyomma parvitarsum (Acari: Ixodidae), with notes on Rickettsia infection. Exp Appl Acarol. (2016) 70:253–9. doi: 10.1007/s10493-016-0071-0
52. Ogrzewalska M, Nieri-Bastos FA, Marcili A, Nava S, González-Acuña D, Muñoz-Leal S, et al. A novel spotted fever group Rickettsia infecting Amblyomma parvitarsum (Acari: Ixodidae) in highlands of Argentina and Chile. Ticks Tick Borne Dis. (2016) 7:439–42. doi: 10.1016/j.ttbdis.2016.01.003
53. Poo-Muñoz DA, Elizondo-Patrone C, Escobar LE, Astorga F, Bermúdez SE, Martínez-Valdebenito C, et al. Fleas and ticks in carnivores from a domestic-wildlife interface: implications for public health and wildlife. J Med Entomol. (2016) 53:1433–43. doi: 10.1093/jme/tjw124
54. Walker DH. Scrub Typhus - Scientific neglect, ever-widening impact. N Engl J Med. (2016) 375:913–5. doi: 10.1056/NEJMp1608499
55. Sepúlveda DA, Zepeda-Paulo F, Ramírez CC, Lavandero B, Figueroa CC. Diversity, frequency, and geographic distribution of facultative bacterial endosymbionts in introduced aphid pests. Insect Sci. (2017) 24:511–21. doi: 10.1111/1744-7917.12313
56. Abarca K, Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G. Tifus de los matorrales, una enfermedad emergente en Chile [Scrub typhus, an emerging infectious disease in Chile]. Rev Chilena Infectol. (2018) 35:696–9. doi: 10.4067/S0716-10182018000600696
57. Cevidanes A, Di Cataldo S, Vera F, Lillo P, Millán J. Molecular detection of vector-borne pathogens in rural dogs and associated Ctenocephalides felis fleas (Siphonaptera: Pulicidae) in Easter Island (Chile). J Med Entomol. (2018) 55:1659–63. doi: 10.1093/jme/tjy141
58. Díaz FE, Abarca K, Kalergis AM. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection. Clin Microbiol Rev. (2018) 31:e00076–17. doi: 10.1128/CMR.00076-17
59. Müller A, Rodríguez E, Walker R, Bittencourt P, Pérez-Macchi S, Gonçalves LR, et al. Occurrence and genetic diversity of Bartonella spp. (Rhizobiales: Bartonellaceae) and Rickettsia spp (Rickettsiales: Rickettsiaceae) in cat fleas (Siphonaptera: Pulicidae) from Chile. J Med Entomol. (2018) 55:1627–32. doi: 10.1093/jme/tjy124
60. Muñoz-Leal S, Martins TF, Luna LR, Rodriguez A, Labruna MB. A new collection of Amblyomma parvitarsum (Acari: Ixodidae) in Peru, with description of a gynandromorph and report of Rickettsia detection. J Med Entomol. (2018) 55:464–7. doi: 10.1093/jme/tjx194
61. Muñoz-Leal S, Marcili A, Fuentes-Castillo D, Ayala M, Labruna MB. A relapsing fever Borrelia and spotted fever Rickettsia in ticks from an Andean valley, central Chile. Exp Appl Acarol. (2019) 78:403–20. doi: 10.1007/s10493-019-00389-x
62. Sacristán I, Sieg M, Acuña F, Aguilar E, García S, López MJ, et al. Molecular and serological survey of carnivore pathogens in free-roaming domestic cats of rural communities in southern Chile. J Vet Med Sci. (2019) 81:1740–8. doi: 10.1292/jvms.19-0208
63. Tapia T, Stenos J, Flores R, Duery O, Iglesias R, Olivares MF, et al. Evidence of Q fever and rickettsial disease in Chile. Trop Med Infect Dis. (2020) 5:99. doi: 10.3390/tropicalmed5020099
64. Müller A, Sepúlveda P, Di Cataldo S, Cevidanes A, Lisón F, Millán J. Molecular investigation of zoonotic intracellular bacteria in Chilean bats. Comp Immunol Microbiol Infect Dis. (2020) 73:101541. doi: 10.1016/j.cimid.2020.101541
65. Weitzel T, Aylwin M, Martínez-Valdebenito C, Acosta-Jamett G, Abarca K. Scrub typhus in Tierra del Fuego: a tropical rickettsiosis in a subantarctic region. Clin Microbiol Infect. (2020) in press. doi: 10.1016/j.cmi.2020.11.023
66. Kuchuloria T, Chitadze N, Gatserelia L, Karchava M, Endeladze M, Mshvidobadze K. Seroprevalence of Coxiella burnetii and rickettsial infections among febrile patients in the Country of Georgia. In: The ESCCAR International Congress on Rickettsia and Other Intracellular Bacteria. Lausanne (2015).
67. Myers TE, Lee JS, Francesconi SC, O'Guinn ML, Tsertsvadze N, Vephkhvadze N, et al. Rickettsia slovaca isolated from Dermacentor marginatus in the Republic of Georgia. In: Am Soc Trop Med & Hyg 57th Annual Meeting. New Orleans, LA. Abst # 2870 (2008).
68. Yarina TR, Myers TE, Lee JS, O'Guinn ML, Tsertsvadze N, Vephkhvadze N, et al. Surveillance of rickettsial pathogens isolated from ticks in the Republic of Georgia. In: Am Soc Trop Med Hyg 58th Annual Meeting. Abst 754. Washington, DC (2009).
69. Jiang J, You BJ, Liu E, Apte A, Yarina TR, Myers TE, et al. Development of three quantitative real-time PCR assays for the detection of Rickettsia raoultii, Rickettsia slovaca and Rickettsia aeschlimannii and their validation with ticks collected from the country of Georgia and the Republic of Azerbaijan. In: 6th International Meeting on Rickettsiae and Rickettsial Diseases. Heraklion (2011).
70. Sukhiashvili R, Zhgenti E, Khmaladze E, Tsertsvadze N, Alkhazashvili M, Francesconi S, et al. Detection of Rickettsia, Ehrlichia and Borrelia species in ticks from different regions of Georgia using real-time PCR assays. In: ASM BioDefense Annual Meeting. Washington DC (2013).
71. Zghenti E, Sukhiashvili R, Khmaladze E, Tsertsvadze N, Pisarcik S, Farris C, et al. Prevalence of Rickettsia, Ehrlichia and Borrelia in arthropods in Georgia. In: 62nd Annual meeting of The American Society of Tropical Medicine and Hygiene. Abst # 744. Atlanta, GA (2013).
72. Zghenti E, Sukhiashvili R, Khmaladze E, Tsertsvadze N, Lee J, Obiso RJ, et al. Rickettsia and Borrelia prevalence among ticks in Georgia. In: 2013 International Society for Disease Surveillance (ISDS) Conference. New Orleans, LA (2013).
73. Sukhiashvili R, Zhgenti E, Khmaladze E, Obiso RJ, Francesconi S, Farris CM, et al. Prevalence of arthropod-borne Rickettsia species in Georgia. In: The ESCCAR International Congress on Rickettsia and Other Intracellular Bacteria. Lausanne (2015).
74. Zhgenti E, Sukhiashvili R, Farris C, Jiang J, Richards AL. Rickettsia species identification among ticks in Georgia. In: 2016 CBRN Applied Sci Consequence Mgmt World Congress. Tbilisi (2016).
75. Zhgenti E, Sukhiashvili R, Obiso RJ, Farris CM, Jiang J, Richards AL. Spotted fever group Rickettsia species identified among ticks in Georgia. In: 28th Meeting of the American Society for Rickettsiology. Abst #93. Big Sky, MT (2016).
76. Sukhiashvili R, Zhgenti E, Jiang J, Richards AL. Identification of spotted fever group rickettsiae (SFGR) species not previously known to exist in Georgia. In: 2017 ASM Biothreats: Research, Response, and Policy. Abst #051. Washington, DC (2017).
77. Sukhiashvili R, Zhgenti E, Jiang J, St. John H, Burjanadze I, Gallagher T, et al. Identification, distribution and prevalence of tick-borne spotted fever group rickettsiae in the country of Georgia. In: One Health - 9th Tick and Tick-borne Pathogen conference and the 1st Asia Pacific Rickettsia Conference. Abst # 249. Cairns, QLD (2017).
78. Sukhiashvili R, Zhgenti E, Khmaladze E, Burjanadze I, Imnadze P, Jiang J, et al. Identification and distribution of nine tick-borne spotted fever group rickettsiae in the country of Georgia. Ticks Tick Borne Dis. (2020) 11:101470. doi: 10.1016/j.ttbdis.2020.101470
79. Khan SA, Bora T, Richards AL, Chattopadhyay S, Jiang J, Laskar B, et al. Molecular phylogenetics of Orientia tsutsugamushi strains circulating in Assam based on 56-kilodalton type-specific antigen gene. In: 17th International Congress on Infectious Diseases. Hyderabad (2016). doi: 10.1016/j.ijid.2016.02.421
80. Khan SA, Bora T, Chattopadhyay S, Richards A. Human case of Rickettsia felis infection in the Indian Subcontinent. In: One Health - 9th Tick and Tick-borne Pathogen Conference and the 1st Asia Pacific Rickettsia Conference. Poster Presentation, Abst # 112. Cairns, QLD (2017).
81. Khan SA, Bora T, Chattopadhyay S, Jiang J, Richards AL, Dutta P. Seroepidemiology of rickettsial infections in Northeast India. Trans Royal Soc Trop Med Hyg. (2016) 110:487–94. doi: 10.1093/trstmh/trw052
82. Khan SA, Bora T, Saikia J, Shah A, Richards AL, Chattopadhyay S, et al. Seroprevalence of typhus group rickettsial infections in the north-east region of India. Indian J Med Res. (2019) 150:203–5. doi: 10.4103/ijmr.IJMR_332_19
83. Varghese GM, Janardhanan J, Mahajan SK, Tariang D, Trowbridge P, Prakash JA, et al. Molecular epidemiology and genetic diversity of Orientia tsutsugamushi from patients with scrub typhus in 3 regions of India. Emerg Infect Dis. (2015) 21:64–9. doi: 10.3201/eid2101.140580
84. Bora T, Khan SA, Jampa L, Laskar B. Genetic diversity of Orientia tsutsugamushi strains circulating in Northeast India. Trans R Soc Trop Med Hyg. (2018) 112:22–30. doi: 10.1093/trstmh/try019
85. Khan SA, Bora T, Thangaraj JWV, Murhekar MV. Spotted fever group rickettsiae among children with acute febrile illness, in Gorakhpur, India. J Trop Pediatr. (2020) fmaa031. fmaa031. doi: 10.1093/tropej/fmaa031
86. Andryushchenko A, Ayazbayev T, Richards A, Pisarcok S. Tick identification in northwestern Kazakhstan using morphological and molecular characteristics. 16th International Congress on Infectious Diseases, Cape Town, South Africa. 2-5 Apr 2014. Abst# 60.014. Int J Infect Dis. (2014) 21S:393. doi: 10.1016/j.ijid.2014.03.1231
87. Andryushchenko AV, Ayazbayev TZ, Bidashko FG, Tanitovsky VA, Farris CM, Richards AL. Detection of rickettsial DNA from Ixodid ticks of the West Kazakhstan region. In: ASM 2014. Abst: #852. Boston, MA (2014).
88. Kyraubayev K, Shapiyeva Z, Utegenova U, Zhandosov S, Beysenaeva M, Ziyadina L, et al. Study of Dermacentor marginatus ticks for rickettsiae in Central Kazakhstan. In: ASM 2014. Abst: #858. Boston, MA (2014).
89. Nurmakhanov T, Sansyzbayev Y, Yeskhodzhayev O, Vilkova A, Berdibekov A, Matzhanova, et al. Presence of Tick-Borne Rickettsia Pathogens in Southern Kazakhstan. Boston, MA: ASM Microbe (2016).
90. Nurmakhanov T, Sansyzbayev Y, St. John H, Farris C, Richards A. Flea-borne rickettsiae in Almaty Oblast, Kazakhstan. 14th Annual International Society for Disease Surveillance Conference, Denver, December 9-10, 2015. Abst #131. J Publ Health Inform. 8 (2016). doi: 10.5210/ojphi.v8i1.6565
91. Sansyzbayev Y, Nurmakhanov T, Yeskhodzhayev O, Vilkova A, Kurmanov B, Begimbayeva E, et al. Effect of Rickettsia spp. upon fitness of Yersinia pestis in fleas that vector plague in the Republic of Kazakhstan. In: ASM Biothreats: Research, Response, and Policy. Abst #049. Washington DC (2017).
92. Jiang J, St John H, Sansyzbayev Y, Nurmakhanov T, Loyola S, Leguia M, et al. Rickettsia asembonensis, Kazakhstan and beyond. In: One Health - 9th Tick and Tick-borne Pathogen conference and the 1st Asia Pacific Rickettsia Conference. Oral Presentation, Abst # 251 Cairns, QLD (2017).
93. Yerubayev T, Nurmakhanov T, Meka-Mechenko T, Abdirassilova A, Yeskhojayev O, Vilkova A, et al. Investigating the presence of Rickettsia spp. and Yersinia pestis in flea from the natural plague foci of Kazakhstan. In: 30th Meeting of the American Society for Rickettsiology. Poster. Abst #15. Santa Fe, NM (2019).
94. Sansyzbayev Y, Nurmakhanov T, Berdibekov A, Vilkova A, Yeskhodzhayev O, St. John HK, et al. Survey for rickettsiae within fleas of Great Gerbils, Almaty Oblast, Kazakhstan. Vector Borne Zoonotic Dis. (2017) 17:172–8. doi: 10.1089/vbz.2016.2049
95. Turebekov N, Abdiyeva K, Yegemberdiyeva R, Dmitrovsky A, Yeraliyeva L, Shapiyeva Z, et al. Prevalence of Rickettsia species in ticks including identification of unknown species in two regions in Kazakhstan. Parasit Vectors. (2019) 12:197. doi: 10.1186/s13071-019-3440-9
96. Rakotonanahary RDL, Harrison A, Maina AN, Jiang J, Richards AL, Rajerison M, et al. Molecular and serological evidence of flea-associated typhus group and spotted fever group rickettsial infections in Madagascar. Parasit Vectors. (2017) 10:125. doi: 10.1186/s13071-017-2061-4
97. Ehlers J, Krüger A, Rakotondranary SJ, Ratovonamana RY, Poppert S, Ganzhorn JU, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. (2020) 205:105339. doi: 10.1016/j.actatropica.2020.105339
98. Keller C, Krüger A, Schwarz NG, Rakotozandrindrainy R, Rakotondrainiarivelo JP, Razafindrabe T, et al. High detection rate of Rickettsia africae in Amblyomma variegatum but low prevalence of anti-rickettsial antibodies in healthy pregnant women in Madagascar. Ticks Tick Borne Dis. (2016) 7:60–5. doi: 10.1016/j.ttbdis.2015.08.005
99. Wilkinson DA, Duron O, Cordonin C, Gomard Y, Ramasindrazana B, Mavingui P, et al. The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: a community shaped by host ecology, bacterial transmission mode, and host-vector specificity. Appl Environ Microbiol. (2016) 82:1778–88. doi: 10.1128/AEM.03505-15
100. Lado P, Qurollo B, Williams C, Junge R, Klompen H. The microbiome of Haemaphysalis lemuris (Acari: Ixodidae), a possible vector of pathogens of endangered lemur species in Madagascar. Ticks Tick Borne Dis. (2018) 9:1252–60. doi: 10.1016/j.ttbdis.2018.05.003
101. Ehlers J, Ganzhorn JU, Silaghi C, Krüger A, Pothmann D, Ratovonamana RY, et al. Tick (Amblyomma chabaudi) infestation of endemic tortoises in southwest Madagascar and investigation of tick-borne pathogens. Ticks Tick Borne Dis. (2016) 7:378–83. doi: 10.1016/j.ttbdis.2015.12.011
102. Phomjareet S, Chaveerach P, Suksawat F, Richards AL. Antibody against spotted fever group rickettsiae in cats residing in the surrounding areas of Rajabhat Maha Sarakham University, Mahasarakham, Thailand. In: 17th Annual Khon Kaen Veterinary Annual International Conference (KVAC) 2016. Khon Kaen (2016).
103. Phomjareet S, Chaveerach P, Jiang J, Suksawat F, Richards AL. Spotted fever group Rickettsia infection of cats and cat fleas in Northeast Thailand. Vector Borne Zoonotic Dis. (2020) 20:566–71. doi: 10.1089/vbz.2019.2564
104. Thipmontree W, Suputtamongkol Y, Tantibhedhyangkul W, Suttinont C, Wongswat E, Silpasakorn S. Human leptospirosis trends: northeast Thailand, 2001-2012. Int J Environ Res Public Health. (2014) 11:8542–51. doi: 10.3390/ijerph110808542
105. Phetsouvanh R, Sonthayanon P, Pukrittayakamee S, Paris DH, Newton PN, Feil EJ, et al. The diversity and geographical structure of Orientia tsutsugamushi strains from scrub typhus patients in Laos. PLoS Negl Trop Dis. 9:e0004024. doi: 10.1371/journal.pntd.0004024
106. Kurhanova I, Loginov J, Tarasyuk O, Chipak N, Kitsara M, Bek N, et al. Surveillance for evidence of typhus group rickettsia infections among people residing in western Ukraine (PDG P364). In: Biological Threat Reduction and Cooperative Biological Engagement Programs (BTRP/CBEP) Annual Science and Disease Surveillance Review. Garmisch-Partenkirchen (2011).
107. Kurhanova I, Tarasyuk O, Chipak N, Kitsara M, Bek N, Loginov J, et al. Seroprevalence of spotted fever group rickettsioses in western Ukraine and prospectives for the future. In: 6th International Meeting on Rickettsiae and Rickettsial Diseases. Heraklion (2011).
108. Hamel D, Silaghi C, Zapadynska S, Kudrin A, Pfister K. Vector-borne pathogens in ticks and EDTA-blood samples collected from client-owned dogs, Kiev, Ukraine. Ticks Tick Borne Dis. (2012) 4:152–5. doi: 10.1016/j.ttbdis.2012.08.005
109. Karbowiak G, Slivinska K, Chmielewski T, Barszcz K, Tylewska-Wierzbanowska S, Werszko J, et al. Rickettsia raoultii in Dermacentor reticulatus ticks, Chernobyl Exclusion Zone, Ukraine, 2010. Emerg Infect Dis. (2016) 22:2214–5. doi: 10.3201/eid2212.160678
110. Didyk YM, Blanárová L, Pogrebnyak S, Akimov I, Petko B, Víchová B. Emergence of tick-borne pathogens (Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Ricketsia raoultii and Babesia microti) in the Kyiv urban parks, Ukraine. Ticks Tick Borne Dis. (2017) 8:219–25. doi: 10.1016/j.ttbdis.2016.10.002
111. Rogovskyy A, Batool M, Gillis DC, Holman PJ, Nebogatkin IV, Rogovska YV, et al. Diversity of Borrelia spirochetes and other zoonotic agents in ticks from Kyiv, Ukraine. Ticks Tick Borne Dis. (2018) 9:404–9. doi: 10.1016/j.ttbdis.2017.12.006
112. Rogovskyy AS, Threadgill DW, Akimov IA, Nebogatkin IV, Rogovska YV, Melnyk MV, et al. Borrelia and other zoonotic pathogens in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Chernobyl Exclusion Zone on the 30th Anniversary of the Nuclear Disaster. Vector Borne Zoonotic Dis. (2019) 19:466–73. doi: 10.1089/vbz.2018.2318
113. Trung NV, Hoi LT, Hoa TM, Dien VM, Lien VN, Luan PT, et al. Clinical presentations of rickettsial diseases in northern Vietnam. In: One Health - 9th Tick and Tick-borne Pathogen conference and the 1st Asia Pacific Rickettsia Conference, Abst # 177. Cairns, QLD (2017).
114. Hoa TM, Ha DT, Hoa LNM, Lien VN, Hoi LT, Trung NV, et al. Phylogenetic analysis of Orientia tsutsugamushi strains based on the sequence homologies of 56 kDa type-specific antigen genes. In: The National Scientific Conference on Infectious Diseases, HIV/AIDS and the 8th ASEAN Conference on Tropical Medicine and Parasitology. Nha Trang (2018).
115. Le TH, Nguyen T, Tran H, Ma H, Nguyen H, Tran G, et al. Q fever among acute undifferentiated fever patients in Vietnam. In: 2nd Asia Pacific Rickettsia Conference. Chiang Rai (2019).
116. Trung NV, Hoi LT, Thuong NTH, Toan TK, Huong TTK, Hoa TM, et al. Seroprevalence of scrub typhus, typhus, and spotted fever among rural and urban populations of Northern Vietnam. Am J Trop Med Hyg. (2017) 96:1084–7. doi: 10.4269/ajtmh.16-0399
117. Trung NV, Hoi LT, Dien VM, Huong DT, Hoa TM, Lien VN, et al. Clinical presentation and molecular diagnosis of rickettsial diseases in northern Vietnam, 2015-2017. Emerg Infect Dis. (2019) 25:633–41. doi: 10.3201/eid2504.180691
118. Trung NV, Hoi LT, Cuong DD, Ha DT, Hoa TM, Lien VN, et al. Analysis of the 56-kDa type specific antigen gene of Orientia tsutsugamushi from northern Vietnam. PLoS One. (2019) 14:e0221588. doi: 10.1371/journal.pone.0221588
119. Duong V, Mai TT, Blasdell K, Lo le V, Morvan C, Lay S, et al. Molecular epidemiology of Orientia tsutsugamushi in Cambodia and Central Vietnam reveals a broad region-wide genetic diversity. Infect Genet Evol. (2013) 15:35–42. doi: 10.1016/j.meegid.2011.01.004
120. Nadjm B, Thuy PT, Trang VD, Ha le D, Kinh NV, Wertheim HF. Scrub typhus in the northern provinces of Vietnam: an observational study of admissions to a national referral hospital. Trans R Soc Trop Med Hyg. (2018) 108:739–40. doi: 10.1093/trstmh/tru145
121. Hamaguchi S, Cuong NC, Tra DT, Doan YH, Shimizu K, Tuan NQ, et al. Clinical and epidemiological characteristics of scrub typhus and murine typhus among hospitalized patients with acute undifferentiated fever in northern Vietnam. Am J Trop Med Hyg. (2015) 92:972–8. doi: 10.4269/ajtmh.14-0806
122. Hotta K, Pham HT, Hoang HT, Trang TC, Vu TN, Ung TT, et al. Prevalence and phylogenetic analysis of Orientia tsutsugamushi in small mammals in Hanoi, Vietnam. Vector Borne Zoonotic Dis. (2016) 16:96–102. doi: 10.1089/vbz.2015.1831
123. Le-Viet N, Phan DT, Le-Viet N, Trinh S, To M, Raoult D, et al. Dual genotype Orientia tsutsugamushi Infection in patient with rash and eschar, Vietnam, 2016. Emerg Infect Dis. (2018) 24:1520–3. doi: 10.3201/eid2408.171622
124. Le Viet N, Laroche M, Thi Pham HL, Viet NL, Mediannikov O, Raoult D, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. PLoS Negl Trop Dis. (2017) 11:e0005397. doi: 10.1371/journal.pntd.0005397
125. Nguyen HLK, Pham HTT, Nguyen TV, Hoang PV, Le MTQ, Takemura T, et al. The genotypes of Orientia tsutsugamushi, identified in scrub typhus patients in northern Vietnam. Trans R Soc Trop Med Hyg. (2017) 111:137–9. doi: 10.1093/trstmh/trx022
126. Hornok S, Szoke K, Meli ML, Sándor AD, Görföl T, Estók P, et al. Molecular detection of vector-borne bacteria in bat ticks (Acari: Ixodidae, Argasidae) from eight countries of the Old and New Worlds. Parasit Vectors. (2019) 12:50. doi: 10.1186/s13071-019-3303-4
127. Le-Viet N, Le VN, Chung H, Phan DT, Phan QD, Cao TV, et al. Prospective case-control analysis of the aetiologies of acute undifferentiated fever in Vietnam. Emerg Microbes Infect. (2019) 8:339–52. doi: 10.1080/22221751.2019.1580539
128. Binh MD, Truong SC, Thanh DL, Ba LC, Van NL, Nhu BD. Identification of trombiculid chigger mites collected on rodents from southern Vietnam and molecular detection of rickettsiaceae pathogen. Korean J Parasitol. (2020) 58:445–50. doi: 10.3347/kjp.2020.58.4.445
129. Le Van N, Pham Van C, Nguyen Dang M, Dao Van T, Le T, Do Q, Vu Hoang H, et al. Clinical features, laboratory characteristics and prognostic factors of severity in patients with rickettsiaceae at two military hospitals, northern Vietnam. Infect Drug Resist. (2020) 13:2129–38. doi: 10.2147/IDR.S253540
130. Nguyen VL, Colella V, Greco G, Fang F, Nurcahyo W, Hadi UK, et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasit Vectors. (2020) 13:420. doi: 10.1186/s13071-020-04288-8
131. Coleman RE, Sangkasuwan V, Suwanabun N, Ching W-M, Sattabongkot J, Eamsila C, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. (2002) 67:497–503. doi: 10.4269/ajtmh.2002.67.497
132. Blacksell SD, Lim C, Tanganuchitcharnchai A, Jintaworn S, Kantipong P, Richards AL, et al. Optimal cut-off and accuracy of an IgM enzyme-linked immunosorbent assay for diagnosis of acute scrub typhus in northern Thailand: an alternative reference method to the IgM immunofluorescence assay. J Clin Microbiol. (2016) 54:1472–8. doi: 10.1128/JCM.02744-15
133. Paris DH, Dumler JS. State of the art of diagnosis of rickettsial diseases: the use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Curren Opin Infect Dis. (2016) 29:433–9. doi: 10.1097/QCO.0000000000000298
134. Renvoise A, Delaunay P, Blanchouin E. Urban family cluster of spotted fever rickettsiosis linked to Rhipicephalus sanguineus infected with Rickettsia conorii subsp. caspia and Rickettsia massiliae Ticks Tick Borne Dis. (2012) 3:389–92. doi: 10.1016/j.ttbdis.2012.10.008
135. Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector Borne Zoonotic Dis. (2011) 11:969–77. doi: 10.1089/vbz.2010.0099
136. Jiang J, Stromdahl EY, Richards AL. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. (2012) 12:175–82. doi: 10.1089/vbz.2011.0614
137. Lee JK, Moraru GM, Stokes JV, Wills RW, Mitchell E, Unz E, et al. Rickettsia parkeri and “Candidatus Rickettsia andeanae” in questing Amblyomma maculatum (Acari: Ixodidae) from Mississippi. J Med Entomol. (2017) 54:476–80. doi: 10.1093/jme/tjw175
138. Eremeeva ME, Capps D, McBride CL, Williams-Newkirk AJ, Dasch GA, Salzer JS, et al. Detection of Rickettsia asembonensis in Fleas (Siphonaptera: Pulicidae, Ceratophyllidae) collected in five counties in Georgia, United States. J Med Entomol. (2020) 57:1246–53. doi: 10.1093/jme/tjaa029
139. Groves MG, Osterman JV. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. (1978) 19:583–8. doi: 10.1128/IAI.19.2.583-588.1978
140. Sunyakumthorn P, Sumonwiriya M, Im-erbsin R, Chumpolkulwong K, Cho N-H, Dunachie SJ, et al. A nonhuman primate model for scrub typhus. In: Joint International Tropical Medicine Meeting. Bangkok (2020).
Keywords: rickettsioses, scrub typhus, cooperative international research, surveillance, orientia
Citation: Jiang J, Farris CM, Yeh KB and Richards AL (2021) International Rickettsia Disease Surveillance: An Example of Cooperative Research to Increase Laboratory Capability and Capacity for Risk Assessment of Rickettsial Outbreaks Worldwide. Front. Med. 8:622015. doi: 10.3389/fmed.2021.622015
Received: 27 October 2020; Accepted: 20 January 2021;
Published: 02 March 2021.
Edited by:
Jeanne Marie Fair, Los Alamos National Laboratory (DOE), United StatesReviewed by:
Andrew W. Bartlow, Los Alamos National Laboratory (DOE), United StatesCopyright © 2021 Jiang, Farris, Yeh and Richards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allen L. Richards, QWxsZW4uUmljaGFyZHNAY29tY2FzdC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.